Potentials of Acetylcholinesterase and Butyrylcholinesterase Alterations in On-Pump Coronary Artery Bypass Surgery in Postoperative Delirium: An Observational Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Preoperative Assessment
2.3. Quantification of AChE and BChE
2.4. Intraoperative Assessment
2.5. Postoperative Assessment
2.6. Statistical Analysis
3. Results
3.1. Patient Sample
3.2. Baseline Characteristics
3.3. Incidence of Delirium
3.4. Intergroup Differences
3.4.1. Preoperative Variables
3.4.2. Intraoperative Variables
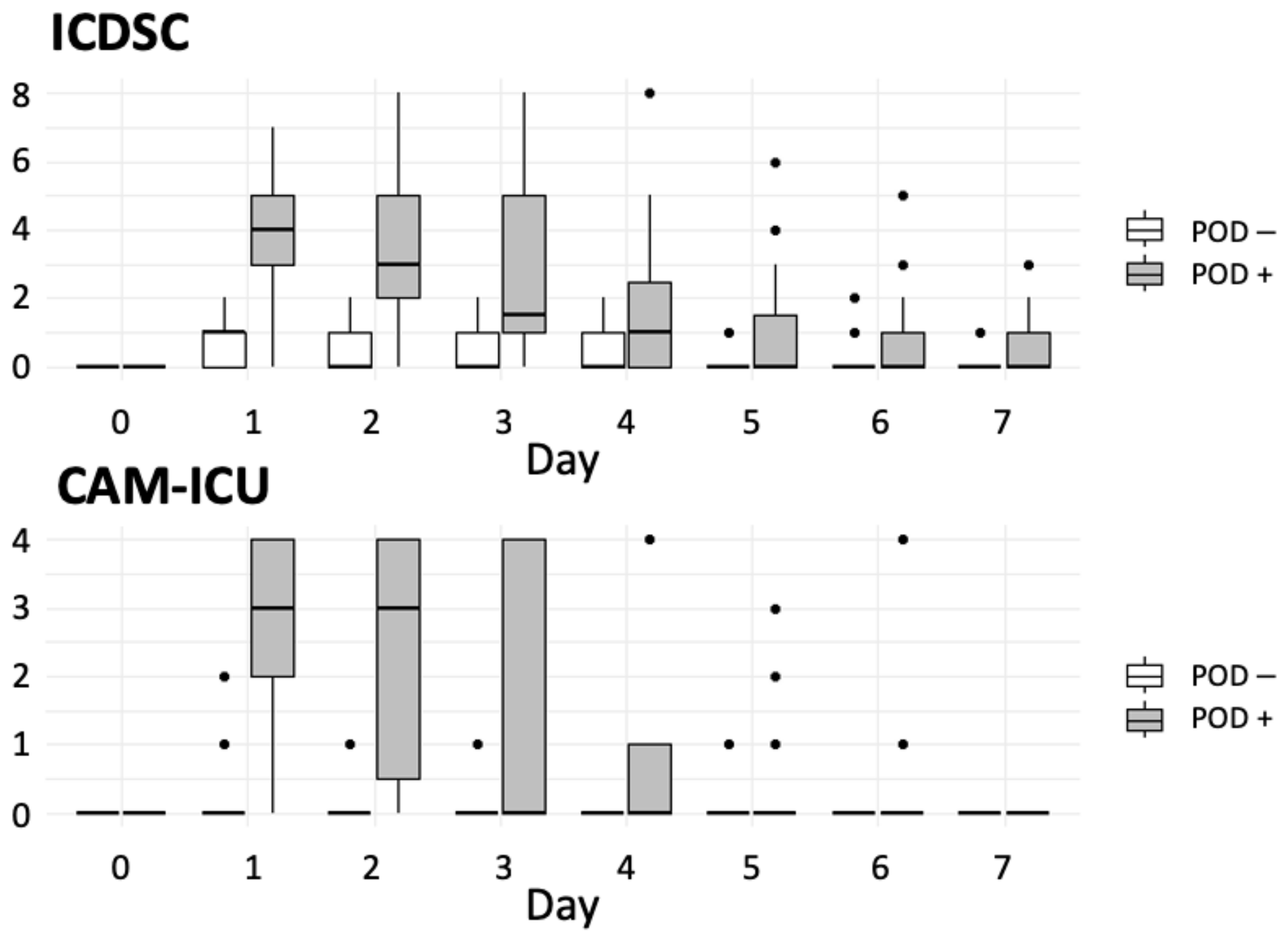
3.4.3. Postoperative Variables
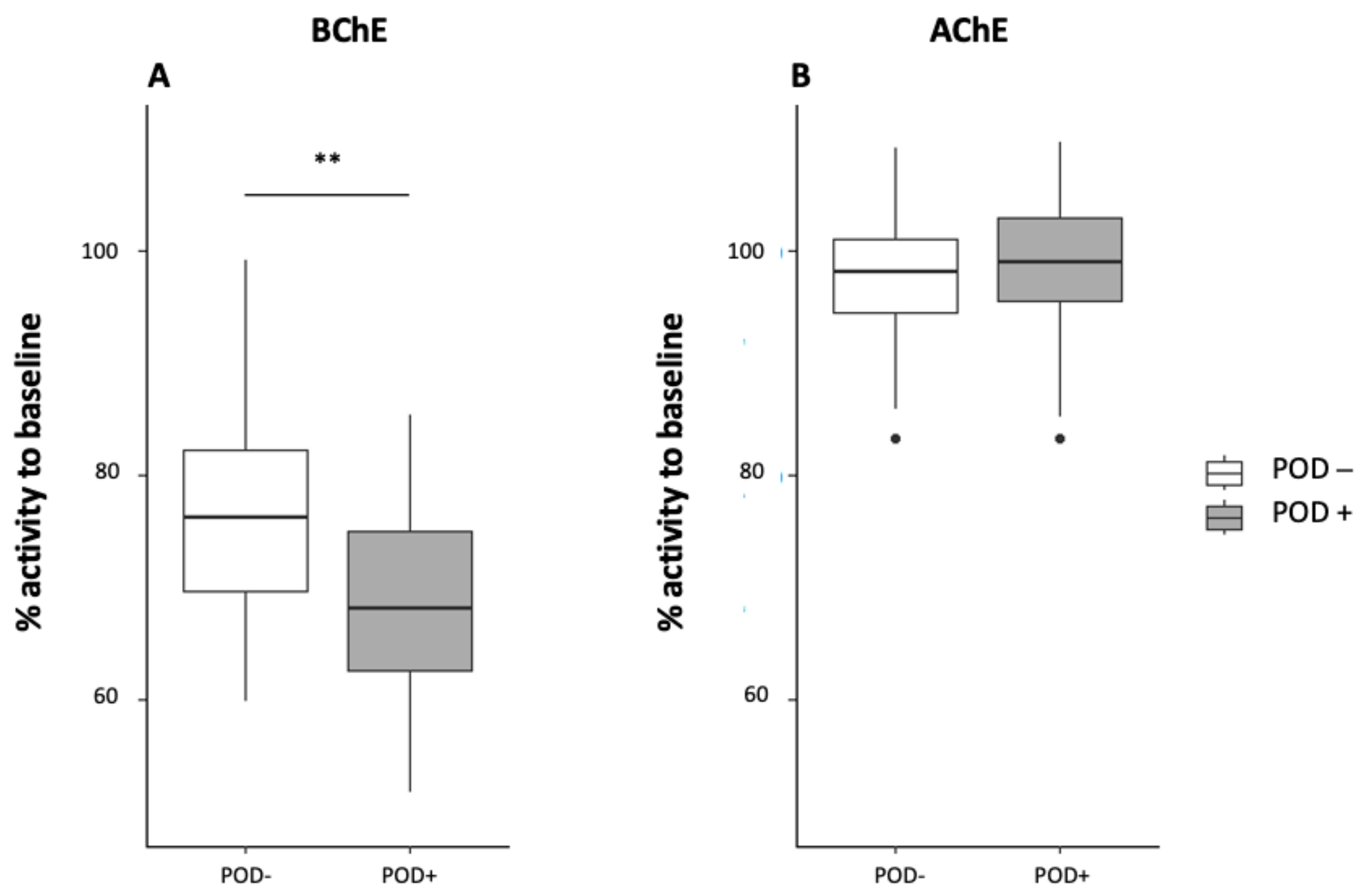
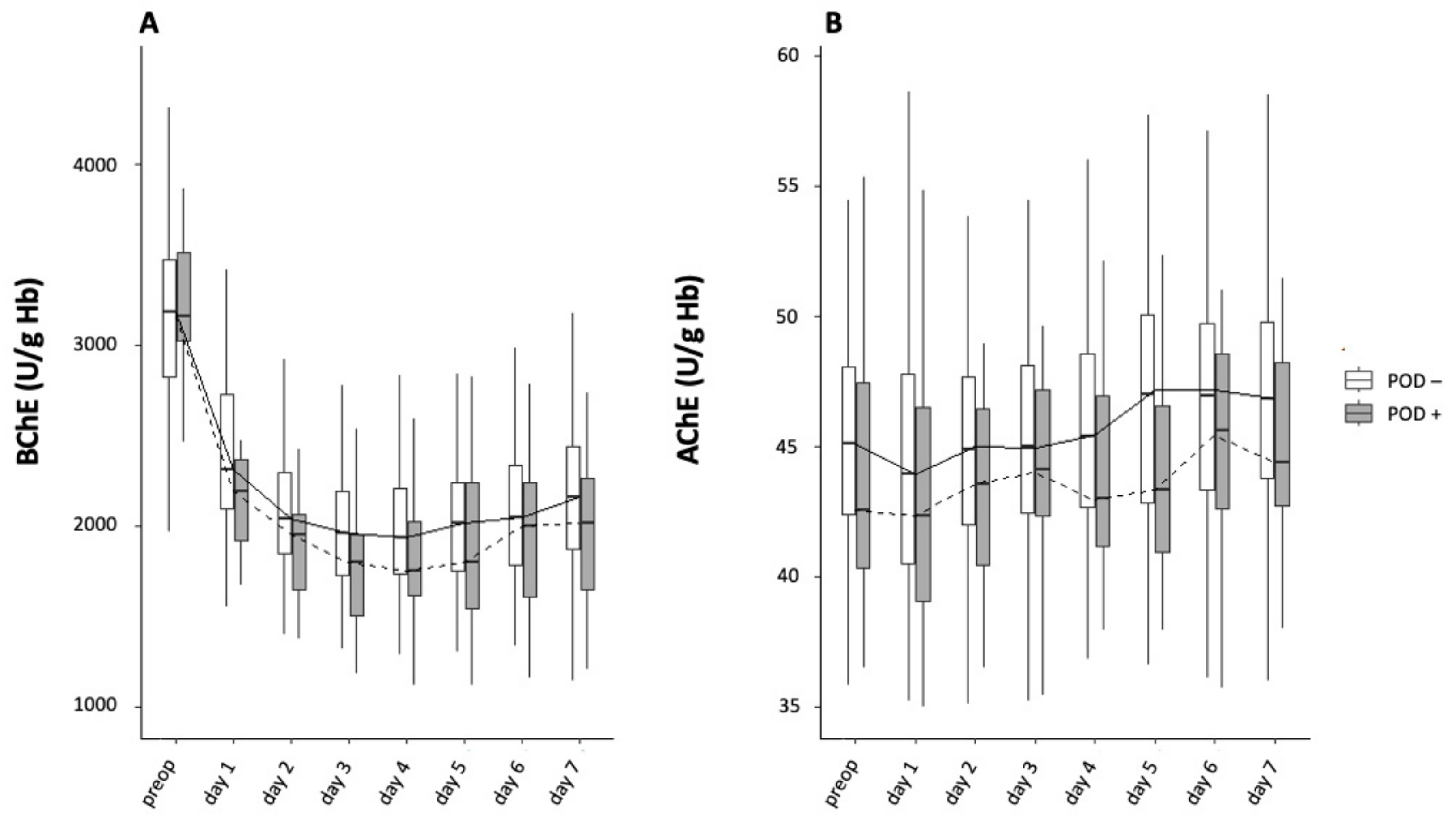
3.4.4. AchE and BChE Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, J.E.; Mart, M.F.; Cunningham, C.; Shehabi, Y.; Girard, T.D.; MacLullich, A.M.J.; Slooter, A.J.C.; Ely, E.W. Delirium. Nat. Rev. Dis. Primers 2020, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Matalanis, G.; Mårtensson, J.; Robbins, R.; Shaw, M.; Seevanayagam, S.; Cowie, D.; Bellomo, R. Predictors and Outcomes of Cardiac Surgery-Associated Delirium. A Single Centre Retrospective Cohort Study. Heart Lung Circ. 2019, 28, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Klein Klouwenberg, P.M.C.; Zaal, I.J.; Spitoni, C.; Ong, D.S.Y.; van der Kooi, A.W.; Bonten, M.J.M.; Slooter, A.J.C.; Cremer, O.L. The attributable mortality of delirium in critically ill patients: Prospective cohort study. BMJ 2014, 349, g6652. [Google Scholar] [CrossRef] [PubMed]
- Cereghetti, C.; Siegemund, M.; Schaedelin, S.; Fassl, J.; Seeberger, M.D.; Eckstein, F.S.; Steiner, L.A.; Goettel, N. Independent Predictors of the Duration and Overall Burden of Postoperative Delirium After Cardiac Surgery in Adults: An Observational Cohort Study. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- American Geriatrics Society. Abstracted clinical practice guideline for postoperative delirium in older adults. J. Am. Geriatr. Soc. 2015, 63, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Adamis, D.; Rooney, S.; Meagher, D.; Mulligan, O.; McCarthy, G. A comparison of delirium diagnosis in elderly medical inpatients using the CAM, DRS-R98, DSM-IV and DSM-5 criteria. Int. Psychogeriatr. 2015, 27, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.R.; Moody, D.M.; Challa, V.R.; Stump, D.A.; Hammon, J.W. Longer duration of cardiopulmonary bypass is associated with greater numbers of cerebral microemboli. Stroke 2000, 31, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Zaal, I.J.; Devlin, J.W.; Peelen, L.M.; Slooter, A.J.C. A systematic review of risk factors for delirium in the ICU. Crit. Care Med. 2015, 43, 40–47. [Google Scholar] [CrossRef]
