Predictors of Extraprostatic Extension in Patients with Prostate Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Population
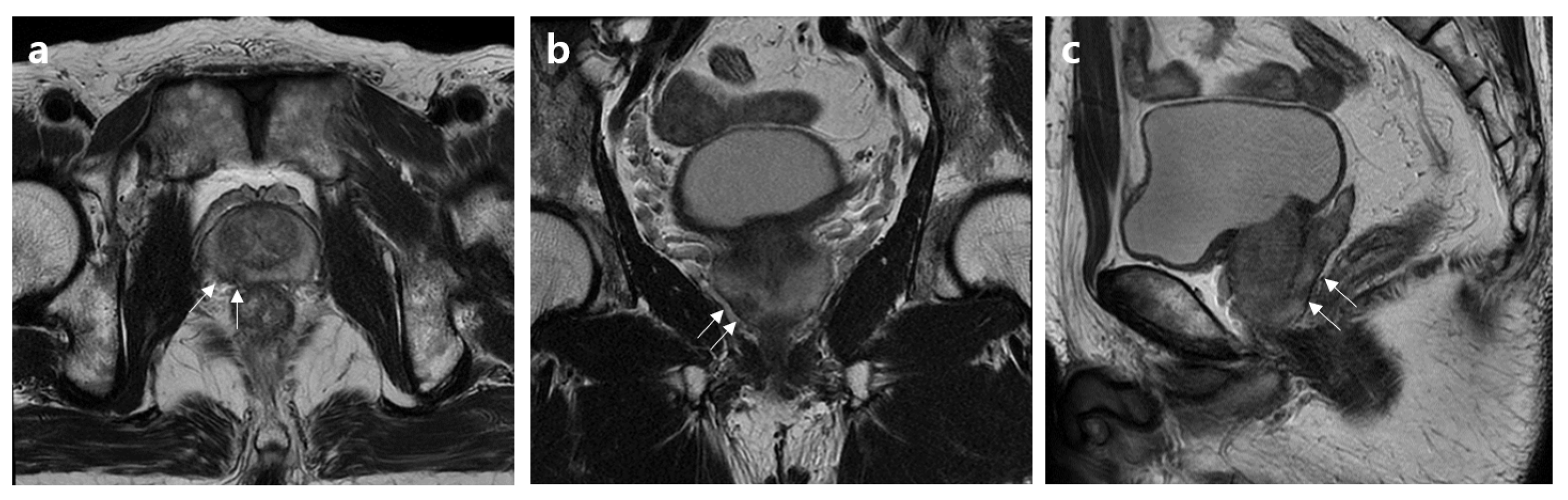
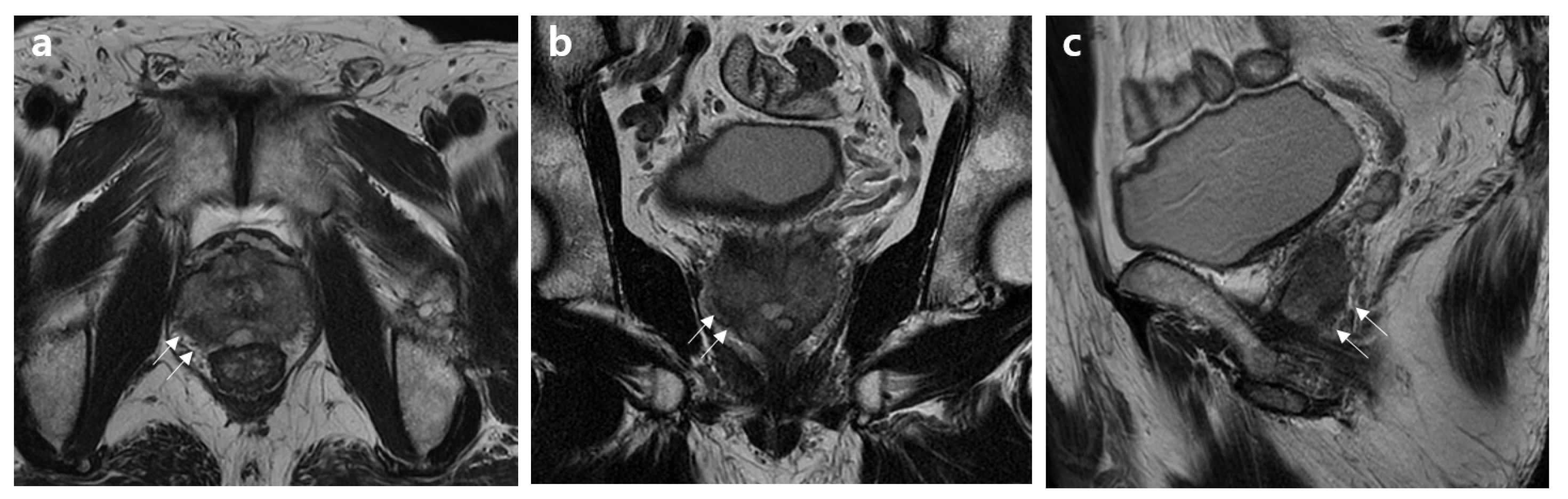
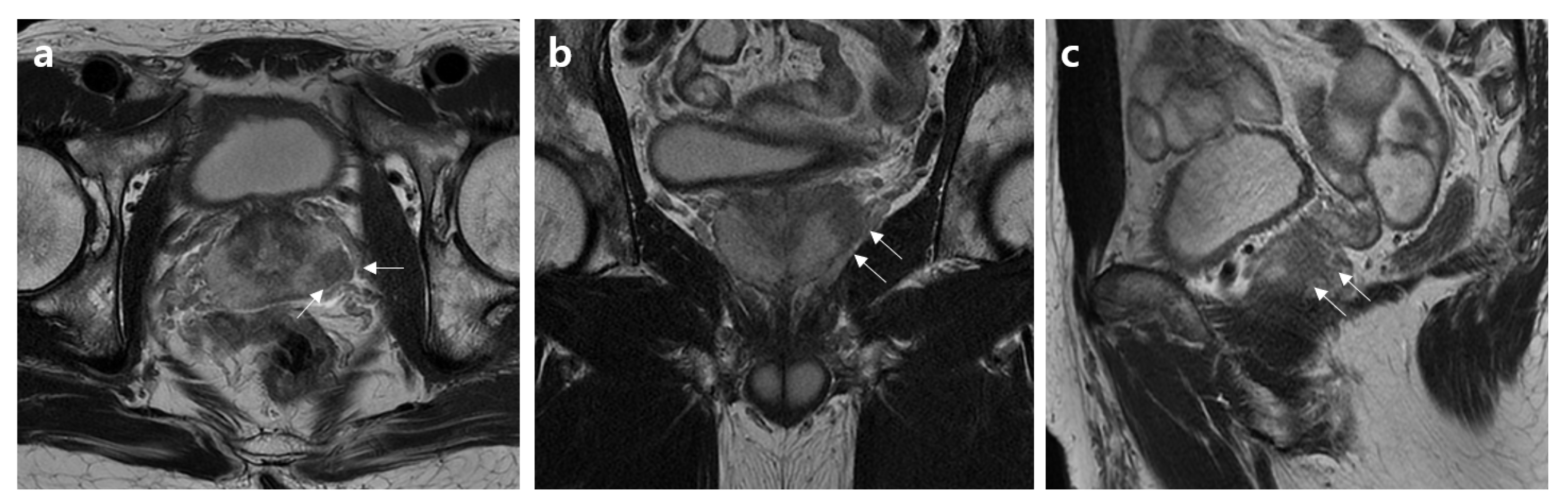
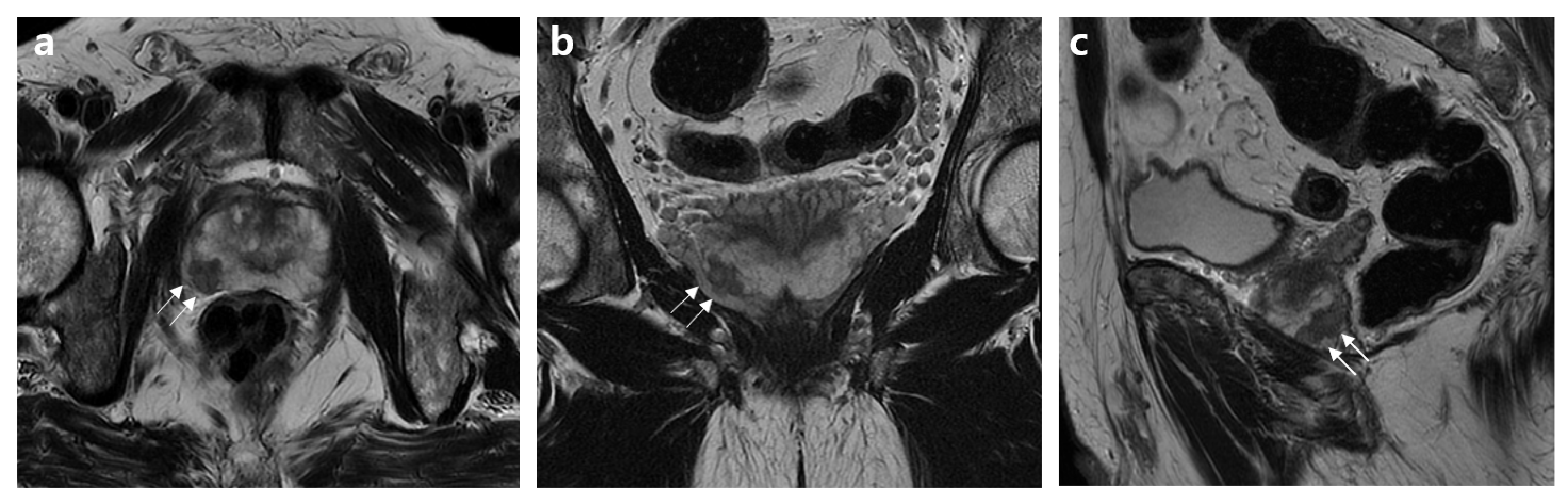
2.2. MRI Assessment
2.3. Histopathology
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tollefson, M.K.; Karnes, R.J.; Rangel, L.J.; Bergstralh, E.J.; Boorjian, S.A. The impact of clinical stage on prostate cancer survival following radical prostatectomy. J. Urol. 2013, 189, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Roethke, M.C.; Lichy, M.P.; Kniess, M.; Werner, M.K.; Claussen, C.D.; Stenzl, A.; Schlemmer, H.P.; Schilling, D. Accuracy of preoperative endorectal MRI in predicting extracapsular extension and influence on neurovascular bundle sparing in radical prostatectomy. World J. Urol. 2013, 31, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.R.; Punnen, S.; Carroll, P.R. Men with low preoperative sexual function may benefit from nerve sparing radical prostatectomy. J. Urol. 2013, 190, 981–986. [Google Scholar] [CrossRef]
- Ball, M.W.; Partin, A.W.; Epstein, J.I. Extent of extraprostatic extension independently influences biochemical recurrence-free survival: Evidence for further pT3 subclassification. Urology 2015, 85, 161–164. [Google Scholar] [CrossRef]
- Danneman, D.; Wiklund, F.; Wiklund, N.P.; Egevad, L. Prognostic significance of histopathological features of extraprostatic extension of prostate cancer. Histopathology 2013, 63, 580–589. [Google Scholar] [CrossRef]
