Real-Life Utilization of Criteria Guidelines for Diagnosis of Cardiac Sarcoidosis (CS)
Abstract
1. Key Messages
2. Introduction
3. Methods
3.1. Study Population
3.2. Data Collection
3.3. Ethical Considerations
4. Results
4.1. Study Participants
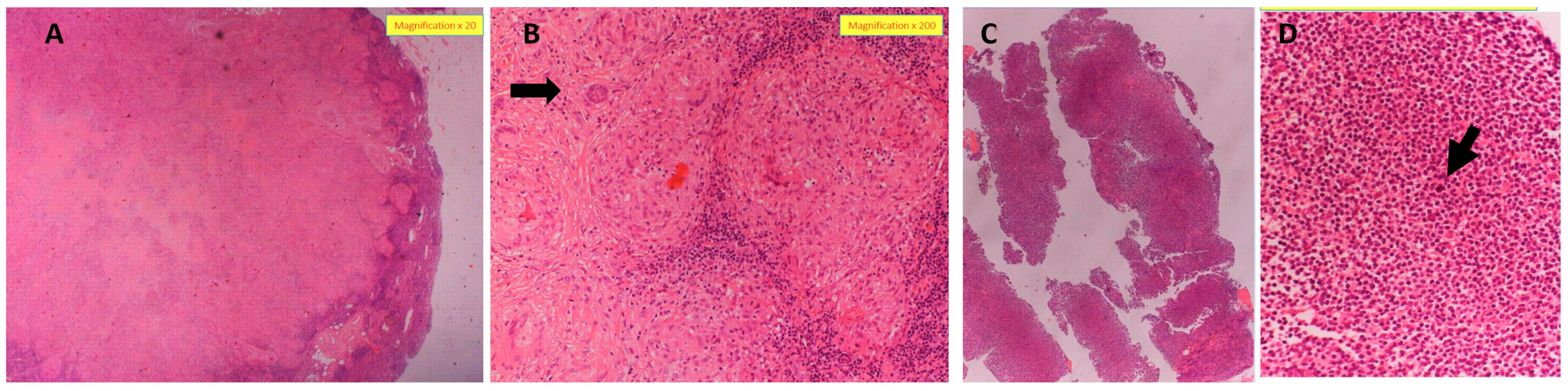
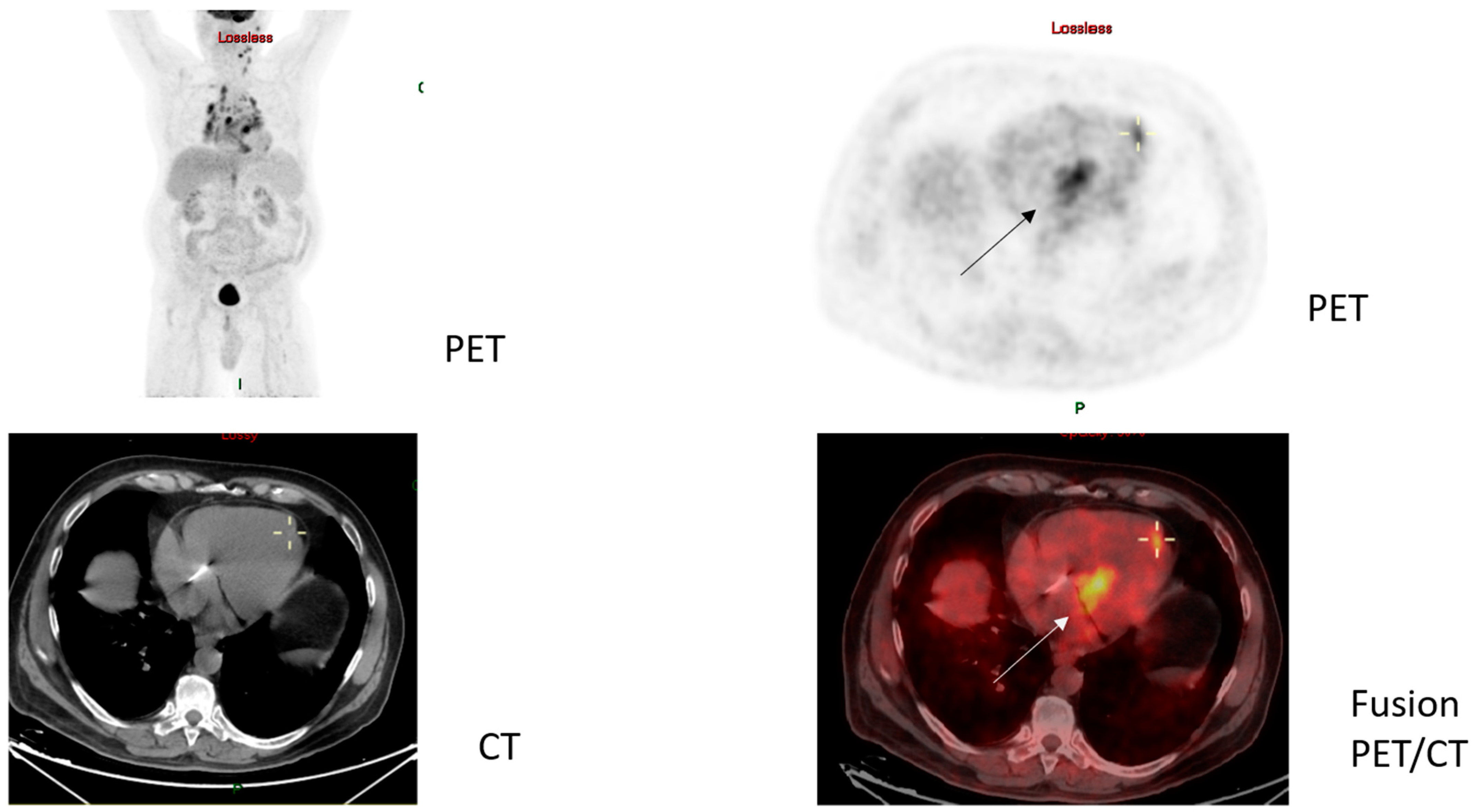
4.2. Patient Diagnosis
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silverman, K.J.; Hutchins, G.M.; Bulkley, B.H. Cardiac sarcoid: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978, 58, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Uemura, A.; Morimoto, S.; Hiramitsu, S.; Kato, Y.; Ito, T.; Hishida, H. Histologic diagnostic rate of cardiac sarcoidosis: Evaluation of endomyocardial biopsies. Am. Heart J. 1999, 138, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Gilotra, N.; Okada, D.; Sharma, A.; Chrispin, J. Management of cardiac sarcoidosis in 2020. Arrhythm. Electrophysiol. Rev. 2020, 9, 182–188. [Google Scholar] [CrossRef]
- Statement on Sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am. J. Respir. Crit. Care Med. 1999, 160, 736–755. [Google Scholar] [CrossRef]
- Birnie, D.H.; Nery, P.B.; Ha, A.C.; Beanlands, R.S.B. Cardiac Sarcoidosis. J. Am. Coll. Cardiol. 2016, 68, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Hulten, E.; Aslam, S.; Osborne, M.; Abbasi, S.; Bittencourt, M.S.; Blankstein, R. Cardiac sarcoidosis-state of the art review. Cardiovasc. Diagn. Ther. 2016, 6, 50–63. [Google Scholar] [CrossRef]
- Bennett, M.K.; Gilotra, N.A.; Harrington, C.; Rao, S.; Dunn, J.M.; Freitag, T.B.; Halushka, M.K.; Russell, S.D. Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from 2000–2009. Circ. Heart Fail. 2013, 6, 676–684. [Google Scholar] [CrossRef]
- Ardehali, H.; Howard, D.L.; Hariri, A.; Qasim, A.; Hare, J.M.; Baughman, K.L.; Kasper, E.K. A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am. Heart J. 2005, 150, 459–463. [Google Scholar] [CrossRef]
- Sandhu, J.S.; Uretsky, B.F.; Zerbe, T.R.; Goldsmith, A.S.; Reddy, P.S.; Kormos, R.L.; Griffith, B.P.; Hardesty, R.L. Coronary artery fistula in the heart transplant patient. A potential complication of endomyocardial biopsy. Circulation 1989, 79, 350–356. [Google Scholar] [CrossRef]