- An, Y.-S.; Jin, Y.; Jin, T.; Hur, E.Y.; Lee, S.-M. Operative and anaesthetic factors influencing on delirium in the intensive care unit: An Analysis of electronic health records. J. Clin. Nurs. 2019, 28, 1327–1335. [Google Scholar] [CrossRef]
- Maldonado, J.R. Delirium pathophysiology: An updated hypothesis of the etiology of acute brain failure. Int. J. Geriatr. Psychiatry 2018, 33, 1428–1457. [Google Scholar] [CrossRef]
- Karmakar, S.; Lal, G. Role of serotonin receptor signaling in cancer cells and anti-tumor immunity. Theranostics 2021, 11, 5296–5312. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- John, M.; Ely, E.W.; Halfkann, D.; Schoen, J.; Sedemund-Adib, B.; Klotz, S.; Radtke, F.; Stehr, S.; Hueppe, M. Acetylcholinesterase and butyrylcholinesterase in cardiosurgical patients with postoperative delirium. J. Intensive Care 2017, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Zivkovic, A.R.; Thiele, M.; Horter, J.; Brenner, T.; Weigand, M.A.; Kleinschmidt, S.; Hofer, S. Point-of-care measured serum cholinesterase activity predicts patient outcome following severe burns. Burns 2021, 47, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Cerejeira, J.; Batista, P.; Nogueira, V.; Firmino, H.; Vaz-Serra, A.; Mukaetova-Ladinska, E.B. Low preoperative plasma cholinesterase activity as a risk marker of postoperative delirium in elderly patients. Age Ageing 2011, 40, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Olbert, M.; Heymann, A.; Zahn, P.K.; Plaschke, K.; von Dossow, V.; Bitzinger, D.; Barth, E.; Meister, M.; Kranke, P.; et al. Relevance of peripheral cholinesterase activity on postoperative delirium in adult surgical patients (CESARO): A prospective observational cohort study. Eur. J. Anaesthesiol. 2019, 36, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Adam, E.H.; Haas, V.; Lindau, S.; Zacharowski, K.; Scheller, B. Cholinesterase alterations in delirium after cardiosurgery: A German monocentric prospective study. BMJ Open 2020, 10, e031212. [Google Scholar] [CrossRef]
