Sex Differences in Prognosis of Heart Failure Due to Ischemic and Nonischemic Cardiomyopathy
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
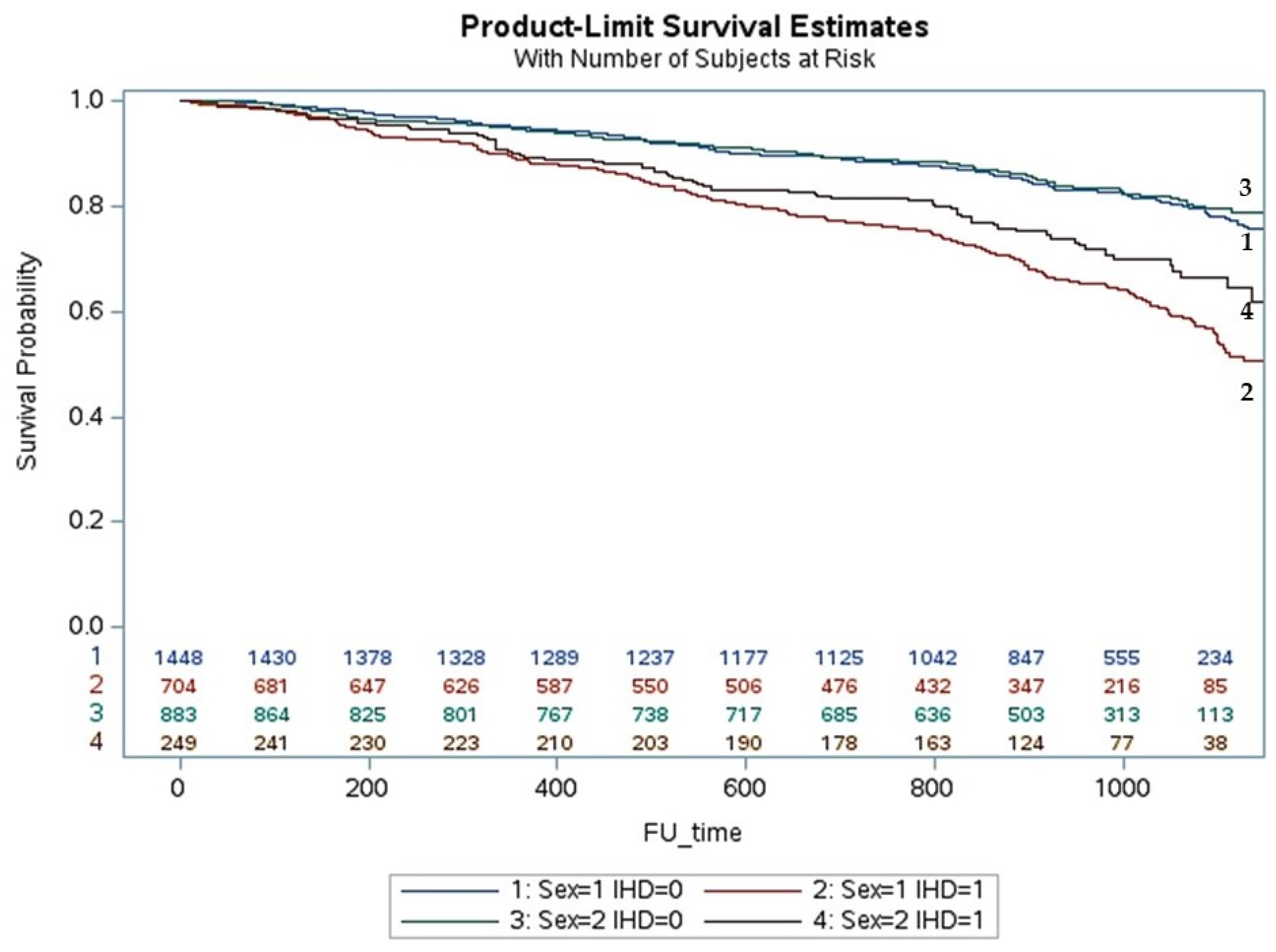
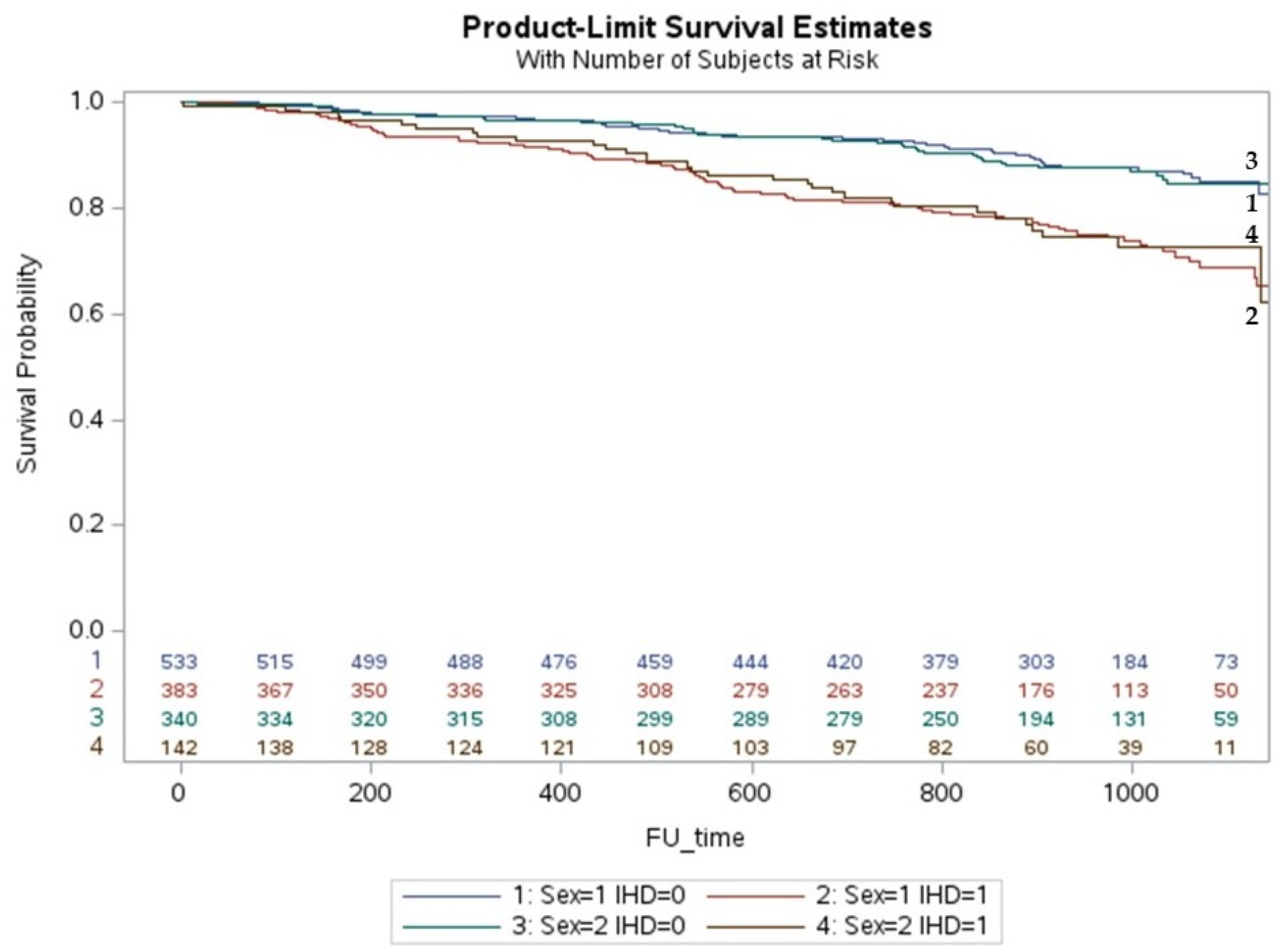
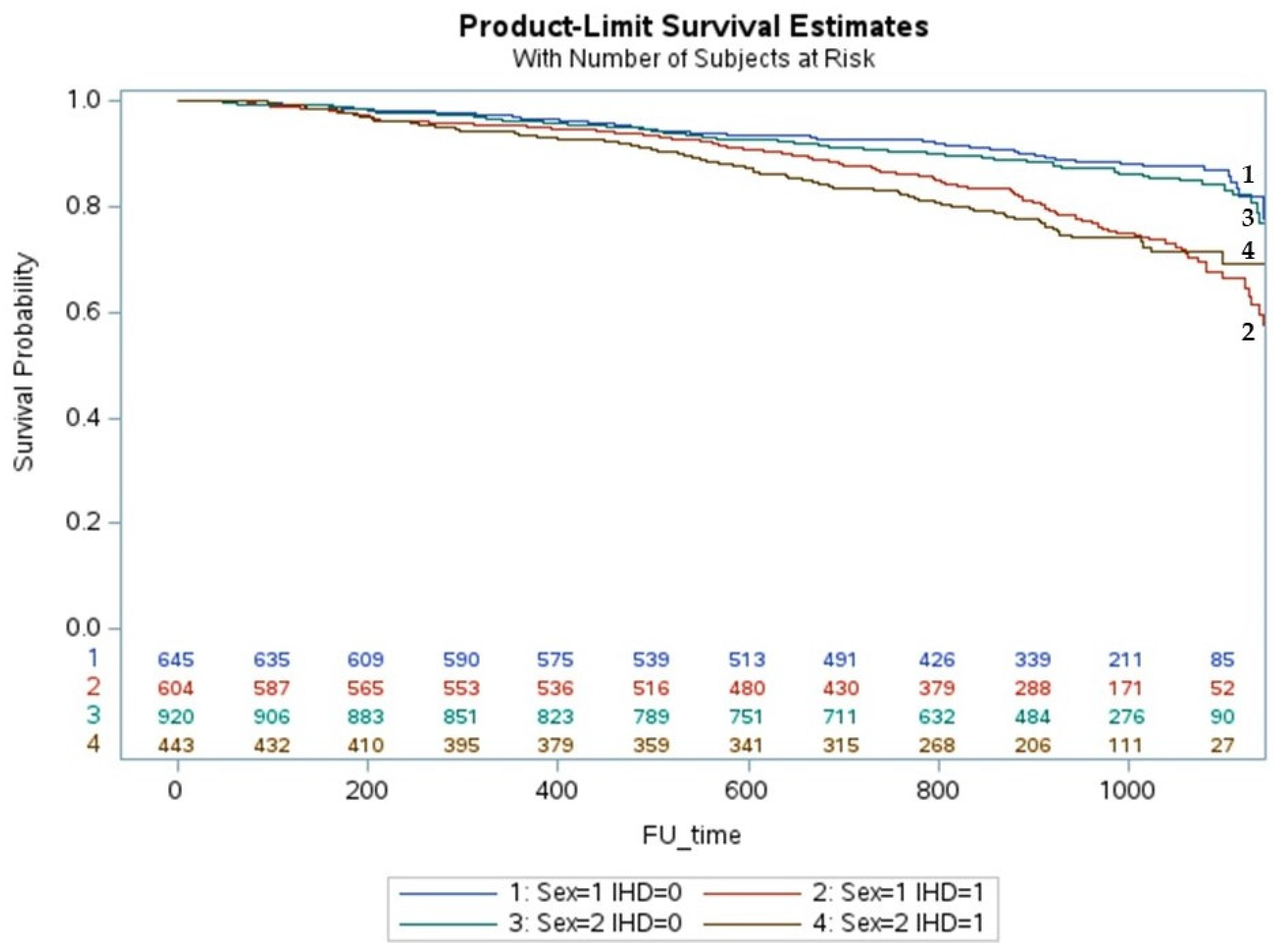
3. Results
Multivariate Analysis and Predictors of All Causes of Death
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar]
- Braunwald, E. The war against heart failure: The Lancet lecture. Lancet 2015, 385, 812–824. [Google Scholar]
- Halliday, B.P.; Gulati, A.; Ali, A.; Newsome, S.J.; Lota, A.S.; Tayal, U.; Vassiliou, V.S.; Aržanauskaitė, M.; Izgi, C.; Krishnathasan, K.; et al. Sex- and age-based differences in the natural history and outcome of dilated cardiomyopathy. Eur. J. Heart Fail. 2018, 20, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Cannatà, A.; Fabris, E.; Merlo, M.; Artico, J.; Gentile, P.; Pio Loco, C.; Ballaben, A.; Ramani, F.; Barbati, G.; Sinagra, G. Sex Differences in the Long-term Prognosis of Dilated Cardiomyopathy. Can. J. Cardiol. 2020, 36, 37–44. [Google Scholar] [CrossRef]
- Felker, G.M.; Shaw, L.K.; O’Connor, C.M. A standardized definition of ischemic cardiomyopathy for use in clinical research. J. Am. Coll. Cardiol. 2002, 39, 210–218. [Google Scholar] [PubMed]
- Brasil. Receita Federal. Brasília. 2022. Available online: https://servicos.receita.fazenda.gov.br/Servicos/CPF/ConsultaSituacao/ConsultaPublica.asp (accessed on 11 April 2022).