- Boesen, L.; Chabanova, E.; Løgager, V.; Balslev, I.; Mikines, K.; Thomsen, H.S. Prostate cancer staging with extracapsular extension risk scoring using multiparametric MRI: A correlation with histopathology. Eur. Radiol. 2015, 25, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Mehralivand, S.; Shih, J.H.; Harmon, S.; Smith, C.; Bloom, J.; Czarniecki, M.; Gold, S.; Hale, G.; Rayn, K.; Merino, M.J.; et al. A Grading System for the Assessment of Risk of Extraprostatic Extension of Prostate Cancer at Multiparametric MRI. Radiology 2019, 290, 709–719. [Google Scholar] [CrossRef]
- Costa, D.N.; Passoni, N.M.; Leyendecker, J.R.; de Leon, A.D.; Lotan, Y.; Roehrborn, C.G.; Otero-Muinelo, S.; Grewal, H.; Xi, Y.; Francis, F.; et al. Diagnostic Utility of a Likert Scale versus Qualitative Descriptors and Length of Capsular Contact for Determining Extraprostatic Tumor Extension at Multiparametric Prostate MRI. AJR Am. J. Roentgenol. 2018, 210, 1066–1072. [Google Scholar] [CrossRef]
- Radtke, J.P.; Hadaschik, B.A.; Wolf, M.B.; Freitag, M.T.; Schwab, C.; Alt, C.; Roth, W.; Duensing, S.; Pahernik, S.A.; Roethke, M.C.; et al. The Impact of Magnetic Resonance Imaging on Prediction of Extraprostatic Extension and Prostatectomy Outcome in Patients with Low-, Intermediate- and High-Risk Prostate Cancer: Try to Find a Standard. J. Endourol. 2015, 29, 1396–1405. [Google Scholar] [CrossRef]
- de Rooij, M.; Hamoen, E.H.; Witjes, J.A.; Barentsz, J.O.; Rovers, M.M. Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur. Urol. 2016, 70, 233–245. [Google Scholar] [CrossRef]
- Epstein, J.I.; Partin, A.W.; Sauvageot, J.; Walsh, P.C. Prediction of progression following radical prostatectomy. A multivariate analysis of 721 men with long-term follow-up. Am. J. Surg. Pathol. 