- Asleh, R.; Briasoulis, A.; Doulamis, I.; Alnsasra, H.; Tzani, A.; Alvarez, P.; Kuno, T.; Kampaktsis, P.; Kushwaha, S. Outcomes after heart transplantation in patients with cardiac sarcoidosis. ESC Heart Fail. 2022, 9, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Kandolin, R.; Lehtonen, J.; Graner, M.; Schildt, J.; Salmenkivi, K.; Kivistö, S.M.; Kupari, M. Diagnosing isolated cardiac sarcoidosis. J. Intern. Med. 2011, 270, 461–468. [Google Scholar] [CrossRef]
- Patel, M.R.; Cawley, P.J.; Heitner, J.F.; Klem, I.; Parker, M.A.; Jaroudi, W.A.; Meine, T.J.; White, J.B.; Elliott, M.D.; Kim, H.W.; et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009, 120, 1969–1977. [Google Scholar] [CrossRef]
- Crouser, E.D.; Ono, C.; Tran, T.; He, X.; Raman, S.V. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am. J. Respir. Crit. Care Med. 2014, 189, 109–112. [Google Scholar] [CrossRef]
- Yang, Y.; Safka, K.; Graham, J.J.; Roifman, I.; Zia, M.I.; Wright, G.A.; Balter, M.; Dick, A.J.; Connelly, K.A. Correlation of late gadolinium enhancement MRI and quantitative T2 measurement in cardiac sarcoidosis. J. Magn. Reson. Imaging 2014, 39, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, S.; Tsujino, I.; Takei, T.; Tsukamoto, E.; Sakaue, S.; Kamigaki, M.; Ito, N.; Ohira, H.; Ikeda, D.; Tamaki, N.; et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur. Heart J. 2005, 26, 1538–1543. [Google Scholar] [CrossRef]
- Youssef, G.; Leung, E.; Mylonas, I.; Nery, P.; Williams, K.; Wisenberg, G.; Gulenchyn, K.Y.; Dekemp, R.A.; Dasilva, J.; Birnie, D.; et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: A systematic review and metaanalysis including the Ontario experience. J. Nucl. Med. 2012, 53, 241–248. [Google Scholar] [CrossRef]
- Coleman, G.C.; Shaw, P.W.; Balfour, P.C.; Gonzalez, J.A.; Kramer, C.M.; Patel, A.R.; Salerno, M. Prognostic value of myocardial scarring on CMR in patients with cardiac sarcoidosis. JACC Cardiovasc. Imaging 2017, 10, 411–420. [Google Scholar] [CrossRef]
- Jaiswal, R.; Vaisyambath, L.; Khayyat, A.; Unachukwu, N.; Nasyrlaeva, B.; Asad, M.; Fabara, S.P.; Balan, I.; Kolla, S.; Rabbani, R. Cardiac sarcoidosis diagnostic challenges and management: A case report and literature review. Cureus 2022, 14, e24850. [Google Scholar] [CrossRef] [PubMed]
- Riasat, M.; Khan, A.; Ehtesham, M.; Meghrajani, V.; Hafez, A. Catastrophic events of cardiac sarcoidosis: A case report. Cureus 2022, 14, e24902. [Google Scholar] [CrossRef]
- Afriyie-Mensah, J.S.; Awindaogo, F.R.; Tagoe, E.N.D.; Ayetey, H. Cardiac sarcoidosis: Two case reports. Clin. Case Rep. 2021, 9, e04270. [Google Scholar] [CrossRef] [PubMed]