- O’Meara, E.; Clayton, T.; McEntegart, M.B.; McMurray, J.J.; Piña, I.L.; Granger, C.B.; Östergren, J.; Michelson, E.L.; Solomon, S.D.; Pocock, S.J.; et al. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: Results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007, 115, 3111–3120. [Google Scholar]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.; Rouleau, J.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Krum, H.; Abraham, W.T.; Dickstein, K.; Køber, L.V.; Desai, A.S.; Solomon, S.D.; Greenlaw, N.; Ali, M.A.; Chiang, Y.; et al. Aliskiren, enalapril, or aliskiren and enalapril in heart failure. N. Engl. J. Med. 2016, 374, 1521–1532. [Google Scholar] [CrossRef]
- Dewan, P.; Rørth, R.; Jhund, P.S.; Shen, L.; Raparelli, V.; Petrie, M.C.; Abraham, W.T.; Desai, A.S.; Dickstein, K.; Køber, L.V.; et al. Differential Impact of Heart Failure with Reduced Ejection Fraction on Men and Women. J. Am. Coll. Cardiol. 2019, 73, 29–40. [Google Scholar]
- Adams, K.F., Jr.; Fonarow, G.C.; Emerman, C.L.; LeJemtel, T.; Costanzo, M.R.; Abraham, W.T.; Berkowitz, R.L.; Galvao, M.; Horton, D.P. Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am. Heart J. 2005, 149, 209–216. [Google Scholar] [CrossRef]
- Lainščak, M.; Milinković, I.; Polovina, M.; Crespo-Leiro, M.G.; Lund, L.H.; Anker, S.D.; Laroche, C.; Ferrari, R.; Coats, A.J.S.; European Society of Cardiology Heart Failure Long-Term Registry Investigators Group; et al. Sex- and age-related differences in the management and outcomes of chronic heart failure: An analysis of patients from the ESC HFA EORP Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2020, 22, 92–102. [Google Scholar]
- Meyer, M.R.; Barton, M. Estrogens and coronary artery disease: New clinical perspectives. Adv. Pharmacol. 2016, 77, 307–360. [Google Scholar]
- Mahmoodzadeh, S.; Dworatzek, E. The Role of 17β-Estradiol and Estrogen Receptors in Regulation of Ca2+ Channels and Mitochondrial Function in Cardiomyocytes. Front. Endocrinol. 2019, 10, 310. [Google Scholar]
- Piro, M.; Della Bona, R.; Abbate, A.; Biasucci, L.M.; Crea, F. Sex-related differences in myocardial remodeling. J. Am. Coll. Cardiol. 2010, 55, 1057–1065. [Google Scholar] [PubMed]
- Powell, B.S.; Dhaher, Y.Y.; Szleifer, I.G. Review of the Multiscale Effects of Female Sex Hormones on Matrix Metalloproteinase-Mediated Collagen Degradation. Crit. Rev. Biomed. Eng. 2015, 43, 401–428. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Moulin, M.; Piquereau, J.; Lemaire, C.; Mericskay, M.; Veksler, V.; Garnier, A. Mitochondria: A central target for sex differences in pathologies. Clin. Sci. 2017, 131, 803–822. [Google Scholar]
- Redfield, M.M.; Jacobsen, S.J.; Burnett, J.C., Jr.; Mahoney, D.W.; Bailey, K.R.; Rodeheffer, R.J. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA 2003, 289, 194–202. [Google Scholar]
- Regitz-Zagrosek, V.; Oertelt-Prigione, S.; Prescott, E.; Franconi, F.; Gerdts, E.; Foryst-Ludwig, A.; Maas, A.H.; Kautzky-Willer, A.; Knappe-Wegner, D.; Kintscher, U.; et al. Gender in cardiovascular diseases: Impact on clinical manifestations, management, and outcomes. Eur. Heart J. 2016, 37, 24–34. [Google Scholar] [PubMed]