1996, 20, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Kayat Bittencourt, L.; Litjens, G.; Hulsbergen-van de Kaa, C.A.; Turkbey, B.; Gasparetto, E.L.; Barentsz, J.O. Prostate Cancer: The European Society of Urogenital Radiology Prostate Imaging Reporting and Data System Criteria for Predicting Extraprostatic Extension by Using 3-T Multiparametric MR Imaging. Radiology 2015, 276, 479–489. [Google Scholar] [CrossRef]
- Baco, E.; Rud, E.; Vlatkovic, L.; Svindland, A.; Eggesbø, H.B.; Hung, A.J.; Matsugasumi, T.; Bernhard, J.C.; Gill, I.S.; Ukimura, O. Predictive value of magnetic resonance imaging determined tumor contact length for extracapsular extension of prostate cancer. J. Urol. 2015, 193, 466–472. [Google Scholar] [CrossRef]
- Turkbey, B.; Mani, H.; Shah, V.; Rastinehad, A.R.; Bernardo, M.; Pohida, T.; Pang, Y.; Daar, D.; Benjamin, C.; McKinney, Y.L.; et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: Histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J. Urol. 2011, 186, 1818–1824. [Google Scholar] [CrossRef]
- Feng, T.S.; Sharif-Afshar, A.R.; Wu, J.; Li, Q.; Luthringer, D.; Saouaf, R.; Kim, H.L. Multiparametric MRI Improves Accuracy of Clinical Nomograms for Predicting Extracapsular Extension of Prostate Cancer. Urology 2015, 86, 332–337. [Google Scholar] [CrossRef]
- Gupta, R.T.; Faridi, K.F.; Singh, A.A.; Passoni, N.M.; Garcia-Reyes, K.; Madden, J.F.; Polascik, T.J. Comparing 3-T multiparametric MRI and the Partin tables to predict organ-confined prostate cancer after radical prostatectomy. Urol. Oncol. 2014, 32, 1292–1299. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Shanbhogue, A.K.; Wang, A.; Kong, M.X.; Babb, J.S.; Taneja, S.S. Length of capsular contact for diagnosing extraprostatic extension on prostate MRI: Assessment at an optimal threshold. J. Magn. Reson. Imaging 2016, 43, 990–997. [Google Scholar] [CrossRef]
