Change in Androgenic Status and Cardiometabolic Profile of Middle-Aged Women with Polycystic Ovary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Measurements
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics at Initial Screening
3.2. Cardiometabolic Parameters at Follow-Up Screening
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef]
- Wild, R.A.; Rizzo, M.; Clifton, S.; Carmina, E. Lipid levels in polycystic ovary syndrome: Systematic review and meta-analysis. Fertil. Steril. 2011, 95, 1073–1079.e11. [Google Scholar] [CrossRef]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin resistance in polycystic ovary syndrome: A systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum. Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Ramezani Tehrani, F.; Behboudi-Gandevani, S.; Bidhendi-Yarandi, R.; Carmina, E. Risk of hypertension in women with polycystic ovary syndrome: A systematic review, meta-analysis and meta-regression. Reprod. Biol. Endocrinol. 2020, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Kakoly, N.S.; Tan, J.W.J.; Fitzgerald, G.; Bahri Khomami, M.; Joham, A.E.; Cooray, S.D.; Misso, M.L.; Norman, R.J.; Harrison, C.L.; et al. Metabolic syndrome in polycystic ovary syndrome: A systematic review, meta-analysis and meta-regression. Obes. Rev. 2019, 20, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.L.; Malek, A.M.; Wild, R.A.; Korytkowski, M.T.; Talbott, E.O. Carotid artery intima-media thickness in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 112–126. [Google Scholar] [CrossRef]
- Christian, R.C.; Dumesic, D.A.; Behrenbeck, T.; Oberg, A.L.; Sheedy, P.F., 2nd; Fitzpatrick, L.A. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 2562–2568. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International, P.N. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef]
- Carmina, E.; Orio, F.; Palomba, S.; Longo, R.A.; Cascella, T.; Colao, A.; Lombardi, G.; Rini, G.B.; Lobo, R.A. Endothelial dysfunction in PCOS: Role of obesity and adipose hormones. Am. J. Med. 2006, 119, 356.e1–356.e6. [Google Scholar] [CrossRef]
- Shroff, R.; Kerchner, A.; Maifeld, M.; Van Beek, E.J.; Jagasia, D.; Dokras, A. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J. Clin. Endocrinol. Metab. 2007, 92, 4609–4614. [Google Scholar] [CrossRef]
- Ramezani Tehrani, F.; Amiri, M.; Behboudi-Gandevani, S.; Bidhendi-Yarandi, R.; Carmina, E. Cardiovascular events among reproductive and menopausal age women with polycystic ovary syndrome: A systematic review and meta-analysis. Gynecol. Endocrinol. 2020, 36, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Landin-Wilhelmsen, K.; Brännström, M.; Dahlgren, E. Cardiovascular disease and risk factors in PCOS women of postmenopausal age: A 21-year controlled follow-up study. J. Clin. Endocrinol. Metab. 2011, 96, 3794–3803. [Google Scholar] [CrossRef] [PubMed]
- Merz, C.N.; Shaw, L.J.; Azziz, R.; Stanczyk, F.Z.; Sopko, G.; Braunstein, G.D.; Kelsey, S.F.; Kip, K.E.; Cooper-DeHoff, R.M.; Johnson, B.D.; et al. Cardiovascular Disease and 10-Year Mortality in Postmenopausal Women with Clinical Features of Polycystic Ovary Syndrome. J. Women’s Health 2016, 25, 875–881. [Google Scholar] [CrossRef]
- Brown, Z.A.; Louwers, Y.V.; Fong, S.L.; Valkenburg, O.; Birnie, E.; de Jong, F.H.; Fauser, B.C.; Laven, J.S. The phenotype of polycystic ovary syndrome ameliorates with aging. Fertil. Steril. 2011, 96, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Carmina, E.; Campagna, A.M.; Lobo, R.A. A 20-year follow-up of young women with polycystic ovary syndrome. Obstet. Gynecol. 2012, 119, 263–269. [Google Scholar] [CrossRef]
- Helvaci, N.; Yildiz, B.O. Polycystic ovary syndrome and aging: Health implications after menopause. Maturitas 2020, 139, 12–19. [Google Scholar] [CrossRef]