- Plitt, A.; Dorbala, S.; Albert, M.A.; Giugliano, R.P. Cardiac sarcoidosis: Case report, workup, and review of the literature. Cardiol. Ther. 2013, 2, 181–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tan, J.L.; Fong, H.K.; Birati, E.Y.; Han, Y. Cardiac Sarcoidosis. Am. J. Cardiol. 2019, 123, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Judson, M.A. Screening sarcoidosis patients for cardiac sarcoidosis: What the data really show. Respir. Med. 2019, 154, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Maier, L.A.; Wilson, K.C.; Bonham, C.A.; Morgenthau, A.S.; Patterson, K.C.; Abston, E.; Bernstein, R.C.; Blankstein, R.; Chen, E.S.; et al. Diagnosis and detection of sarcoidosis. An official american thoracic society clinical practice guideline. Am. J. Respir. Crit. Care Med. 2020, 201, e26–e51. [Google Scholar] [CrossRef] [PubMed]
- Judson, M.A.; Costabel, U.; Drent, M.; Wells, A.; Maier, L.; Koth, L.; Shigemitsu, H.; Culver, D.A.; Gelfand, J.; Valeyre, D.; et al. The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool. Sarcoidosis Vasc. Diffuse Lung Dis. 2014, 31, 19–27. [Google Scholar]
- Terasaki, F.; Azuma, A.; Anzai, T.; Ishizaka, N.; Ishida, Y.; Isobe, M.; Inomata, T.; Ishibashi-Ueda, H.; Eishi, Y.; Kitakaze, M.; et al. JCS 2016 Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis—Digest Version. Circ. J. 2019, 83, 2329–2388. [Google Scholar] [CrossRef]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S.; Judson, M.A.; Kron, J.; Mehta, D.; Cosedis Nielsen, J.; et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014, 11, 1305–1323. [Google Scholar] [CrossRef]
- Trivieri, M.G.; Spagnolo, P.; Birnie, D.; Liu, P.; Drake, W.; Kovacic, J.C.; Baughman, R.; Fayad, Z.A.; Judson, M.A. Challenges in Cardiac and Pulmonary Sarcoidosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1878–1901. [Google Scholar] [CrossRef]
- Markatis, E.; Afthinos, A.; Antonakis, E.; Papanikolaou, I.C. Cardiac sarcoidosis: Diagnosis and management. Rev. Cardiovasc. Med. 2020, 21, 321–338. [Google Scholar] [CrossRef]
- Ribeiro Neto, M.L.; Jellis, C.; Hachamovitch, R.; Wimer, A.; Highland, K.B.; Sahoo, D.; Khabbaza, J.E.; Pande, A.; Bindra, A.; Southern, B.D.; et al. Performance of diagnostic criteria in patients clinically judged to have cardiac sarcoidosis: Is it time to regroup? Am. Heart J. 2020, 223, 106–109. [Google Scholar] [CrossRef]
