Hemoglobin–Albumin–Lymphocyte–Platelet (HALP) Score as a Predictive Model for the Success of Reconstruction of Head and Neck Defects with Free Microvascular Flaps
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
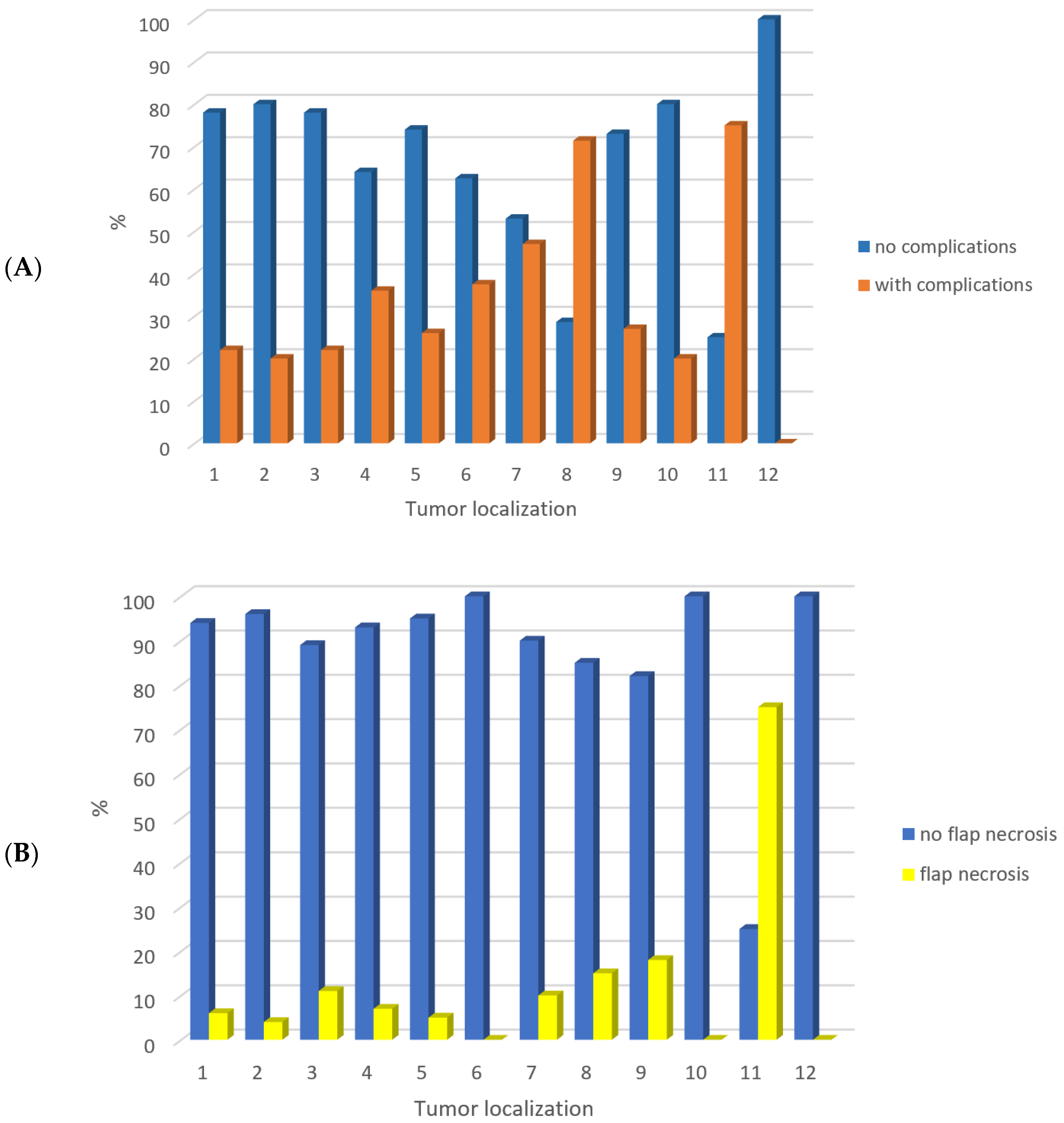
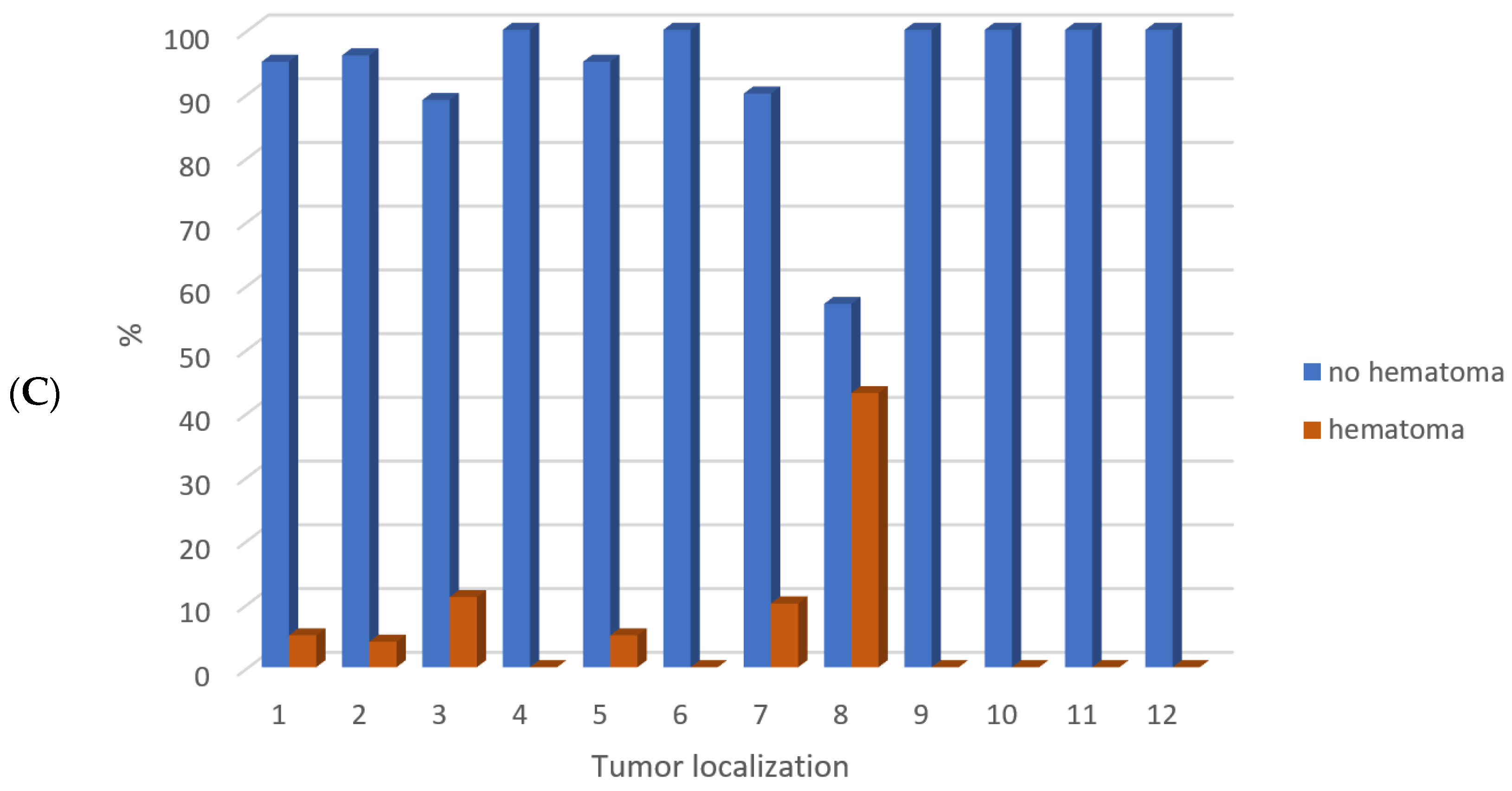
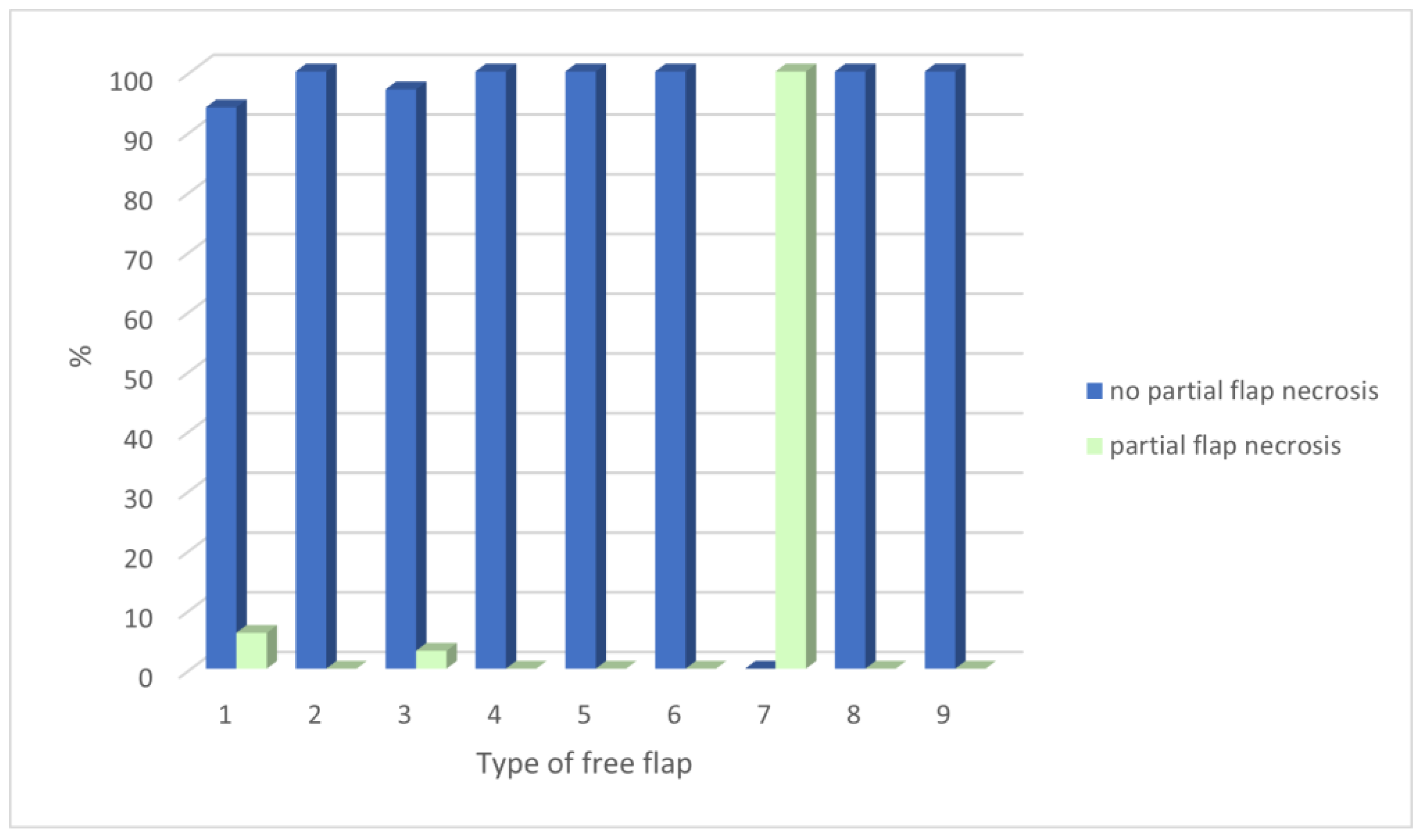
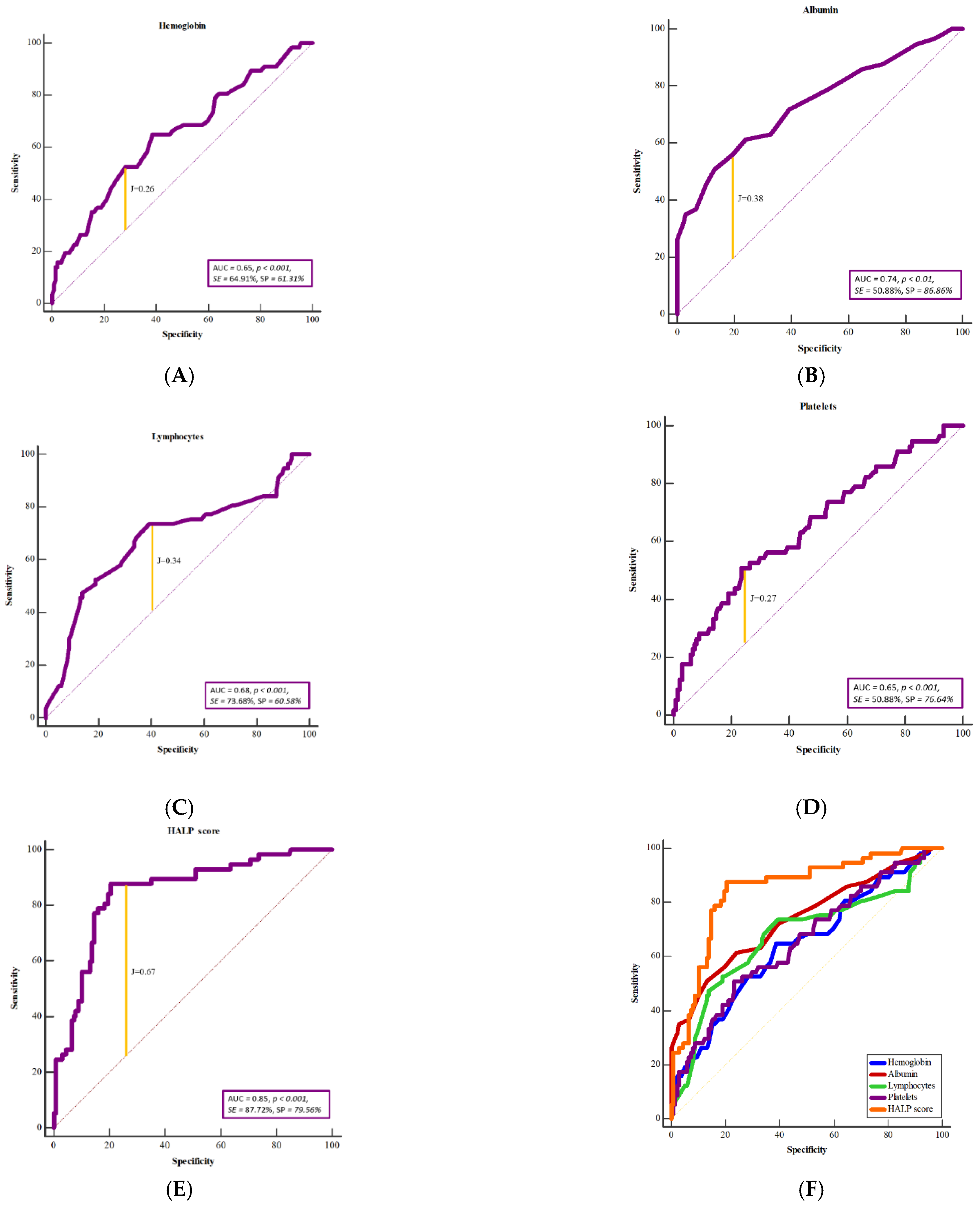
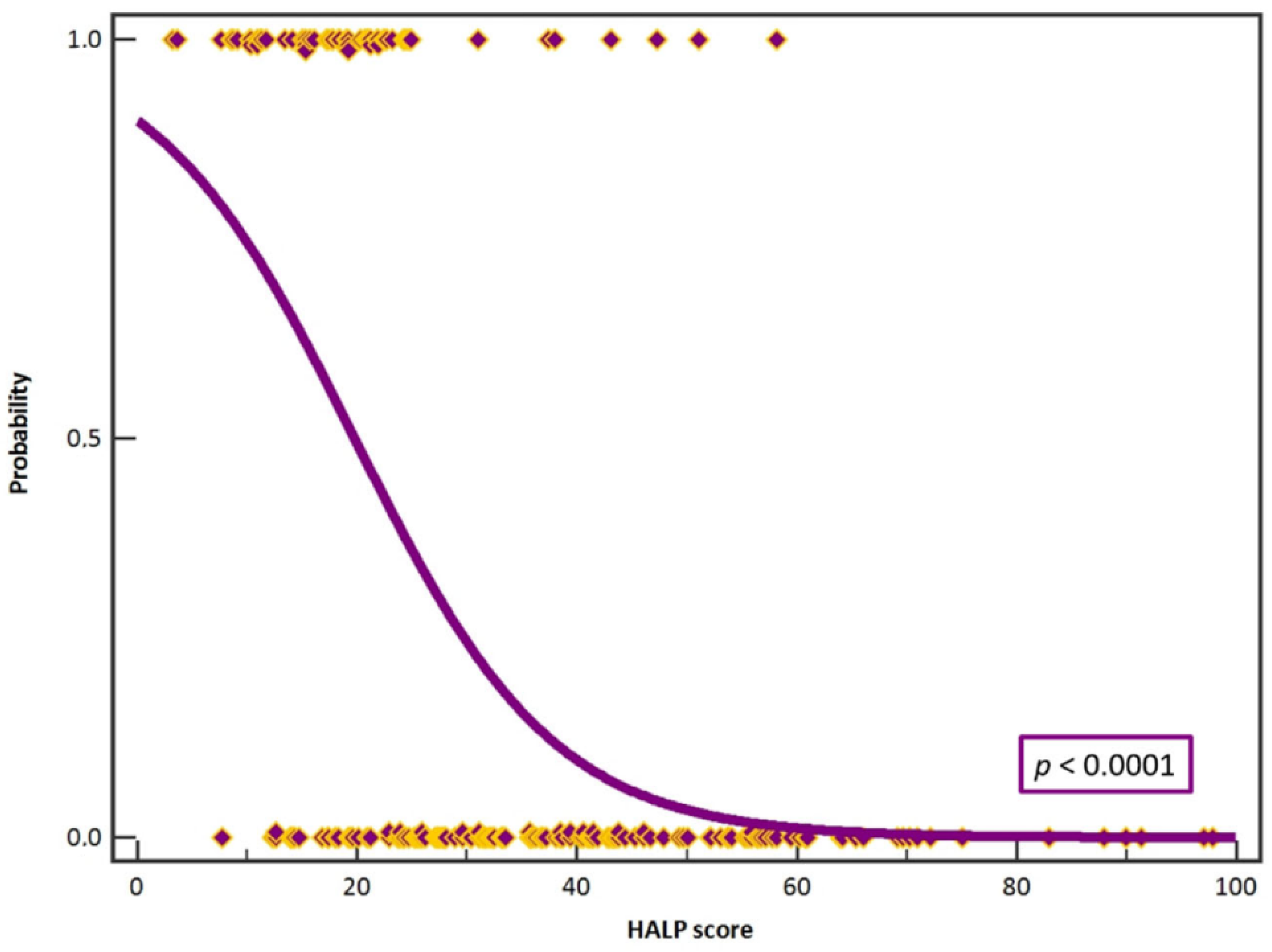
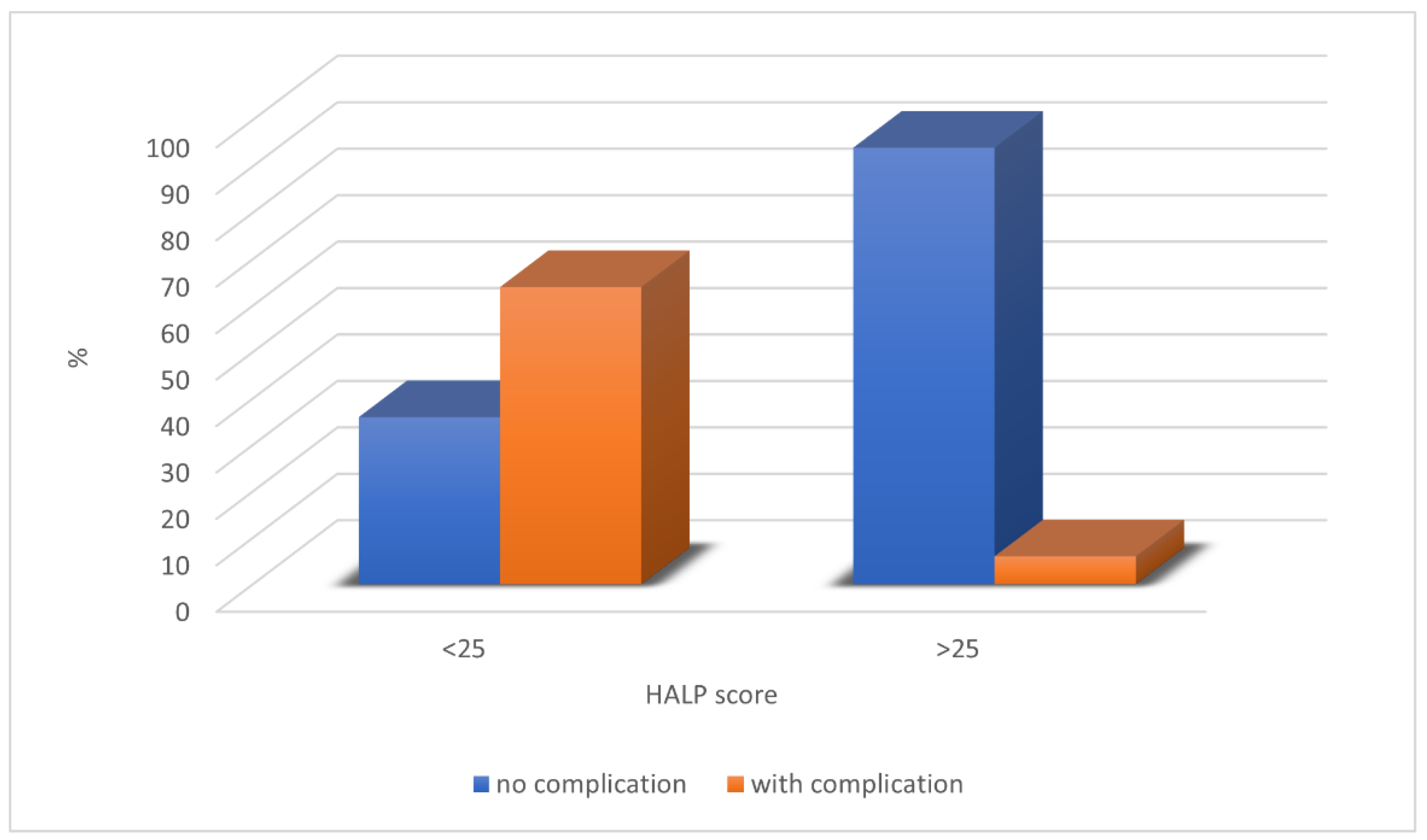
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabrysz-Forget, F.; Tabet, P.; Rahal, A.; Bissada, E.; Christopoulos, A.; Ayad, T. Free versus pedicled flaps for reconstruction of head and neck cancer defects: A systematic review. J. Otolaryngol. Head Neck Surg. 2019, 48, 13. [Google Scholar] [CrossRef] [PubMed]
- Daniel, R.K.; Taylor, G.I. Distant transfer of an island flap by microvascular anastomoses. A clinical technique. Plast Reconstr. Surg. 1973, 52, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.; Wei, F.C. Microsurgical free flap in head and neck reconstruction. Head Neck 2010, 32, 1236–1245. [Google Scholar] [CrossRef]
