Italian Physicians’ Perceptions about the Role of Asciminib in Later Lines Chronic Myeloid Leukemia in Clinical Practice: A GIMEMA Survey
Abstract
1. Introduction
2. Materials and Methods
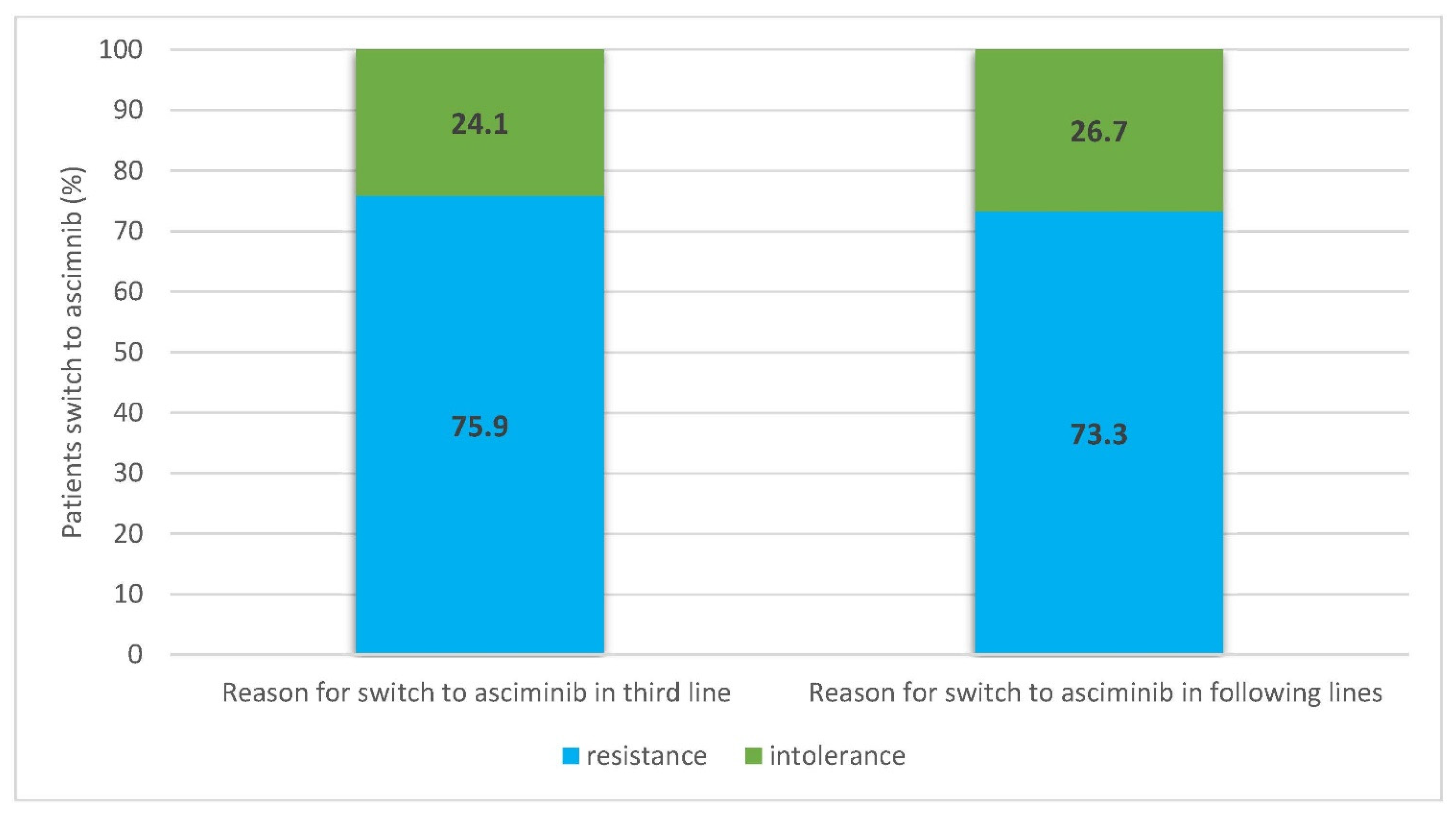
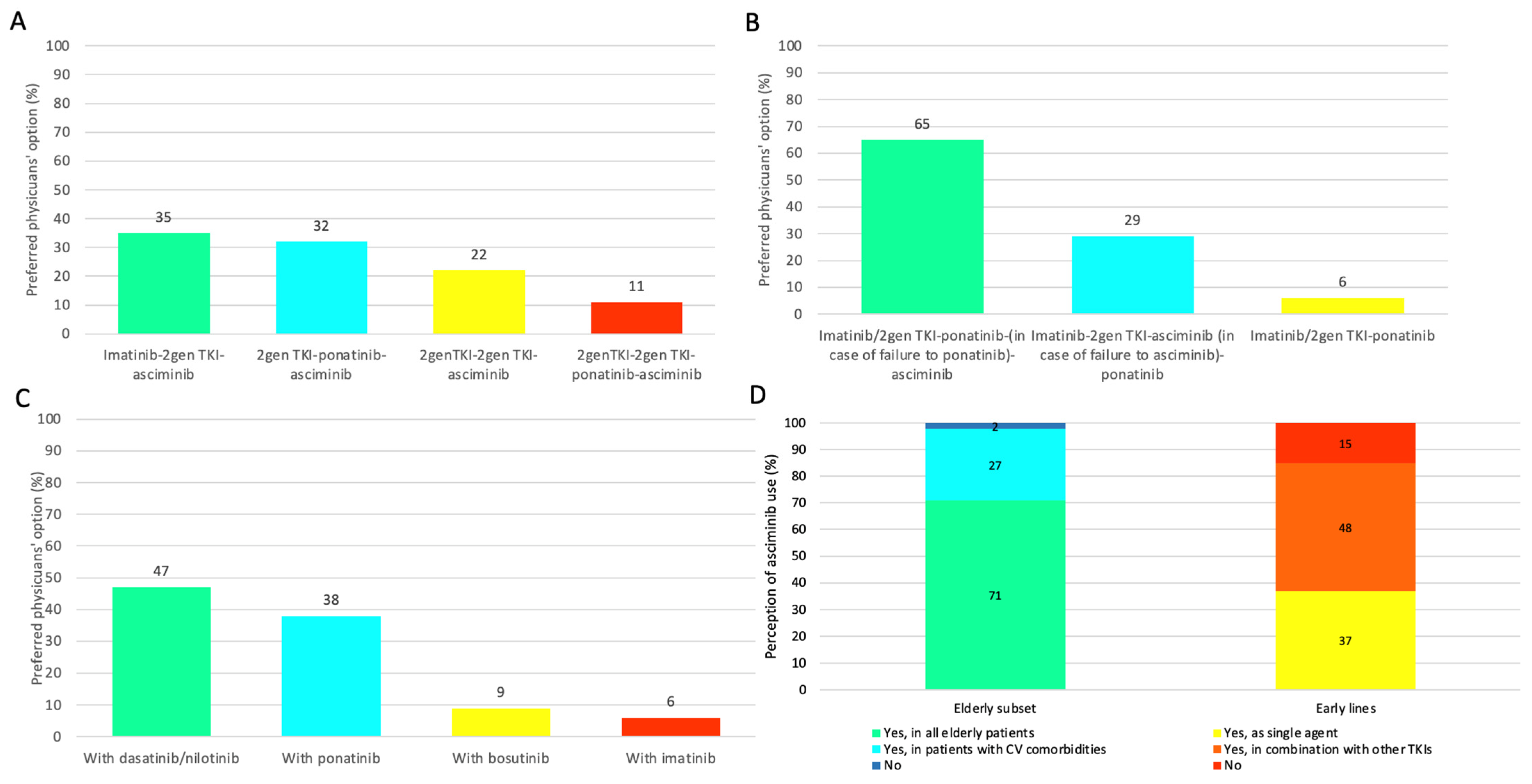
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| AOU OSPEDALI RIUNITI “UMBERTO I—G.M. LANCISI—G. SALESI”—ANCONA—SOD CLINICA EMATOLOGICA, Serena Rupoli |
|---|
| IRCCS CENTRO DI RIFERIMENTO ONCOLOGICO DI AVIANO—SOSD ONCOEMATOLOGIA TRAPIANTI EMOPOIETICI E TERAPIE CELLULARI, Maria Grazia Michieli |
| AOU CONSORZIALE POLICLINICO—BARI—UO EMATOLOGIA CON TRAPIANTO, Pellegrino Musto |
| ASST PAPA GIOVANNI XXIII—OSPEDALE DI BERGAMO—SC EMATOLOGIA, Alessandro Rambaldi |
| AOU DI BOLOGNA—POLICLINICO S. ORSOLA-MALPIGHI—UOC EMATOLOGIA, Fausto Castagnetti |
| AO BROTZU, PRESIDIO OSPEDALIERO A. BUSINCO—CAGLIARI—SC EMATOLOGIA E CTMO, Giovanni Caocci |
| CTC U.O DI EMATOLOGIA CON TRAPIANTO DI MIDOLLO OSSEO—CATANIA, Francesco Di Raimondo |
| AO DI CATANZARO “PUGLIESE-CIACCIO”, PRESIDIO OSPEDALIERO “CIACCIO—DE LELLIS”—EMATOLOGIA, Luciano Levato |
| AOU CAREGGI—FIRENZE—SOD EMATOLOGIA, Antonella Gozzini |
| AO DI RILIEVO NAZIONALE ANTONIO CARDARELLI—NAPOLI—UOC EMATOLOGIA CON TRAPIANTO DI MIDOLLO, Mario Annunziata |
| AOU FEDERICO II—NAPOLI—UOC EMATOLOGIA, Fabrizio Pane |
| AOU POLICLINICO P. GIACCONE—PALERMO—UO EMATOLOGIA, Sergio Siragusa |
| AO OSPEDALI RIUNITI VILLA SOFIA CERVELLO—PALERMO—UO EMATOLOGIA AD INDIRIZZO ONCOLOGICO, Caterina Patti |
| AO DI PERUGIA, OSPEDALE S. MARIA DELLA MISERICORDIA—EMATOLOGIA E TRAPIANTO MIDOLLO OSSEO, Cristina Mecucci |
| AO OSPEDALI RIUNITI MARCHE NORD—OSPEDALE SAN SALVATORE—PESARO—UOC EMATOLOGIA E CENTRO TRAPIANTI, Giuseppe Visani |
| 027, FONDAZIONE POLICLINICO UNIVERSITARIO AGOSTINO GEMELLI IRCCS—ROMA—AREA EMATOLOGICA, Simona Sica |
| UNIVERSITA’ DEGLI STUDI DI ROMA “SAPIENZA”—DIPARTIMENTO DI MEDICINA TRASLAZIONALE E DI PRECISIONE—U.O.C. EMATOLOGIA, Massimo Breccia |
| ASL ROMA 2, OSPEDALE S. EUGENIO—OSPEDALE S.EUGENIO—UOC EMATOLOGIA, Elisabetta Abruzzese |