- Saha, S.; Karaca, K.; Jebran, A.F.; Waezi, N.; Ort, K.; Brandes, I.; Hagl, C.; Niehaus, H. Diagnostic Value of Cholinesterase Activity for the Development of Postoperative Delirium after Cardiac Surgery. Thorac. Cardiovasc. Surg. 2021, 69, 693–699. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Nashef, S.; Ali, J.; Smith, C.; Saxton, D.; Maxine, T.; Tadd, P. The Official Site: EuroSCORE: European System for Cardiac Operative Risk Evaluation. Available online: https://www.euroscore.org/index.php?id=1 (accessed on 18 July 2023).
- Worek, F.; Mast, U.; Kiderlen, D.; Diepold, C.; Eyer, P. Improved determination of acetylcholinesterase activity in human whole blood. Clin. Chim. Acta 1999, 288, 73–90. [Google Scholar] [CrossRef]
- Securetec Detektions-Systeme, A.G. Che Check Mobile: Portable Cholinesterase Testing System. 2013. Available online: https://www.securetec.net/app/uploads/2018/08/Schnelltest-Bestimmung-Cholinesterase_Brochure_ChE_Enzymtest_military_use_70507_v03_EN_Email.pdf (accessed on 22 July 2023).
- ICD-10-CM: The Complete Official Codebook, 2019th ed.; American Medical Association: Chicago, MI, USA, 2018; ISBN 9781622027736.
- Zivkovic, A.R.; Bender, J.; Brenner, T.; Hofer, S.; Schmidt, K. Reduced butyrylcholinesterase activity is an early indicator of trauma-induced acute systemic inflammatory response. J. Inflamm. Res. 2016, 9, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Zivkovic, A.R.; Tourelle, K.M.; Brenner, T.; Weigand, M.A.; Hofer, S.; Schmidt, K. Reduced serum cholinesterase activity indicates splenic modulation of the sterile inflammation. J. Surg. Res. 2017, 220, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Bitzinger, D.I.; Gruber, M.; Tümmler, S.; Malsy, M.; Seyfried, T.; Weber, F.; Redel, A.; Graf, B.M.; Zausig, Y.A. In Vivo Effects of Neostigmine and Physostigmine on Neutrophil Functions and Evaluation of Acetylcholinesterase and Butyrylcholinesterase as Inflammatory Markers during Experimental Sepsis in Rats. Mediat. Inflamm. 2019, 2019, 8274903. [Google Scholar] [CrossRef]
- Fernandez-Cabezudo, M.J.; Lorke, D.E.; Azimullah, S.; Mechkarska, M.; Hasan, M.Y.; Petroianu, G.A.; al-Ramadi, B.K. Cholinergic stimulation of the immune system protects against lethal infection by Salmonella enterica serovar Typhimurium. Immunology 2010, 130, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Al-Barazie, R.M.; Bashir, G.H.; Qureshi, M.M.; Mohamed, Y.A.; Al-Sbiei, A.; Tariq, S.; Lammers, W.J.; al-Ramadi, B.K.; Fernandez-Cabezudo, M.J. Cholinergic Activation Enhances Resistance to Oral Salmonella Infection by Modulating Innate Immune Defense Mechanisms at the Intestinal Barrier. Front. Immunol. 