- Patel, R.S.; McCluskey, S.A.; Goldstein, D.P.; Minkovich, L.; Irish, J.C.; Brown, D.H.; Gullane, P.J.; Lipa, J.E.; Gilbert, R.W. Clinicopathologic and therapeutic risk factors for perioperative complications and prolonged hospital stay in free flap reconstruction of the head and neck. Head Neck 2010, 32, 1345–1353. [Google Scholar] [CrossRef]
- Eskander, A.; Kang, S.; Tweel, B.; Sitapara, J.; Old, M.; Ozer, E.; Agrawal, A.; Carrau, R.; Rocco, J.W.; Teknos, T.N. Predictors of Complications in Patients Receiving Head and Neck Free Flap Reconstructive Procedures. Otolaryngol. Head Neck Surg. 2018, 158, 839–847. [Google Scholar] [CrossRef]
- Patel, V.M.; Stern, C.; Miglani, A.; Weichman, K.E.; Lin, J.; Ow, T.J.; Garfein, E.S. Evaluation of the Relationship between Age and Outcome after Microvascular Reconstruction among Patients with Recurrent Head and Neck Squamous Cell Carcinoma. J. Reconstr. Microsurg. 2017, 33, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hong, J.P.; Suh, H.P.; Park, J.Y.; Kim, D.H.; Ha, S.; Lee, J.; Hwang, J.H.; Kim, Y.K. Prognostic Nutritional Index is a Predictor of Free Flap Failure in Extremity Reconstruction. Nutrients 2020, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Sanati-Mehrizy, P.; Massenburg, B.B.; Rozehnal, J.M.; Ingargiola, M.J.; Hernandez