| AOU DI SASSARI—CLINICHE UNIVERSITARIE—STABILIMENTO CLINICHE DI SAN PIETRO —UOC EMATOLOGIA, Claudio Fozza |
| ASUI DI UDINE—PRESIDIO OSPEDALIERO “SANTA MARIA DELLA MISERICORDIA”—CLINICA EMATOLOGICA, Mario Tiribelli |
| AULSS 8 BERICA—OSPEDALE DI VICENZA—UOC EMATOLOGIA, Maria Cristina Miggiano |
| IRCCS AOU SAN MARTINO—GENOVA—UO EMATOLOGIA E TRAPIANTI, Germana Beltrami |
| AOU CITTA’ DELLA SALUTE E DELLA SCIENZA, OSPEDALE S. GIOVANNI BATTISTA MOLINETTE—TORINO—SC EMATOLOGIA 2, Patrizia Pregno |
| AOU DI PARMA—SC EMATOLOGIA E CENTRO TRAPIANTI MIDOLLO OSSEO, Monica Crugnola |
| FONDAZIONE IRCSS POLICLINICO SAN MATTEO—PAVIA—UO EMATOLOGIA, Luca Arcaini |
| AOU INTEGRATA DI VERONA, POLICLINICO G.B. ROSSI—UOC EMATOLOGIA, Massimiliano Bonifacio |
| FONDAZIONE IRCCS CA’ GRANDA, OSPEDALE MAGGIORE POLICLINICO—MILANO—EMATOLOGIA—PADIGLIONE MARCORA, Alessandra Iurlo |
| ASO S. CROCE E CARLE—CUNEO—SC EMATOLOGIA, Davide Rapezzi |
| IRCCS OSPEDALE S. RAFFAELE—MILANO—UO ONCOEMATOLOGIA, Fabio Ciceri |
| ASST DEGLI SPEDALI CIVILI DI BRESCIA—UO EMATOLOGIA, Alessandra Tucci |
| ASL NAPOLI 1 CENTRO, PRESIDIO OSPEDALIERO ASCALESI—OSPEDALE S.MARIA DI LORETO NUOVO, Angiolina Rocino |
| AUSL DI REGGIO EMILIA —ARCISPEDALE SANTA MARIA NUOVA, IRCCS—SC EMATOLOGIA, Francesco Merli |
| ASL BRINDISI, OSPEDALE ‘PERRINO’—BRINDISI—UO EMATOLOGIA, Domenico Pastore |
| AOU ARCISPEDALE SANT’ANNA—CONA (FE)—UOC EMATOLOGIA E FISIOPATOLOGIA DELLA COAGULAZIONE, Francesco Cavazzini |
| AOU PISANA—UO EMATOLOGIA UNIVERSITARIA, Sara Galimberti |
| AOU SENESE—UOC EMATOLOGIA E TRAPIANTI, Monica Bocchia |
| AULSS 3 SERENISSIMA, OSPEDALE DELL’ANGELO—MESTRE—UO EMATOLOGIA, Renato Bassan |
| UOSD di Ematologia Presidi Osp. S.Spirito-Nuovo Regina Margherita—S.Filippo Neri, Tommaso Caravita di Toritto |
| APSS TRENTINO, OSPEDALE DI TRENTO—PRESIDIO OSPEDALIERO S.CHIARA—SSD EMATOLOGIA, Anna Guella |
| ASST FATEBENEFRATELLI SACCO OSPEDALE LUIGI SACCO—POLO UNIVERSITARIO —EMATOLOGIA E MEDICINA TRASFUSIONALE (SIMT), MARIA CRISTINA CARRARO |