- Rosenbaum, A.N.; Kolluri, N.; Elwazir, M.Y.; Kapa, S.; Abou Ezzeddine, O.F.; Bois, J.P.; Chareonthaitawee, P.; Schmidt, T.J.; Cooper, L.T. Identification of a novel presumed cardiac sarcoidosis category for patients at high risk of disease. Int. J. Cardiol. 2021, 335, 66–72. [Google Scholar] [CrossRef]
- Brincker, H. The sarcoidosis-lymphoma syndrome. Br. J. Cancer 1986, 54, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, I.C.; Sharma, O.P. The relationship between sarcoidosis and lymphoma. Eur. Respir. J. 2010, 36, 1207–1209. [Google Scholar] [CrossRef] [PubMed]
- Viles-Gonzalez, J.F.; Pastori, L.; Fischer, A.; Wisnivesky, J.P.; Goldman, M.G.; Mehta, D. Supraventricular arrhythmias in patients with cardiac sarcoidosis prevalence, predictors, and clinical implications. Chest 2013, 143, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Selan, J.C.; Michaelson, M.; Fanburg, B.L.; Estes, N.A.M. Evaluation and management of heart rhythm disturbances due to cardiac sarcoidosis. Heart Lung Circ. 2014, 23, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Banba, K.; Kusano, K.F.; Nakamura, K.; Morita, H.; Ogawa, A.; Ohtsuka, F.; Ogo, K.O.; Nishii, N.; Watanabe, A.; Nagase, S.; et al. Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis. Heart Rhythm 2007, 4, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Sadek, M.M.; Yung, D.; Birnie, D.H.; Beanlands, R.S.; Nery, P.B. Corticosteroid therapy for cardiac sarcoidosis: A systematic review. Can. J. Cardiol. 2013, 29, 1034–1041. [Google Scholar] [CrossRef]
- Chiu, C.-Z.; Nakatani, S.; Zhang, G.; Tachibana, T.; Ohmori, F.; Yamagishi, M.; Kitakaze, M.; Tomoike, H.; Miyatake, K. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am. J. Cardiol. 2005, 95, 143–146. [Google Scholar] [CrossRef]
- Rosenthal, D.G.; Parwani, P.; Murray, T.O.; Petek, B.J.; Benn, B.S.; De Marco, T.; Gerstenfeld, E.P.; Janmohamed, M.; Klein, L.; Lee, B.K.; et al. Long-Term Corticosteroid-Sparing Immunosuppression for Cardiac Sarcoidosis. J. Am. Heart Assoc. 2019, 8, e010952. [Google Scholar] [CrossRef]
- Birnie, D.; Beanlands, R.S.B.; Nery, P.; Aaron, S.D.; Culver, D.A.; DeKemp, R.A.; Gula, L.; Ha, A.; Healey, J.S.; Inoue, Y.; et al. Cardiac Sarcoidosis multi-center randomized controlled trial (CHASM CS- RCT). Am. Heart J. 2020, 220, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Bussinguer, M.; Danielian, A.; Sharma, O.P. Cardiac sarcoidosis: Diagnosis and management. Curr. Treat. Options Cardiovasc. Med. 2012, 14, 652–664. [Google Scholar] [CrossRef]