Rosa, J.; Taub, P.J. Risk Factors Leading to Free Flap Failure: Analysis from the National Surgical Quality Improvement Program Database. J. Craniofac. Surg. 2016, 27, 1956–1964. [Google Scholar] [CrossRef]
- Farag, C.M.; Antar, R.; Akosman, S.; Ng, M.; Whalen, M.J. What is hemoglobin, albumin, lymphocyte, platelet (HALP) score? A comprehensive literature review of HALP’s prognostic ability in different cancer types. Oncotarget 2023, 14, 153–172. [Google Scholar] [CrossRef]
- Chen, X.L.; Xue, L.; Wang, W.; Chen, H.N.; Zhang, W.H.; Liu, K.; Chen, X.Z.; Yang, K.; Zhang, B.; Chen, Z.X.; et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: A retrospective cohort study. Oncotarget 2015, 6, 41370–41382. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, X.; Ai, J.; Yang, L. Hemoglobin, albumin, lymphocyte, and platelet (HALP) score and cancer prognosis: A systematic review and meta-analysis of 13,110 patients. Int. Immunopharmacol. 2023, 114, 109496. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Lin, P.Y.; Chew, K.Y.; Kuo, Y.R. Free tissue transfers in head and neck reconstruction: Complications, outcomes and strategies for management of flap failure: Analysis of 2019 