| AOU DI PADOVA—UO EMATOLOGIA, Gianni Binotto |
| CASA DI CURA LA MADDALENA S.P.A.—DIPARTIMENTO ONCOLOGICO DI III LIVELLO—PALERMO—UO ONCOEMATOLOGIA E TMO, Maurizio Musso |
| ISTITUTI FISIOTERAPICI OSPITALIERI—IFO—ISTITUTO REGINA ELENA—ROMA—UOSD EMATOLOGIA, Andrea Mengarelli |
| ASP MESSINA, PRESIDIO OSPEDALIERO ‘S. VINCENZO’—TAORMINA—UOC EMATOLOGIA E LABORATORIO DI BIOLOGIA MOLECOLARE, Giuseppe Mineo |
| AREA VASTA N. 5 ASCOLI PICENO—S. BENEDETTO DEL TRONTO, PRESIDIO OSPEDALIERO AV5 OSP. GEN. PROV.LE “C.G.MAZZONI” —UOC EMATOLOGIA, Piero Galieni |
| AOU POLICLINICO “G. MARTINO”—MESSINA—UOC EMATOLOGIA, Caterina Musolino |
| AOU MAGGIORE DELLA CARITA’ DI NOVARA—SCDU EMATOLOGIA, Monia Lunghi |
| AOU SANT`ANDREA—ROMA—UOC EMATOLOGIA, Agostino Tafuri |
| AZIENDA SANITARIA UNIVERSITARIA INTEGRATA DI TRIESTE—SC EMATOLOGIA, Francesco Zaja |
| ASL LECCE, OSPEDALE ‘V. FAZZI’—UO EMATOLOGIA, Nicola Di Renzo |
| ASST DEGLI SPEDALI CIVILI DI BRESCIA—SSVD CENTRO TRAPIANTI MIDOLLO PER ADULTI—CATTEDRA DI EMATOLOGIA, Domenico Russo |
| ASL DELLA PROVINCIA DI BARLETTA, ANDRIA, TRANI, OSPEDALE “MONS. DIMICCOLI”—BARLETTA—UO EMATOLOGIA, Giuseppe Tarantini |
| I.R.S.T. SRL IRCCS—MELDOLA—SC ONCOLOGIA MEDICA, Alessandro Lucchesi |
| U.O.C. ONCOEMATOLOGIA “ISTITUTO ONCOLOGICO VENETO IRCCS, PRESIDIO OSPEDALIERO S. GIACOMO APOSTOLO, Roberto Sartori |
| ASL LATINA, PRESIDIO OSPEDALIERO NORD—OSPEDALE SANTA MARIA GORETTI—UOC EMATOLOGIA, Alessandro Pulsoni |
| ARNAS GARIBALDI, PO GARIBALDI-NESIMA—CATANIA—UOC EMATOLOGIA, Ugo Consoli |
| IRCCS ONCOLOGICO ISTITUTO TUMORI GIOVANNI PAOLO II—BARI —UO EMATOLOGIA, Attilio Guarini |
| ASP DI RAGUSA—UO IMMUNOEMATOLOGIA E MEDICINA TRASFUSIONALE, Sergio Cabibbo |
| ASP CALTANISSETTA—UO EMATOLOGIA, Maria Flavia Fiorenza |
| OSPEDALE MAURIZIANO UMBERTO I—TORINO—SCDU EMATOLOGIA, Carmen Fava |
| OSPEDALE DI SASSUOLO SPA—EMATOLOGIA, Sara Bigliardi |
| ASL TO 2, TORINO NORD EMERGENZA SAN GIOVANNI BOSCO—SSD EMATOLOGIA, Massimo Pini |
| OSPEDALE S.ANDREA DI VERCELLI—ASL VERCELLI—SS EMATOLOGIA, LORENZO DE PAOLI |