2018, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Zujalovic, B.; Mayer, B.; Hafner, S.; Balling, F.; Barth, E. AChE-activity in critically ill patients with suspected septic encephalopathy: A prospective, single-centre study. BMC Anesthesiol. 2020, 20, 287. [Google Scholar] [CrossRef]
- Zimmer, K.R.; Lencina, C.L.; Zimmer, A.R.; Thiesen, F.V. Influence of physical exercise and gender on acetylcholinesterase and butyrylcholinesterase activity in human blood samples. Int. J. Environ. Health Res. 2012, 22, 279–286. [Google Scholar] [CrossRef]
- Lane, R.M.; He, Y. Butyrylcholinesterase genotype and gender influence Alzheimer’s disease phenotype. Alzheimers. Dement. 2013, 9, e1–e73. [Google Scholar] [CrossRef]
- Brimijoin, S.; Tye, S. Favorable Impact on Stress-Related Behaviors by Modulating Plasma Butyrylcholinesterase. Cell Mol. Neurobiol. 2017, 38, 7–12. [Google Scholar] [CrossRef]
- Mear, Y.; Enjalbert, A.; Thirion, S. GHS-R1a constitutive activity and its physiological relevance. Front. Neurosci. 2013, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Wang, L.; Taché, Y. Stress-related alterations of acyl and desacyl ghrelin circulating levels: Mechanisms and functional implications. Peptides 2011, 32, 2208–2217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gahete, M.D.; Córdoba-Chacón, J.; Kineman, R.D.; Luque, R.M.; Castaño, J.P. Role of ghrelin system in neuroprotection and cognitive functions: Implications in Alzheimer’s disease. Peptides 2011, 32, 2225–2228. [Google Scholar] [CrossRef] [PubMed]
- Michels, B.; Holzamer, A.; Graf, B.M.; Bredthauer, A.; Petermichl, W.; Müller, A.; Zausig, Y.A.; Bitzinger, D.I. Butyrylcholinesterase as a perioperative complication marker in patients after transcatheter aortic valve implantation: A prospective observational study. BMJ Open 2021, 11, e042857. [Google Scholar] [CrossRef] [PubMed]
- Nadorp, B.; Soreq, H. Predicted overlapping microRNA regulators of acetylcholine packaging and degradation in neuroinflammation-related disorders. Front. Mol. Neurosci. 