- Mansur, A.P.; Del Carlo, C.H.; Gonçalinho, G.H.F.; Avakian, S.D.; Ribeiro, L.C.; Ianni, B.M.; Fernandes, F.; Cesar, L.A.; Bocchi, E.A.; Pereira-Barretto, A.C. Sex Differences in Heart Failure Mortality with Preserved, Mildly Reduced and Reduced Ejection Fraction: A Retrospective, Single-Center, Large-Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 16171. [Google Scholar]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Omland, T.; Wolf, P.A.; Vasan, R.S. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N. Engl. J. Med. 2004, 350, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, K.; Minami, Y.; Otsubo, S.; Sato, N.; Investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) Registry. Sex Differences in Left Ventricular Cavity Dilation and Outcomes in Acute Heart Failure Patients with Left Ventricular Systolic Dysfunction. Can. J. Cardiol. 2018, 34, 477–484. [Google Scholar] [CrossRef]
- Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: An individual patient data meta-analysis. Eur. Heart J. 2012, 33, 1750–1757. [Google Scholar]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [PubMed]
- Van Rooij, E.; Olson, E.N. MicroRNAs: Powerful new regulators of heart disease and provocative therapeutic targets. J. Clin. Investig. 2007, 117, 2369–2376. [Google Scholar] [PubMed]
- Hershberger, R.E.; Morales, A.; Siegfried, J.D. Clinical and genetic issues in dilated cardiomyopathy: A review for genetics professionals. Genet. Med. 2010, 12, 655–667. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]

| All Patients | niCMP | iCMP | p | |
|---|---|---|---|---|
| n = 7483 | n = 4883 (65.2) | n = 2600 (34.8) | ||
| Age (Years) | 64.26 ± 14.23 | 61.90 ± 14.97 | 68.71 ± 11.46 | <0.001 |
| Female (%) | 3066 (41.0) | 2205 (45.2) | 861 (33.1) | <0.001 |
| Comorbidities | ||||
| Myocardial infarction (%) | 1350 (18.0) | 3 (0.04) | 1347 (51.8) | <0.001 |
| Diabetes (%) | 1496 (20.0) | 630 (12.9) | 866 (34.8) | <0.001 |
| Chronic kidney disease (%) | 817 (10.9) | 401 (8.2) | 416 (16.0) | <0.001 |
| Stroke (%) | 317 (4.2) | 152 (3.1) | 165 (6.4) | <0.001 |
| Atrial fibrillation (%) | 1356 (18.1) | 897 (18.4) | 459 (17.7) | 0.4439 |
| Number of comorbidities (%) | 3659 (48.9) | 1636 (33.5) | 2023 (77.8) | <0.001 |
| n = 1 | 2281 (30.5) | 1209 (24.8) | 1072 (41.2) | |
| n = 2 | 1009 (13.5) | 348 (7.13) | 661 (25.4) | |
| n = 3 | 300 (4.0) | 72 (1.47) | 228 (8.77) | |
| n ≥ 4 | 69 (0.9) | 7 (0.14) | 62 (2.38) | |
| Medication | ||||
| ACE inhibitor or BRA | 4482 (59.9) | 2940 (60.2) | 1549 (59.6) | 0.790 |
| Beta-blocker | 3839 (51.3) | 2471 (50.6) | 1344 (51.7) | 0.610 |
| Spironolactone | 2095 (28.0) | 1323 (27.1) | 762 (29.3) | 0.128 |
| Diuretics | 2499 (33.4) | 2217 (45.4) | 1199 (46.1) | 0.719 |
| Surgical intervention | ||||
| Coronary artery bypass graft (%) | 741 (9.9) | 0 (0.00) | 741 (28.5) | <0.001 |
| Percutaneous coronary intervention (%) | 277 (3.7) | 1 (0.02) | 276 (10.6) | <0.001 |
| Pacemaker implantation (%) | 467 (6.2) | 372 (7.6) | 95 (3.7) | <0.001 |
| Implantable cardioverter defibrillators (%) | 224 (3.0) | 116 (2.4) | 108 (4.2) | <0.001 |
| Cardiac resynchronization therapy (%) | 275 (3.7) | 221 (4.5) | 54 (2.1) | <0.001 |
| Transplant (%) | 175 (2.3) | 141 (2.9) | 34 (1.3) | <0.001 |