- Lim, C.; Flood, T.A.; Hakim, S.W.; Shabana, W.M.; Quon, J.S.; El-Khodary, M.; Thornhill, R.E.; El Hallani, S.; Schieda, N. Evaluation of apparent diffusion coefficient and MR volumetry as independent associative factors for extra-prostatic extension (EPE) in prostatic carcinoma. J. Magn. Reson. Imaging 2016, 43, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Morlacco, A.; Sharma, V.; Viers, B.R.; Rangel, L.J.; Carlson, R.E.; Froemming, A.T.; Karnes, R.J. The Incremental Role of Magnetic Resonance Imaging for Prostate Cancer Staging before Radical Prostatectomy. Eur. Urol. 2017, 71, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Zapała, P.; Dybowski, B.; Bres-Niewada, E.; Lorenc, T.; Powała, A.; Lewandowski, Z.; Gołębiowski, M.; Radziszewski, P. Predicting side-specific prostate cancer extracapsular extension: A simple decision rule of PSA, biopsy, and MRI parameters. Int. Urol. Nephrol. 2019, 51, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Caglic, I.; Kovac, V.; Barrett, T. Multiparametric MRI—Local staging of prostate cancer and beyond. Radiol. Oncol. 2019, 53, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Caglic, I.; Povalej Brzan, P.; Warren, A.Y.; Bratt, O.; Shah, N.; Barrett, T. Defining the incremental value of 3D T2-weighted imaging in the assessment of prostate cancer extracapsular extension. Eur. Radiol. 2019, 29, 5488–5497. [Google Scholar] [CrossRef]
- Sankineni, S.; George, A.K.; Brown, A.M.; Rais-Bahrami, S.; Wood, B.J.; Merino, M.J.; Pinto, P.A.; Choyke, P.L.; Turkbey, B. Posterior subcapsular prostate cancer: Identification with mpMRI and MRI/TRUS fusion-guided biopsy. Abdom. Imaging 2015, 40, 2557–2565. [Google Scholar] [CrossRef][Green Version]