flaps in single institute. Microsurgery 2014, 34, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Peters, T.T.; Post, S.F.; van Dijk, B.A.; Roodenburg, J.L.; van der Laan, B.F.; Werker, P.M.; Halmos, G.B. Free flap reconstruction for head and neck cancer can be safely performed in both young and elderly patients after careful patient selection. Eur. Arch. Otorhinolaryngol. 2015, 272, 2999–3005. [Google Scholar] [CrossRef]
- McCarty, J.L.; Corey, A.S.; El-Deiry, M.W.; Baddour, H.M.; Cavazuti, B.M.; Hudgins, P.A. Imaging of Surgical Free Flaps in Head and Neck Reconstruction. AJNR Am. J. Neuroradiol. 2019, 40, 5–13. [Google Scholar] [CrossRef]
- Walia, A.; Lee, J.J.; Jackson, R.S.; Hardi, A.C.; Bollig, C.A.; Graboyes, E.M.; Zenga, J.; Puram, S.V.; Pipkorn, P. Management of Flap Failure After Head and Neck Reconstruction: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2022, 167, 224–235. [Google Scholar] [CrossRef]
- Tsai, M.H.; Chuang, H.C.; Lin, Y.T.; Lu, H.; Chen, W.C.; Fang, F.M.; Chien, C.Y. Clinical impact of albumin in advanced head and neck cancer patients with free flap reconstruction-a retrospective study. PeerJ 2018, 6, e4490. [Google Scholar] [CrossRef]