2014, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, J.L.; Babikian, V.L.; Treanor, P.; Pochay, V.E.; Wigginton, J.B.; Crittenden, M.D.; Marcantonio, E.R. Mi-croemboli are not associated with delirium after coronary artery bypass graft surgery. Perfusion 2009, 24, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Bokeriia, L.A.; Golukhova, E.Z.; Breskina, N.Y.; Polunina, A.G.; Davydov, D.M.; Begachev, A.V.; Kazanovskaya, S.N. Asymmetric cerebral embolic load and postoperative cognitive dysfunction in cardiac surgery. Cerebrovasc. Dis. 2007, 23, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Blauth, C.I. Macroemboli and microemboli during cardiopulmonary bypass. The Annals of Thoracic Surgery 1995, 59, 1300–1303. [Google Scholar] [CrossRef]
- Martin, K.K.; Wigginton, J.B.; Babikian, V.L.; Pochay, V.E.; Crittenden, M.D.; Rudolph, J.L. Intraoperative cerebral high-intensity transient signals and postoperative cognitive function: A systematic review. Am. J. Surg. 2009, 197, 55–63. [Google Scholar] [CrossRef]
- Patel, N.; Minhas, J.S.; Chung, E.M.L. Intraoperative Embolization and Cognitive Decline After Cardiac Surgery: A Systematic Review. Semin. Cardiothorac. Vasc. Anesth. 2016, 20, 225–231. [Google Scholar] [CrossRef]
- Kruis, R.W.J.; Vlasveld, F.A.E.; van Dijk, D. The (un)importance of cerebral microemboli. Semin. Cardiothorac. Vasc. Anesth. 2010, 14, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Behrends, M.; DePalma, G.; Sands, L.; Leung, J. Intraoperative Blood Transfusions are Associated with Early Postoperative Delirium in Older Patients. J. Am. Geriatr. Soc. 2013, 61, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Kunz, J.V.; Spies, C.D.; Bichmann, A.; Sieg, M.; Mueller, A. Postoperative anaemia might be a risk factor for postoperative delirium and prolonged hospital stay: A secondary analysis of a prospective cohort study. PLoS ONE 2020, 15, e0229325. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, E.L.; Behrends, M. Blood Transfusion and Postoperative Delirium. Curr. Anesthesiol. Rep. 2015, 5, 24–32. [Google Scholar] [CrossRef]
- Habeeb-Allah, A.; Alshraideh, J.A. Delirium post-cardiac surgery: Incidence and associated factors. Nurs. Crit. Care 2021, 26, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Maniar, H.S.; Lindman, B.R.; Escallier, K.; Avidan, M.; Novak, E.; Melby, S.J.; Damiano, M.S.; Lasala, J.; Quader, N.; Rao, R.S.; et al. Delirium after surgical and transcatheter aortic valve replacement is associated with increased mortality. J. Thorac. Cardiovasc. Surg. 2016, 151, 815–823.e2. [Google Scholar] [CrossRef] [PubMed]