- Sertdemir, M.; Weidner, A.M.; Schoenberg, S.O.; Morelli, J.N.; Haecker, A.; Kirchner, M.; Weiss, C.; Hausmann, D.; Dinter, D.J.; Attenberger, U.I. Is There a Role for Functional MRI for the Assessment of Extracapsular Extension in Prostate Cancer? Anticancer Res. 2018, 38, 427–432. [Google Scholar] [CrossRef]
- Jambor, I.; Falagario, U.; Ratnani, P.; Perez, I.M.; Demir, K.; Merisaari, H.; Sobotka, S.; Haines, G.K.; Martini, A.; Beksac, A.T.; et al. Prediction of biochemical recurrence in prostate cancer patients who underwent prostatectomy using routine clinical prostate multiparametric MRI and decipher genomic score. J. Magn. Reson. Imaging 2020, 51, 1075–1085. [Google Scholar] [CrossRef]
- Albisinni, S.; De Groote, A.; Deneft, F.; Thoma, P.; Catteau, X.; Roumeguère, T.; Wildschutz, T. Can preoperative prostate MRI before radical prostatectomy predict extracapsular extension and the side of the index lesion? Prog. Urol. 2016, 26, 281–286. [Google Scholar] [CrossRef]
| All Patients (n = 800) | No-EPE Patients (n = 565) | EPE Patients (n = 235) | p-Value | Missing, n (%) | |
|---|---|---|---|---|---|
| Age (years) | 65.1 (61–69) | 64.6 (60–67) | 67.4 (59–69) | 0.451 | 0 (0) |
| Serum PSA (ng/mL) | 10.8 (8.2–15.8) | 8.4 (7.5–17.9) | 15.6 (13.5–24.8) | 0.023 | 0 (0) |
| Prostate volume on MRI (mL) | 42.7 (35.3–46.8) | 40.3 (32.6–49.2) | 42.7 (33.9–52.5) | 0.562 | 0 (0) |
| MRI to surgery (day) | 24.5 (11–56) | 36.8 (10–53) | 30.6 (12–57) | 0.496 | 0 (0) |
| Positive biopsy cores (%) | 33.5 (20–50) | 31.3 (30–55) | 37.8 (34–62) | 0.097 | 55 (6.8) |
| Clinical T stage | 45 (5.6) | ||||
| cT1c | 480 | 390 | 90 | 0.074 | |
| cT2a | 115 | 54 | 61 | 0.782 | |
| cT2b | 79 | 61 | 18 | 0.062 | |
| cT2c | 20 | 6 | 14 | 0.058 | |
| cT3a/b | 61 | 34 | 27 | 0.672 | |