- Wu, H.; Liu, F.; Ji, F.; Guo, M.; Wang, Y.; Cao, M. Identification of Independent Risk Factors for Complications: A Retrospective Analysis of 163 Fibular Free Flaps for Mandibulofacial Reconstruction. J. Oral. Maxillofac. Surg. 2018, 76, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Shum, J.; Markiewicz, M.R.; Park, E.; Bui, T.; Lubek, J.; Bell, R.B.; Dierks, E.J. Low prealbumin level is a risk factor for microvascular free flap failure. J. Oral. Maxillofac. Surg. 2014, 72, 169–177. [Google Scholar] [CrossRef]
- Xu, H.; Han, Z.; Ma, W.; Zhu, X.; Shi, J.; Lin, D. Perioperative Albumin Supplementation is Associated with Decreased Risk of Complications Following Microvascular Head and Neck Reconstruction. J. Oral. Maxillofac. Surg. 2021, 79, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Woo, K.J.; Park, B.Y.; Kang, S.R. Effects of Transfusion on Free Flap Survival: Searching for an Optimal Hemoglobin Threshold for Transfusion. J. Reconstr. Microsurg. 2018, 34, 610–615. [Google Scholar] [CrossRef]
- Sanchez-Porro Gil, L.; Leon Vintro, X.; Lopez Fernandez, S.; Vega Garcia, C.; Pons Playa, G.; Fernandez Garrido, M.; Masia Ayala, J. The Effect of Perioperative Blood Transfusions on Microvascular Anastomoses. J. Clin. Med. 2021, 10, 1333. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.B.; Patel, A.; Del Corral, G.A.; Sexton, K.W.; Ehrenfeld, J.M.; Guillamondegui, O.D.; Shack, R.B. Preoperative anemia predicts thrombosis and free flap failure in microvascular reconstruction. Ann. Plast. Surg. 2012, 69, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, H.; Saito, J.; Takekawa, D.; Ohyama, T.; Kushikata, T.; Hirota, K. Availability of preoperative neutrophil-lymphocyte ratio to predict postoperative delirium after head and neck free-flap reconstruction: A retrospective study. PLoS ONE 2021, 16, e0254654. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.H.; Bauder, A.R.; Centkowski, S.; Shammas, R.L.; Mundy, L.; Kovach, S.J.; Levin, L.S.; Hollenbeck, S.T. Preoperative Platelet Count Predicts Lower Extremity Free Flap Thrombosis: A Multi-Institutional Experience. Plast Reconstr. Surg. 2017, 139, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Tarabishy, S.P.; Inglesby, D.; Tapp, M.; Corral, G.D.; Herrera, F.A. Thrombocytosis is associated with complications after microvascular surgery: An NSQIP data analysis. Microsurgery 2020, 40, 288–297. [Google Scholar] [CrossRef]
| Total | Males | Females | |
|---|---|---|---|
| Number of patients | 194 | 134 | 60 |
| Age (years) | 60 ± 12.54 | 60 ± 11.46 | 60.41 ± 14.78 |
| Hospital stay (days) | 33.41 ± 18.41 | 33.64 ± 17.90 | 32.90 ± 19.66 |
| Number of overall flap complication | 57 | 39 | 18 |
| Type of Free Flap | Number of Free Flaps | Overall Complications | Flap Revision | Necrosis | Infection | Fistula | Hematoma | Partial Necrosis |
|---|---|---|---|---|---|---|---|---|
| Radial forearm flap | 95 | 26 | 9 | 5 | 14 | 10 | 6 | 6 |
| Fibular flap | 47 | 12 | 6 | 4 | 5 | 4 | 3 | 0 |
| ALT flap | 37 | 14 | 5 | 4 | 7 | 5 | 2 | 1 |
| DCIA flap | 9 | 2 | 1 | 1 | 1 | 0 | 0 | 0 |
| Scapular flap | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Latissimus dorsi flap | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| DIEP flap | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
| Rectus muscle flap | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 |
| Serratus muscle flap | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 194 | 57 (29.4%) | 22 (11.2%) | 16 (8.2%) | 29 (14.9%) | 20 (10.3%) | 11 (5.7%) | 8 (4.