- Salluh, J.I.F.; Wang, H.; Schneider, E.B.; Nagaraja, N.; Yenokyan, G.; Damluji, A.; Serafim, R.B.; Stevens, R.D. Outcome of delirium in critically ill patients: Systematic review and meta-analysis. BMJ 2015, 350, h2538. [Google Scholar] [CrossRef]
- Hasin, Y.; Avidan, N.; Bercovich, D.; Korczyn, A.D.; Silman, I.; Beckmann, J.S.; Sussman, J.L. Analysis of genetic polymorphisms in acetylcholinesterase as reflected in different populations. Curr. Alzheimer Res. 2005, 2, 207–218. [Google Scholar] [CrossRef]
- Carnahan, R.M.; Lund, B.C.; Perry, P.J.; Culp, K.R.; Pollock, B.G. The relationship of an anticholinergic rating scale with serum anticholinergic activity in elderly nursing home residents. Psychopharmacol. Bull. 2002, 36, 14–19. [Google Scholar]
- Rump, A.F.E.; Schierholz, J.; Biederbick, W.; Theisohn, M.; Diefenbach, C.; Abel, M.; Börner, U.; Buzello, W.; Klaus, W. Pseudocholinesterase-Activity Reduction during Cardiopulmonary Bypass. Gen. Pharmacol. Vasc. Syst. 1999, 32, 65–69. [Google Scholar] [CrossRef]
- Salahudeen, M.S.; Duffull, S.B.; Nishtala, P.S. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: A systematic review. BMC Geriatr. 2015, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Carnahan, R.M.; Lund, B.C.; Perry, P.J.; Pollock, B.G.; Culp, K.R. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: Associations with serum anticholinergic activity. J. Clin. Pharmacol. 2006, 46, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, M.M.J.; Roes, K.C.B.; Honing, M.L.H.; Kuiper, M.A.; Karakus, A.; van der Jagt, M.; Spronk, P.E.; van Gool, W.A.; van der Mast, R.C.; Kesecioglu, J.; et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: A multicentre, double-blind, place-bo-controlled randomised trial. Lancet 2010, 376, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Burry, L.; Hutton, B.; Williamson, D.R.; Mehta, S.; Adhikari, N.K.; Cheng, W.; Ely, E.W.; Egerod, I.; Fergusson, D.A.; Rose, L. Pharmacological interventions for the treatment of delirium in critically ill adults. Cochrane Database Syst. Rev. 2019, 9, CD011749. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.K.; Bogardus, S.T.; Baker, D.I.; Leo-Summers, L.; Cooney, L.M. The Hospital Elder Life Program: A model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J. Am. Geriatr. Soc. 2000, 48, 1697–1706. [Google Scholar] [CrossRef]