| ISUP grade group (biopsy) | 3 (2–5) | 2.1 (1.5 –2.8) | 3.6 (2.8–4.2) | 0.024 | 0 (0) |
| ISUP grade group (surgical) | 0 (0) | ||||
| 1 | 23 | 18 | 5 | 0.067 | |
| 2 | 290 | 270 | 20 | 0.093 | |
| 3 | 273 | 210 | 63 | 0.105 | |
| 4 | 146 | 49 | 97 | 0.026 | |
| 5 | 68 | 18 | 50 | 0.013 | |
| Histopathology (TMN stage) | |||||
| pT2a | 42 | N/A | N/A | ||
| pT2b | 23 | N/A | N/A | ||
| pT2c | 511 | N/A | N/A | ||
| pT3a | 171 | N/A | N/A | ||
| pT3b | 50 | N/A | N/A | ||
| pT4 | 3 | N/A | N/A | ||
| Risk group (D’Amico) | 0 (0) | ||||
| Low | 26 | 21 | 5 | 0.102 | |
| Intermediate | 496 | 440 | 56 | 0.089 | |
| High | 278 | 104 | 174 | 0.071 | |
| PI-RADS v2.1 of the leading lesion | 3 (2–5) | 2 (1–3) | 4 (4–5) | 0.017 | 0 (0) |
| Mehralivand EPE grade | 2 (1–3) | 1 (0–2) | 2 (2–3) | 0.009 | 0 (0) |
| Variables | Observed EPE % (n/Total n) | Unadjusted Odds Ratios * (95% CI) |
|---|---|---|
| ISUP grade group (biopsy) | ||
| 1 | 21.7 (10/46) | Reference |
| 2 | 12.4 (38/305) | 3.04 (0.65 to 11.7) |
| 3 | 21.3 (60/281) | 2.86 (0.83 to 14.3) |
| 4–5 | 75.5 (127/168) | 10.3 (2.57 to 35.9) |
| PI-RADS v2.1 of the leading lesion | ||
| 1–2 | 14.1 (12/85) | Reference |
| 3 | 26.4 (9/34) | 1.13 (0.16 to 5.83) |
| 4 | 26.6 (63/236) | 7.25 (0.81 to 20.7) |
| 5 | 33.9 (151/445) | 13.4 (1.81 to 82.5) |
| Mehralivand EPE grade | ||
| 0 | 11.0 (11/100) | Reference |
| 1 | 15.8 (24/151) | 1.29 (0.33 to 5.15) |
| 2 | 26.5 (98/369) | 8.09 (1.55 to 8.49) |
| 3 | 56.6 (102/180) | 20.1 (3.67 to 72.4) |
| Variables | Unadjusted Odds Ratios (95% CI) | Adjusted * ORs (95% CI) |
|---|---|---|
| ISUP grade group (biopsy) 4–5 | 4.12 (1.61 to 10.5) | 3.23 (0.84 to 6.62) |
| PI-RADS v2.1 score of 5 | 6.34 (1.59 to 16.9) | 5.89 (1.12 to 19.7) |