| ASL DI SALERNO— PO SAN LUCA, Giovanni D’Arena |
| ASST-MANTOVA-Struttura Complessa-Servizio immunoematologia e Medicina Trasfusionale-Ospedale San Carlo Poma, Massimo Franchini |
| LSS n.7-PEDEMONTANA- OSPEDALE DI BASSANO DEL GRAPPA UOC ONCOEMATOLOGIA, Eros Di Bona |
References
- Bower, H.; Björkholm, M.; Dickman, P.W.; Höglund, M.; Lambert, P.C.; Andersson, T.M.L. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J. Clin. Oncol. 2016, 34, 2851–2857. [Google Scholar] [CrossRef] [PubMed]
- Breccia, M.; Olimpieri, P.P.; Olimpieri, O.; Pane, F.; Iurlo, A.; Foggi, P.; Cirilli, A.; Colatrella, A.; Cuomo, M.; Gozzo, L.; et al. How many chronic myeloid leukemia patients who started a frontline second-generation tyrosine kinase inhibitor have to switch to a second-line treatment? A retrospective analysis from the monitoring registries of the Italian medicines agency (AIFA). Cancer Med. 2020, 9, 4160–4165. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Lang, F. Third-line therapy for chronic myeloid leukemia: Current status and future directions. J. Hematol. Oncol. 2021, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Steegmann, J.L.; Baccarani, M.; Breccia, M.; Casado, L.F.; García-Gutiérrez, V.; Hochhaus, A.; Kim, D.-W.; Kim, T.D.; Khoury, H.J.; Le Coutre, P.; et al. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukemia. Leukemia 2016, 30, 1648–1671. [Google Scholar] [CrossRef] [PubMed]
- Senapati, J.; Sasaki, K.; Issa, G.C.; Lipton, J.H.; Radich, J.P.; Jabbour, E.; Kantarjian, H.M. Management of chronic myeloid leukemia in 2023-common ground and common sense. Blood Cancer J. 2023, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, N.; Hughes, T.P. Asciminib for chronic myeloid leukaemia: Next questions. Br. J. Haematol. 2022, 199, 322–331. [Google Scholar] [CrossRef]