- Hshieh, T.T.; Yang, T.; Gartaganis, S.L.; Yue, J.; Inouye, S.K. Hospital Elder Life Program: Systematic Review and Meta-analysis of Effectiveness. Am. J. Geriatr. Psychiatry 2018, 26, 1015–1033. [Google Scholar] [CrossRef]
| Characteristic | POD+ (n = 20) | POD− (n = 73) | p-Value |
|---|---|---|---|
| Age (years) | 71.5 (63.8–76.3) | 64 (56–71) | 0.003 |
| Female sex | 3 (15.0) | 9 (12.3) | 0.717 |
| Body mass index (kg/m²) | 28.9 (25.7–32.4) | 29.4 (27–32.1) | 0.581 |
| Atrial fibrillation | 3 (15.0) | 19 (26.0) | 0.385 |
| COPD | 5 (25.0) | 6 (8.2) | 0.054 |
| DM II | 13 (65.0) | 22 (30.1) | 0.008 |
| EuroSCORE | 1.4 (1.2–1.8) | 0.9 (0.7–1.2) | <0.001 |
| ASA–PSCS | |||
| II | 0 | 7 (9.6) | - |
| III | 19 (95.0) | 57 (78.1) | - |
| IV | 1 (5.0) | 9 (12.3) | - |
| Characteristic | POD+ | POD− | p-Values |
|---|---|---|---|
| Preoperative | |||
| ALT (U/L) | 21 (17.0–32.5) | 25.5 (17.75–43.0) | 0.289 |
| GGT (U/L) | 26.5 (19.0–39.5) | 34.0 (25.0–48.25) | 0.161 |
| HBA1c (%) | 6.5 (6.0–7.0) | 5.8 (5.5–6.4) | 0.003 |
| Glucose (mg/dL) | 164 (154.0–199.0) | 102.5 (92.3–126.5) | 0.039 |
| Intraoperative | |||
| Hb (g/dL) | 10.0 (9.3–11.2) | 11.1 (10.0 –12.2) | 0.016 |
| Thrombocytes (×106/L) | 157.5 (106.0–167.0) | 169 (140.25 –205.5) | 0.042 |
| GGT (U/L) | 13 (10.8–21.3) | 12 (16.5–34.0) | 0.018 |
| Postoperative | |||
| Hb (g/dL) | 97.5 (89.5–104.5) | 109 (97.0–117.0) | 0.002 |
| Erythrocytes (×106/µL) | 3.3 (2.9–3.5) | 3.6 (3.2–3.9) | 0.009 |
| Hematocrit (%) | 0.30 (0.26–0.32) | 0.32 (0.28–0.34) | 0.016 |
| MCHC (g/dL) | 336.5 (328.8–339.3) | 341 (334.0–347.0) | 0.002 |
| Thrombocytes (×106/L) | 173.5 (121.8–196.0) | 187.0 (160.0–220.0) | 0.015 |
| GGT (U/L) | 20 (12.0–28.0) | 24.0 (20.5–33.5) | 0.076 |
| ALP (U/L) | 41 (35.0–44.0) | 54.5 (43.5–63.8) | 0.009 |
| Characteristic | POD+ (n = 20) | POD− (n = 73) | p-Values |
|---|---|---|---|
| Surgical and anesthetic characteristics | |||
| CPB-time (min) | 107.0 (93.3–117.0) | 77.0 (66.8–90.0) | <0.001 |
| Cross-clamp time (min) | 71.0 (55.5–80.3) | 53.0 (45.0–67.0) | 0.011 |
| Anesthesia duration (min) | 298.5 (261.0–327.0) | 262.0 (245.0–303.0) | 0.024 |
| Surgery duration (min) | 223.0 (195.8–238.9) | 192.5 (164.0–225.0) | 0.046 |
| RBC transfusion during observation period (mL) | 450.0 (0.0–600.0) | 0.0 (0.0–0.0) | <0.001 |
| Ventilation duration (h) | 20.8 (17.0–24.2) | 12.3 (9.4–16.6) | <0.001 |
| LOS-ICU (d) | 2.9 (5.2–7.2) | 1.1 (0.9–2.0) | <0.001 |
| LOS-Hospital (d) | 11.0 (9.3–13.8) | 10.0 (8.0–12.0) | 0.125 |