| Hospitalization (%) | 2236 (30.0) | 1279 (26.2) | 957 (36.8) | <0.001 |
| Echocardiogram | ||||
| LVEF baseline (%) | 43.01 ± 15.39 | 41.87 ± 15.55 | 45.14 ± 14.86 | <0.001 |
| LVEF final (%) | 44.87 ± 14.82 | 44.76 ± 14.99 a | 45.08 ± 14.47 | 0.364 |
| LVDD baseline (mm) | 58.22 ± 9.57 | 59.22 ± 9.88 | 56.31 ± 8.66 | <0.001 |
| LVDD final (mm) | 57.72 ± 9.94 | 58.15 ± 10.31 b | 56.90 ± 9.15 b | <0.001 |
| Type of heart failure | ||||
| Reduced EF (%) | 3359 (44.9) | 2383 (48.8) | 976 (37.5) | <0.001 |
| Mildly reduced EF (%) | 1436 (19.2) | 896 (18.4) | 540 (20.8) | 0.0114 |
| Preserved EF (%) | 2688 (35.9) | 1604 (32.9) | 1084 (41.7) | <0.001 |
| Death (%) | 1475 (19.5) | 745 (15.3) | 730 (28.1) | <0.001 |
| niCMP n = 4883 (65.2) | iCMP n = 2600 (34.8) | |||||
|---|---|---|---|---|---|---|
| Men n = 2678 (54.8) | Women n = 2205 (45.2) | p | Men n = 1736 (66.9) | Women n = 861 (33.1) | p | |
| Age (Years) | 60.18 ± 14.24 | 64.0 ± 15.5 | <0.001 | 68.12 ± 11.05 | 69.9 ± 12.18 | <0.001 |
| Comorbidities | ||||||
| Myocardial infarction (%) | 0 (0.00) | 3 (0.14) | 0.056 | 921 (53.0) | 426 (49.5) | 0.094 |
| Diabetes (%) | 306 (11.4) | 324 (14.7) | <0.001 | 561 (32.3) | 305 (35.4) | 0.107 |
| Chronic kidney disease (%) | 245 (9.2) | 156 (7.1) | 0.009 | 308 (17.7) | 108 (12.5) | <0.001 |
| Stroke (%) | 94 (3.5) | 58 (2.6) | 0.078 | 111 (6.4) | 54 (6.3) | 0.913 |
| Atrial fibrillation (%) | 539 (20.1) | 358 (16.2) | <0.001 | 328 (18.9) | 131 (15.2) | 0.022 |
| Number of comorbidities (%) | 916 (34.2) | 720 (32.7) | 0.334 | 1362 (78.5) | 661 (76.8) | 0.126 |
| n = 1 | 669 (25.0) | 540 (24.5) | 705 (40.5) | 367 (42.6) | ||
| n = 2 | 203 (7.6) | 145 (6.6) | 449 (25.8) | 212 (24.6) | ||
| n = 3 | 42 (1.6) | 30 (1.4) | 159 (9.1) | 69 (8.0) | ||
| n ≥ 4 | 2 (0.1) | 5 (0.2) | 49 (2.3) | 13 (1.5) | ||
| Medication | ||||||
| ACE inhibitor or ARB | 1649 (61.6) | 1291 (58.5) | 0.283 | 1062 (61.2) | 487 (56.6) | 0.254 |
| Beta-blocker | 1344 (50.2) | 1127 (51.1) | 0.713 | 910 (52.4) | 434 (50.4) | 0.585 |
| Spironolactone | 744 (27.8) | 579 (26.3) | 0.366 | 519 (29.9) | 243 (28.2) | 0.514 |
| Diuretics | 1245 (46.5) | 972 (44.1) | 0.302 | 783 (45.1) | 416 (48.3) | 0.350 |
| Surgical intervention | ||||||
| Coronary artery bypass graft (%) | 0 (0) | 0 (0) | 511 (29.4) | 230 (26.7) | 0.156 | |
| Percutaneous coronary intervention (%) | 1 (0.04) | 0 (0) | 187 (10.6) | 89 (10.3) | 0.746 | |
| Pacemaker implantation (%) | 162 (6.1) | 210 (9.5) | <0.001 | 64 (3.7) | 31 (3.6) | 0.919 |
| Implantable cardioverter defibrillators (%) | 74 (2.8) | 42 (1.9) | 0.050 | 88 (5.1) | 20 (2.3) | 0.001 |
| Cardiac resynchronization therapy (%) | 103 (3.9) | 118 (5.4) | 0.012 | 45 (2.6) | 9 (1.1) | 0.009 |
| Transplant (%) | 90 (3.4) | 51 (2.3) | 0.030 | 24 (1.4) | 10 (1.2) | 0.644 |
| Hospitalization (%) | 676 (25.2) | 603 (27.4) | 0.096 | 654 (37.6) | 303 (35.2) | 0.229 |
| Echocardiogram | ||||||
| LVEF baseline (%) | 39.0 ± 14.44 | 45.4 ± 16.12 | <0.001 | 43.3 ± 14.48 | 48.8 ± 14.95 | <0.001 |
| LVEF final (%) | 42.14 ± 14.64 a | 47.9 ± 14.8 a | <0.001 | 43.3 ± 14.19 | 48.6 ± 14.38 | <0.001 |
| LVDD baseline (mm) | 61.9 ± 9.67 | 56.0 ± 9.121 | <0.001 | 57.8 ± 8.42 | 53.2 ± 8.30 | <0.001 |