- Mauro, M.; Hughes, T.P.; Kim, D.W.; Rea, D.; Cortes, J.E.; Hochhaus, A.; Sasaki, K.; Breccia, M.; Talpaz, M.; Ottmann, O.; et al. Asciminib monotherapy in patients with CML-CP without BCR::ABL1 T315I mutations treated with at least two prior TKIs: 4-year phase 1 safety and efficacy results. Leukemia 2023, 37, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Réa, D.; Boquimpani, C.; Minami, Y.; Cortes, J.E.; Hughes, T.P.; Apperley, J.F.; Lomaia, E.; Voloshin, S.; Turkina, A.; et al. Asciminib vs. bosutinib in chronic-phase chronic myeloid leukemia previously treated with at least two tyrosine kinase inhibitors: Longer-term follow-up of ASCEMBL. Leukemia 2023, 37, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.; Rea, D.; Boquimpani, C.; Minami, Y.; Mauro, M.; Cortes, J.E.; Apperley, J.F.; Gutiérrez, V.G.; Kapoor, S.; Allepuz, A.; et al. Dynamics of response and response factors in patients with chronic myeloid leukemia in chronic phase (CML-CP) after >2 prior tyrosine kinase inhibitors (TKIs) in the phase 3 Ascembl study. Blood 2022, 140 (Suppl. S1), 6757–6759. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, V.; Breccia, M.; Jabbour, E.; Mauro, M.; Cortes, J.E. A clinician perspective on the treatment of chronic myeloid leukemia in the chronic phase. J. Hematol. Oncol. 2022, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, A.; Gambacorti-Passerini, C.; Abboud, C.; Gjertsen, B.T.; Brümmendorf, T.H.; Smith, B.D.; Ernst, T.; Giraldo-Castellano, P.; Olsson-Strömberg, U.; Saussele, S.; et al. Bosutinib for pretreated patients with chronic phase chronic myeloid leukemia: Primary results of the phase 4 BYOND study. Leukemia 2020, 34, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Apperley, J.; Lomaia, E.; Moiraghi, B.; Undurraga Sutton, M.; Pavlovsky, C.; Chuah, C.; Sacha, T.; Lipton, J.H.; Schiffer, C.A.; et al. Ponatinib dose-ranging study in chronic phase chronic myeloid leukemia: A randomized, open-label phase 2 clinical trial. Blood 2021, 138, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Breccia, M.; Russo Rossi, A.; Giai, V.; Martino, B.; Fava, C.; Annunziata, M.; Abruzzese, E.; Binotto, G.; Baratè, C.; Nardozza, A.P.; et al. Real-World Efficacy Profile of Asciminib in An Italian, Multi-Resistant Chronic Phase Chronic Myeloid Leukemia (CML-CP) Patient Population. HemaSphere 2023, 7, 1197. [Google Scholar] [CrossRef]