- Griffin, J.M.; Chasler, J.; Wand, A.L.; Okada, D.R.; Smith, J.N.; Saad, E.; Tandri, H.; Chrispin, J.; Sharp, M.; Kasper, E.K.; et al. Management of Cardiac Sarcoidosis Using Mycophenolate Mofetil as a Steroid-Sparing Agent. J. Card. Fail. 2021, 27, 1348–1358. [Google Scholar] [CrossRef]
- Kikuchi, N.; Nunoda, S.; Serizawa, N.; Suzuki, A.; Suzuki, T.; Fukushima, K.; Uto, K.; Shiga, T.; Shoda, M.; Hagiwara, N. Combination therapy with corticosteroid and mycophenolate mofetil in a case of refractory cardiac sarcoidosis. J. Cardiol. Cases 2016, 13, 125–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Demeter, S.L. Myocardial sarcoidosis unresponsive to steroids. Treatment with cyclophosphamide. Chest 1988, 94, 202–203. [Google Scholar] [CrossRef]
- Harper, L.J.; McCarthy, M.; Ribeiro Neto, M.L.; Hachamovitch, R.; Pearson, K.; Bonanno, B.; Shaia, J.; Brunken, R.; Joyce, E.; Culver, D.A. Infliximab for refractory cardiac sarcoidosis. Am. J. Cardiol. 2019, 124, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T.; Nabeta, T.; Naruse, Y.; Taniguchi, T.; Yoshioka, K.; Miyakoshi, C.; Kurashima, S.; Miyoshi, Y.; Tanaka, H.; Okumura, T.; et al. Comparisons between biopsy-proven versus clinically diagnosed cardiac sarcoidosis. Heart 2022, 108, 1887–1894. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazitt, T.; Kharouf, F.; Feld, J.; Haddad, A.; Hijazi, N.; Kibari, A.; Fuks, A.; Sabo, E.; Mor, M.; Peleg, H.; et al. Real-Life Utilization of Criteria Guidelines for Diagnosis of Cardiac Sarcoidosis (CS). J. Clin. Med. 2023, 12, 5278. https://doi.org/10.3390/jcm12165278
Gazitt T, Kharouf F, Feld J, Haddad A, Hijazi N, Kibari A, Fuks A, Sabo E, Mor M, Peleg H, et al. Real-Life Utilization of Criteria Guidelines for Diagnosis of Cardiac Sarcoidosis (CS). Journal of Clinical Medicine. 2023; 12(16):5278. https://doi.org/10.3390/jcm12165278
Chicago/Turabian StyleGazitt, Tal, Fadi Kharouf, Joy Feld, Amir Haddad, Nizar Hijazi, Adi Kibari, Alexander Fuks, Edmond Sabo, Maya Mor, Hagit Peleg, and et al. 2023. "Real-Life Utilization of Criteria Guidelines for Diagnosis of Cardiac Sarcoidosis (CS)" Journal of Clinical Medicine 12, no. 16: 5278. https://doi.org/10.3390/jcm12165278
APA StyleGazitt, T., Kharouf, F., Feld, J., Haddad, A., Hijazi, N., Kibari, A., Fuks, A., Sabo, E., Mor, M., Peleg, H., Asleh, R., & Zisman, D. (2023). Real-Life Utilization of Criteria Guidelines for Diagnosis of Cardiac Sarcoidosis (CS). Journal of Clinical Medicine, 12(16), 5278. https://doi.org/10.3390/jcm12165278