| In-hospital mortality | 0 | 0 | - |
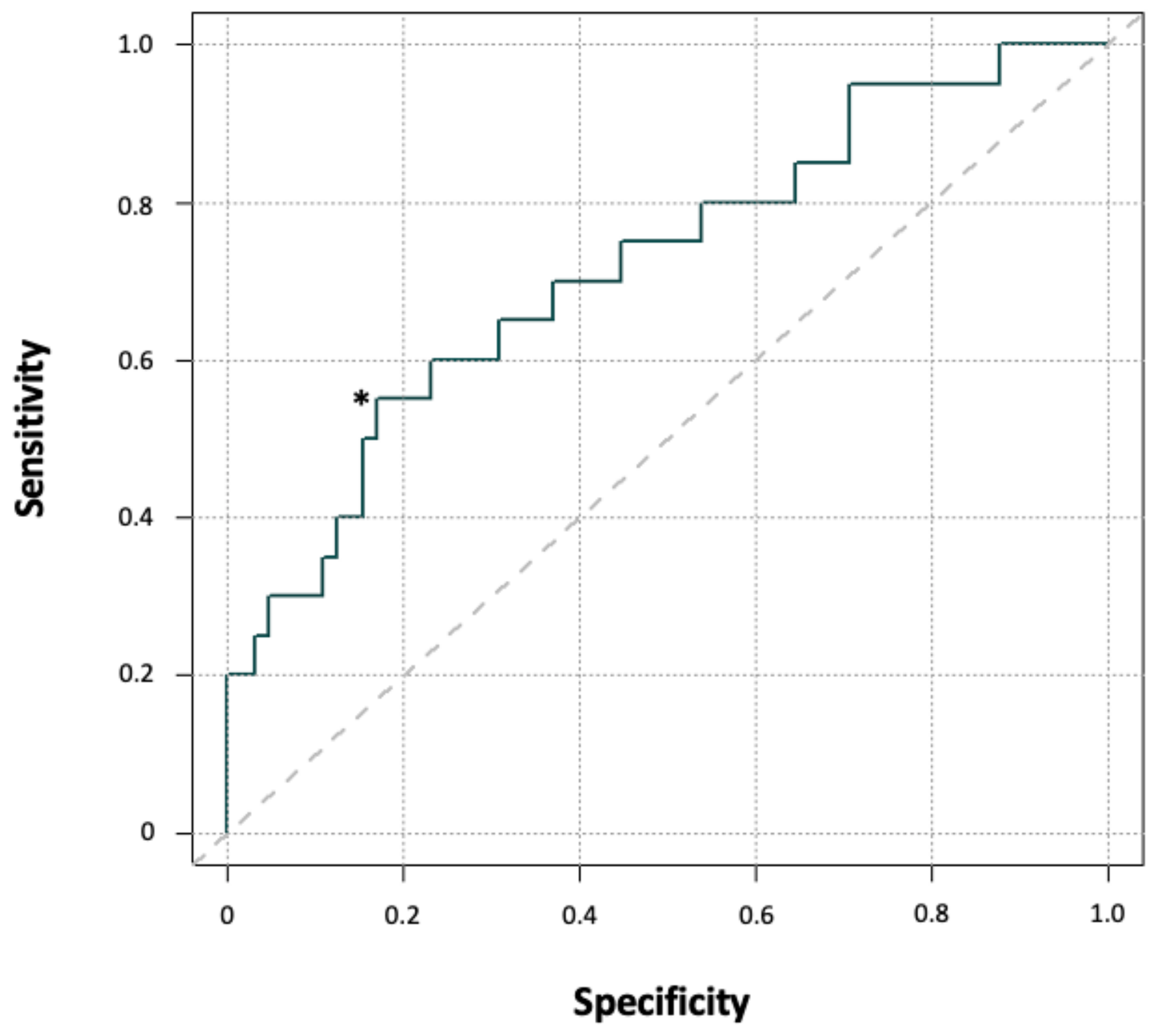
| Timepoint | Drop to Baseline (%) | Odd´s Ratio | p-Values |
|---|---|---|---|
| Day 1 | 20 | 3.89 (0.81–37.62) | 0.08 |
| Day 1 | 25 | 3.62 (1.10–14.22 | 0.02 |
| Day 1 | 30 | 3.88 (1.24–12.86) | 0.01 |
| Day 1 | 35 | 4.66 (1.18–18.77) | 0.01 |
| Day 1 | 40 | 16.27 (1.48–844.38) | 0.01 |
| Day 1 | 45 | N.A. | 0.01 |
| Day 2 | 20 | N.A. | 0.57 |
| Day 2 | 25 | N.A. | 0.03 |
| Day 2 | 30 | 2.52 (0.63–14.80) | 0.26 |
| Day 2 | 35 | 1.90 (0.62–6.32) | 0.31 |
| Day 2 | 40 | 5.47 (1.71–18.63) | 0.002 |
| Day 2 | 45 | 5.51 (1.22–26.45) | 0.01 |
| Day 3 | 20 | N.A. | 1.0 |
| Day 3 | 25 | N.A. | 0.33 |
| Day 3 | 30 | 2.78 (0.34–129.17) | 0.45 |
| Day 3 | 35 | 5.90 (1.25–13.11) | 0.01 |
| Day 3 | 40 | 3.66 (1.15–13.11) | 0.02 |
| Day 3 | 45 | 3.58 (0.95–13.28) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajonz, T.S.; Kunzemann, C.; Schreiner, A.L.; Beckert, F.; Schneck, E.; Boening, A.; Markmann, M.; Sander, M.; Koch, C. Potentials of Acetylcholinesterase and Butyrylcholinesterase Alterations in On-Pump Coronary Artery Bypass Surgery in Postoperative Delirium: An Observational Trial. J. Clin. Med. 2023, 12, 5245. https://doi.org/10.3390/jcm12165245
Zajonz TS, Kunzemann C, Schreiner AL, Beckert F, Schneck E, Boening A, Markmann M, Sander M, Koch C. Potentials of Acetylcholinesterase and Butyrylcholinesterase Alterations in On-Pump Coronary Artery Bypass Surgery in Postoperative Delirium: An Observational Trial. Journal of Clinical Medicine. 2023; 12(16):5245. https://doi.org/10.3390/jcm12165245
Chicago/Turabian StyleZajonz, Thomas S., Christian Kunzemann, Anna Lena Schreiner, Frauke Beckert, Emmanuel Schneck, Andreas Boening, Melanie Markmann, Michael Sander, and Christian Koch. 2023. "Potentials of Acetylcholinesterase and Butyrylcholinesterase Alterations in On-Pump Coronary Artery Bypass Surgery in Postoperative Delirium: An Observational Trial" Journal of Clinical Medicine 12, no. 16: 5245. https://doi.org/10.3390/jcm12165245
APA StyleZajonz, T. S., Kunzemann, C., Schreiner, A. L., Beckert, F., Schneck, E., Boening, A., Markmann, M., Sander, M., & Koch, C. (2023). Potentials of Acetylcholinesterase and Butyrylcholinesterase Alterations in On-Pump Coronary Artery Bypass Surgery in Postoperative Delirium: An Observational Trial. Journal of Clinical Medicine, 12(16), 5245. https://doi.org/10.3390/jcm12165245