- Breccia, M.; Chiodi, F.; Nardozza, A.P.; Valsecchi, D.; Perrone, V.; Sangiorgi, D.; Giacomini, E.; Rendace, M.C.; Coco, P.; Premoli, E.; et al. Real-world analysis of therapeutic management and disease burden in chronic myeloid leukemia patients with later lines in Italy. J. Clin. Med. 2022, 11, 3597. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breccia, M.; Piciocchi, A.; Abruzzese, E.; Cilloni, D.; Messina, M.; Soddu, S.; Castagnetti, F.; Stagno, F.; Fazi, P.; Iurlo, A.; et al. Italian Physicians’ Perceptions about the Role of Asciminib in Later Lines Chronic Myeloid Leukemia in Clinical Practice: A GIMEMA Survey. J. Clin. Med. 2023, 12, 5267. https://doi.org/10.3390/jcm12165267
Breccia M, Piciocchi A, Abruzzese E, Cilloni D, Messina M, Soddu S, Castagnetti F, Stagno F, Fazi P, Iurlo A, et al. Italian Physicians’ Perceptions about the Role of Asciminib in Later Lines Chronic Myeloid Leukemia in Clinical Practice: A GIMEMA Survey. Journal of Clinical Medicine. 2023; 12(16):5267. https://doi.org/10.3390/jcm12165267
Chicago/Turabian StyleBreccia, Massimo, Alfonso Piciocchi, Elisabetta Abruzzese, Daniela Cilloni, Monica Messina, Stefano Soddu, Fausto Castagnetti, Fabio Stagno, Paola Fazi, Alessandra Iurlo, and et al. 2023. "Italian Physicians’ Perceptions about the Role of Asciminib in Later Lines Chronic Myeloid Leukemia in Clinical Practice: A GIMEMA Survey" Journal of Clinical Medicine 12, no. 16: 5267. https://doi.org/10.3390/jcm12165267
APA StyleBreccia, M., Piciocchi, A., Abruzzese, E., Cilloni, D., Messina, M., Soddu, S., Castagnetti, F., Stagno, F., Fazi, P., Iurlo, A., Caocci, G., Gozzini, A., Intermesoli, T., D’Adda, M., & Pane, F. (2023). Italian Physicians’ Perceptions about the Role of Asciminib in Later Lines Chronic Myeloid Leukemia in Clinical Practice: A GIMEMA Survey. Journal of Clinical Medicine, 12(16), 5267. https://doi.org/10.3390/jcm12165267