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarle, M.; Čvrljević, I.; Raguž, M.; Lukšić, I. Hemoglobin–Albumin–Lymphocyte–Platelet (HALP) Score as a Predictive Model for the Success of Reconstruction of Head and Neck Defects with Free Microvascular Flaps. J. Clin. Med. 2023, 12, 5314. https://doi.org/10.3390/jcm12165314
Tarle M, Čvrljević I, Raguž M, Lukšić I. Hemoglobin–Albumin–Lymphocyte–Platelet (HALP) Score as a Predictive Model for the Success of Reconstruction of Head and Neck Defects with Free Microvascular Flaps. Journal of Clinical Medicine. 2023; 12(16):5314. https://doi.org/10.3390/jcm12165314
Chicago/Turabian StyleTarle, Marko, Igor Čvrljević, Marina Raguž, and Ivica Lukšić. 2023. "Hemoglobin–Albumin–Lymphocyte–Platelet (HALP) Score as a Predictive Model for the Success of Reconstruction of Head and Neck Defects with Free Microvascular Flaps" Journal of Clinical Medicine 12, no. 16: 5314. https://doi.org/10.3390/jcm12165314
APA StyleTarle, M., Čvrljević, I., Raguž, M., & Lukšić, I. (2023). Hemoglobin–Albumin–Lymphocyte–Platelet (HALP) Score as a Predictive Model for the Success of Reconstruction of Head and Neck Defects with Free Microvascular Flaps. Journal of Clinical Medicine, 12(16), 5314. https://doi.org/10.3390/jcm12165314