| Mehralivand EPE grade 3 | 12.6 (3.46 to 40.2) | 9.23 (2.43 to 30.5) |
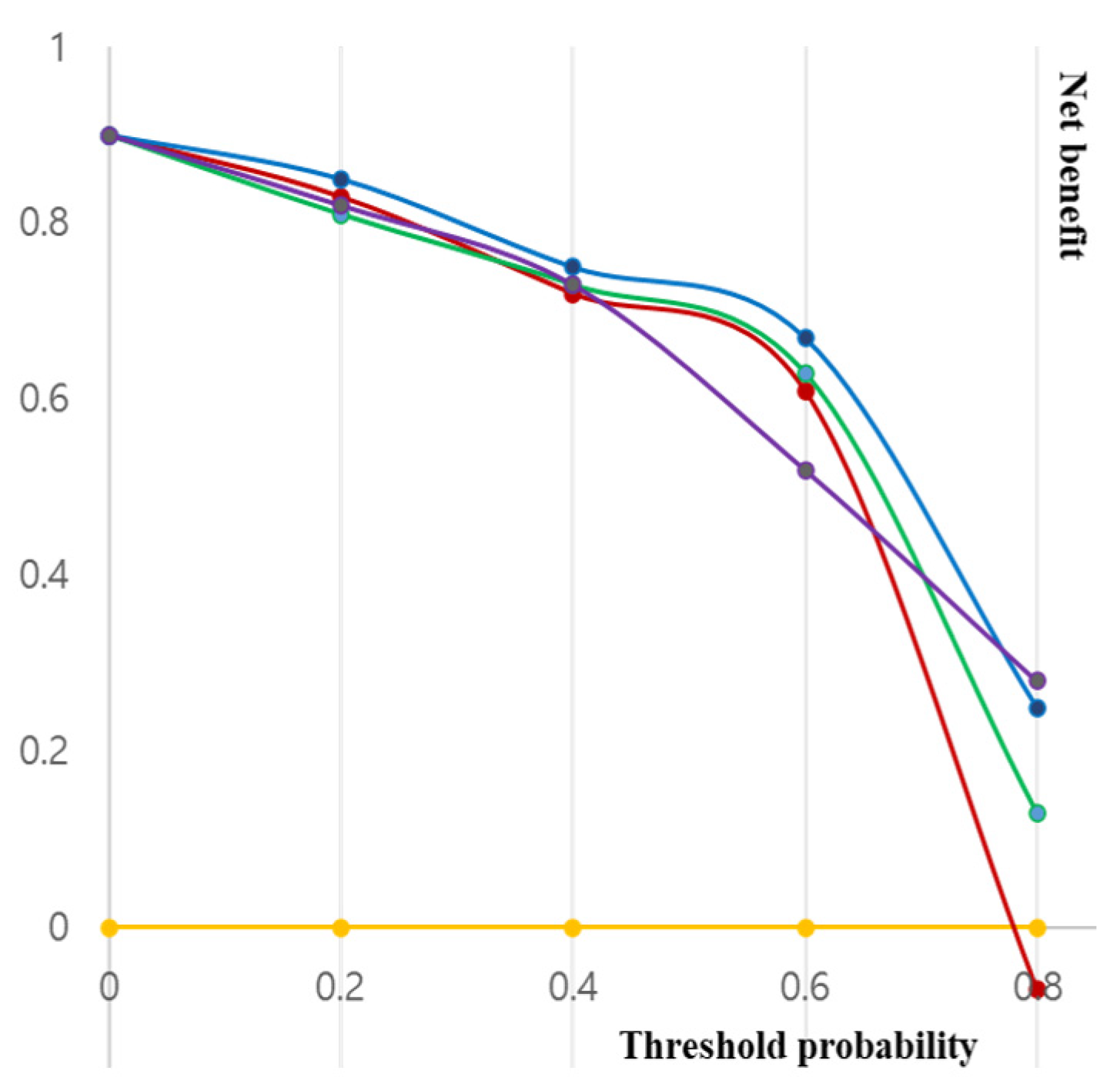
| Predictor | AUC (95% CI) | p (vs. Base) |
|---|---|---|
| Basic model | 0.607 (0.518–0.702) | - |
| Model 1 | 0.802 (0.671–0.817) | 0.023 |
| Model 2 | 0.879 (0.858–0.904) | 0.009 |
| Probability | All | Basic Model | Model 1 | Model 2 |
|---|---|---|---|---|
| 10 | 0.857 | 0.857 | 0.857 | 0.887 |
| 15 | 0.754 | 0.754 | 0.762 | 0.845 |
| 20 | 0.703 | 0.638 | 0.735 | 0.817 |
| 25 | 0.686 | 0.626 | 0.723 | 0.799 |
| 30 | 0.337 | 0.608 | 0.675 | 0.751 |
| 35 | 0.226 | 0.279 | 0.523 | 0.742 |
| 40 | −0.027 | 0.098 | 0.407 | 0.465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Cho, S.H.; Kim, W.H.; Kim, H.J.; Park, J.M.; Kim, G.C.; Ryeom, H.K.; Yoon, Y.S.; Cha, J.G. Predictors of Extraprostatic Extension in Patients with Prostate Cancer. J. Clin. Med. 2023, 12, 5321. https://doi.org/10.3390/jcm12165321
Kim SH, Cho SH, Kim WH, Kim HJ, Park JM, Kim GC, Ryeom HK, Yoon YS, Cha JG. Predictors of Extraprostatic Extension in Patients with Prostate Cancer. Journal of Clinical Medicine. 2023; 12(16):5321. https://doi.org/10.3390/jcm12165321
Chicago/Turabian StyleKim, See Hyung, Seung Hyun Cho, Won Hwa Kim, Hye Jung Kim, Jong Min Park, Gab Chul Kim, Hun Kyu Ryeom, Yu Sung Yoon, and Jung Guen Cha. 2023. "Predictors of Extraprostatic Extension in Patients with Prostate Cancer" Journal of Clinical Medicine 12, no. 16: 5321. https://doi.org/10.3390/jcm12165321
APA StyleKim, S. H., Cho, S. H., Kim, W. H., Kim, H. J., Park, J. M., Kim, G. C., Ryeom, H. K., Yoon, Y. S., & Cha, J. G. (2023). Predictors of Extraprostatic Extension in Patients with Prostate Cancer. Journal of Clinical Medicine, 12(16), 5321. https://doi.org/10.3390/jcm12165321