| LVDD final (mm) | 60.3 ± 10.46 b | 54.8 ± 9.51 b | <0.001 | 58.3 ± 8.90 b | 53.3 ± 8.43 c | <0.001 |
| Type of heart failure | ||||||
| Reduced EF (%) | 1479 (55.2) | 904 (41.0) | <0.001 | 719 (41.4) | 257 (29.8) | <0.001 |
| Mildly reduced EF (%) | 543 (20.3) | 353 (16.0) | <0.001 | 392 (22.5) | 483 (17.2) | 0.002 |
| Preserved EF (%) | 656 (24.5) | 948 (43.0) | <0.001 | 628 (36.1) | 456 (53.0) | <0.001 |
| Death (%) | 420 (15.7) | 325 (14.7) | 0.361 | 519 (29.8) | 211 (24.5) | 0.004 |
| Variable | Hazard Ratio | 95% Confidence Limits | Variable | Score of Chi-Square Test | p | ||
|---|---|---|---|---|---|---|---|
| All patients | CKD | 3.24 | 2.89 | 3.63 | CKD | 976.46 | <0.001 |
| Stroke | 2.62 | 2.25 | 3.05 | Diabetes | 251.47 | <0.001 | |
| Diabetes | 2.22 | 1.98 | 2.48 | Stroke | 224.30 | <0.001 | |
| MI | 1.42 | 1.25 | 1.61 | Age | 74.90 | <0.001 | |
| Device | 1.31 | 1.11 | 1.54 | LVEF baseline | 73.43 | <0.001 | |
| Revascularization | 1.19 | 1.03 | 1.38 | MI | 33.01 | <0.001 | |
| Idiopathic CMP | 1.15 | 1.00 | 1.32 | Device | 8.69 | 0.003 | |
| Age | 1.02 | 1.02 | 1.03 | Revascularization | 4.49 | 0.034 | |
| LVEF baseline | 0.99 | 0.98 | 0.99 | Idiopathic | 3.88 | 0.049 | |
| Women | CKD | 3.54 | 2.90 | 4.30 | CKD | 446.85 | <0.001 |
| Stroke | 3.07 | 2.37 | 3.99 | Diabetes | 119.93 | <0.001 | |
| Diabetes | 2.57 | 2.15 | 3.07 | Stroke | 106.32 | <0.001 | |
| AF | 2.02 | 1.66 | 2.45 | AF | 65.22 | <0.001 | |
| Revascularization | 1.30 | 1.02 | 1.66 | Age | 39.31 | <0.001 | |
| MI | 1.25 | 1.00 | 1.55 | LVEF baseline | 13.93 | <0.001 | |
| Age | 1.03 | 1.02 | 1.03 | Revascularization | 8.10 | 0.004 | |
| LVEF baseline | 0.99 | 0.98 | 0.99 | MI | 3.92 | 0.048 | |
| Men | CKD | 2.92 | 2.54 | 3.36 | CKD | 557.33 | <0.001 |
| Stroke | 2.24 | 1.85 | 2.71 | Stroke | 136.14 | <0.001 | |
| Diabetes | 1.92 | 1.67 | 2.20 | Diabetes | 123.55 | <0.001 | |
| AF | 1.91 | 1.67 | 2.20 | AF | 83.88 | <0.001 | |
| MI | 1.44 | 1.24 | 1.66 | LVEF baseline | 52.02 | <0.001 | |
| Device | 1.34 | 1.09 | 1.64 | Age | 43.16 | <0.001 | |
| Revascularization | 1.26 | 1.06 | 1.50 | MI | 34.55 | <0.001 | |
| Age | 1.02 | 1.01 | 1.02 | Device | 5.77 | 0.016 | |
| LVEF baseline | 0.98 | 0.97 | 0.98 | Revascularization | 4.87 | 0.027 | |
| Variables | Hazard Ratio | 95% Confidence Limits | Variables | Score of Chi-Square Test | p | ||
|---|---|---|---|---|---|---|---|
| All patients | CKD | 3.59 | 3.03 | 4.24 | CKD | 608.58 | <0.001 |
| Stroke | 3.00 | 2.39 | 3.77 | AF | 149.38 | <0.001 | |
| Diabetes | 2.66 | 2.25 | 3.14 | Diabetes | 136.74 | <0.001 | |
| AF | 2.27 | 1.94 | 2.66 | Stroke | 93.62 | <0.001 | |
| Device | 1.41 | 1.14 | 1.75 | LVEF baseline | 26.90 | <0.001 | |
| Idiopathic | 1.28 | 1.09 | 1.52 | Age | 35.18 | <0.001 | |
| Age | 1.02 | 1.01 | 1.02 | Device | 11.82 | 0.001 | |
| LVEF baseline | 0.98 | 0.98 | 0.99 | Idiopathic | 8.53 | 0.004 | |
| Women | CKD | 3.82 | 2.94 | 4.96 | CKD | 301.64 | <0.001 |
| Diabetes | 2.89 | 2.29 | 3.64 | Diabetes | 86.44 | <0.001 | |
| Stroke | 2.39 | 1.63 | 3.52 | AF | 49.24 | <0.001 | |
| AF | 2.00 | 1.56 | 2.57 | Stroke | 20.37 | <0.001 | |
| Age | 1.02 | 1.02 | 1.03 | Age | 18.78 | <0.001 | |
| LVEF baseline | 0.99 | 0.98 | 0.99 | LVEF baseline | 13.44 | <0.001 | |
| Men | CKD | 3.75 | 3.01 | 4.68 | CKD | 325.64 | <0.001 |
| Stroke | 3.70 | 2.78 | 4.92 | Stroke | 116.60 | <0.001 | |
| AF | 2.60 | 2.12 | 3.19 | AF | 95.47 | <0.001 | |
| Diabetes | 2.31 | 1.83 | 2.93 | Diabetes | 37.23 | <0.001 | |
| Device | 1.52 | 1.13 | 2.05 | LVEF baseline | 29.34 | <0.001 | |
| Idiopathic | 1.50 | 1.18 | 1.90 | Device | 14.15 | <0.001 | |
| Age | 1.01 | 1.00 | 1.02 | Idiopathic | 8.20 | 0.004 | |
| LVEF baseline | 0.98 | 0.97 | 0.99 | Age | 8.63 | 0.003 | |
| Variables | Hazard Ratio | 95% Confidence Limits | Variables | Score of Chi-Square Test | p | ||
|---|---|---|---|---|---|---|---|
| All patients | CKD | 2.53 | 2.16 | 2.95 | CKD | 286.12 | <0.001 |
| Stroke | 2.09 | 1.71 | 2.57 | Stroke | 85.80 | <0.001 | |
| Diabetes | 1.93 | 1.66 | 2.24 | Diabetes | 73.63 | <0.001 | |
| AF | 1.58 | 1.34 | 1.86 | AF | 35.05 | <0.001 | |
| MI | 1.45 | 1.24 | 1.68 | LVEF baseline | 24.25 | <0.001 | |
| Revascularization | 1.18 | 1.01 | 1.37 | Age | 39.57 | <0.001 | |
| Age | 1.02 | 1.02 | 1.03 | MI | 22.10 | <0.001 | |
| LVEF baseline | 0.98 | 0.98 | 0.99 | Revascularization | 4.61 | 0.032 | |
| Women | Stroke | 3.91 | 2.72 | 5.62 | CKD | 116.26 | <0.001 |
| CKD | 3.39 | 2.51 | 4.58 | Stroke | 61.25 | <0.001 | |
| Diabetes | 2.27 | 1.71 | 3.01 | Diabetes | 27.92 | <0.001 | |
| AF | 1.93 | 1.40 | 2.66 | AF | 26.06 | <0.001 | |
| Revascularization | 1.38 | 1.05 | 1.83 | Age | 16.72 | <0.001 | |
| MI | 1.35 | 1.01 | 1.80 | Revascularization | 4.58 | 0.032 | |
| Age | 1.03 | 1.01 | 1.04 | MI | 4.26 | 0.039 | |
| Men | CKD | 2.41 | 2.00 | 2.89 | CKD | 175.89 | <0.001 |
| Diabetes | 1.87 | 1.56 | 2.23 | Diabetes | 45.81 | <0.001 | |
| Stroke | 1.80 | 1.40 | 2.30 | Stroke | 40.28 | <0.001 | |
| AF | 1.48 | 1.22 | 1.80 | LVEF baseline | 29.06 | <0.001 | |
| MI | 1.45 | 1.21 | 1.74 | Age | 26.61 | <0.001 | |
| Age | 1.02 | 1.01 | 1.03 | MI | 18.16 | <0.001 | |
| LVEF baseline | 0.98 | 0.97 | 0.99 | AF | 15.71 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansur, A.d.P.; Pereira-Barretto, A.C.; del Carlo, C.H.; Avakian, S.D.; Nakagawa, N.K.; Cesar, L.A.M.; Bocchi, E.A. Sex Differences in Prognosis of Heart Failure Due to Ischemic and Nonischemic Cardiomyopathy. J. Clin. Med. 2023, 12, 5323. https://doi.org/10.3390/jcm12165323
Mansur AdP, Pereira-Barretto AC, del Carlo CH, Avakian SD, Nakagawa NK, Cesar LAM, Bocchi EA. Sex Differences in Prognosis of Heart Failure Due to Ischemic and Nonischemic Cardiomyopathy. Journal of Clinical Medicine. 2023; 12(16):5323. https://doi.org/10.3390/jcm12165323
Chicago/Turabian StyleMansur, Antonio de Padua, Antonio Carlos Pereira-Barretto, Carlos Henrique del Carlo, Solange Desirée Avakian, Naomi Kondo Nakagawa, Luiz Antonio Machado Cesar, and Edimar Alcides Bocchi. 2023. "Sex Differences in Prognosis of Heart Failure Due to Ischemic and Nonischemic Cardiomyopathy" Journal of Clinical Medicine 12, no. 16: 5323. https://doi.org/10.3390/jcm12165323
APA StyleMansur, A. d. P., Pereira-Barretto, A. C., del Carlo, C. H., Avakian, S. D., Nakagawa, N. K., Cesar, L. A. M., & Bocchi, E. A. (2023). Sex Differences in Prognosis of Heart Failure Due to Ischemic and Nonischemic Cardiomyopathy. Journal of Clinical Medicine, 12(16), 5323. https://doi.org/10.3390/jcm12165323