- Honigberg, M.C.; Zekavat, S.M.; Aragam, K.; Finneran, P.; Klarin, D.; Bhatt, D.L.; Januzzi, J.L., Jr.; Scott, N.S.; Natarajan, P. Association of Premature Natural and Surgical Menopause with Incident Cardiovascular Disease. JAMA 2019, 322, 2411–2421. [Google Scholar] [CrossRef]
- Harvey, R.E.; Coffman, K.E.; Miller, V.M. Women-specific factors to consider in risk, diagnosis and treatment of cardiovascular disease. Women’s Health 2015, 11, 239–257. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar]
- Pinola, P.; Puukka, K.; Piltonen, T.T.; Puurunen, J.; Vanky, E.; Sundström-Poromaa, I.; Stener-Victorin, E.; Lindén Hirschberg, A.; Ravn, P.; Skovsager Andersen, M.; et al. Normo- and hyperandrogenic women with polycystic ovary syndrome exhibit an adverse metabolic profile through life. Fertil. Steril. 2017, 107, 788–795.e782. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Panidis, D. Unravelling the phenotypic map of polycystic ovary syndrome (PCOS): A prospective study of 634 women with PCOS. Clin. Endocrinol. 2007, 67, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Zoet, G.A.; Meun, C.; Benschop, L.; Boersma, E.; Budde, R.P.J.; Fauser, B.; de Groot, C.J.M.; van der Lugt, A.; Maas, A.; Moons, K.G.M.; et al. Cardiovascular RiskprofilE-IMaging and gender-specific disOrders (CREw-IMAGO): Rationale and design of a multicenter cohort study. BMC Women’s Health 2017, 17, 60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daan, N.M.; Louwers, Y.V.; Koster, M.P.; Eijkemans, M.J.; de Rijke, Y.B.; Lentjes, E.W.; Fauser, B.C.; Laven, J.S. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: Who is really at risk? Fertil. Steril. 2014, 102, 1444–1451.e3. [Google Scholar] [CrossRef]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Zhao, X.; Ni, R.; Li, L.; Mo, Y.; Huang, J.; Huang, M.; Azziz, R.; Yang, D. Defining hirsutism in Chinese women: A cross-sectional study. Fertil. Steril. 2011, 96, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Piercy, K.L.; Troiano, R.P. Physical Activity Guidelines for Americans From the US Department of Health and Human Services. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e005263. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Polotsky, A.J.; Allshouse, A.; Crawford, S.L.; Harlow, S.D.; Khalil, N.; Santoro, N.; Legro, R.S. Relative contributions of oligomenorrhea and hyperandrogenemia to the risk of metabolic syndrome in midlife women. J. Clin. Endocrinol. Metab. 2012, 97, E868–E877. [Google Scholar] [CrossRef]
- Dapas, M.; Lin, F.T.J.; Nadkarni, G.N.; Sisk, R.; Legro, R.S.; Urbanek, M.; Hayes, M.G.; Dunaif, A. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. 2020, 17, e1003132. [Google Scholar] [CrossRef]
- Ehrmann, D.A.; Liljenquist, D.R.; Kasza, K.; Azziz, R.; Legro, R.S.; Ghazzi, M.N.; Group, P.C.T.S. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 48–53. [Google Scholar] [CrossRef]
- Dunaif, A.; Graf, M.; Mandeli, J.; Laumas, V.; Dobrjansky, A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J. Clin. Endocrinol. Metab. 1987, 65, 499–507. [Google Scholar] [CrossRef]
- Panidis, D.; Tziomalos, K.; Macut, D.; Delkos, D.; Betsas, G.; Misichronis, G.; Katsikis, I. Cross-sectional analysis of the effects of age on the hormonal, metabolic, and ultrasonographic features and the prevalence of the different phenotypes of polycystic ovary syndrome. Fertil. Steril. 2012, 97, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Kiconco, S.; Tay, C.T.; Rassie, K.L.; Azziz, R.; Teede, H.J.; Joham, A.E. Where are we in understanding the natural history of polycystic ovary syndrome? A systematic review of longitudinal cohort studies. Hum. Reprod. 2022, 37, 1255–1273. [Google Scholar] [CrossRef]
- Forslund, M.; Schmidt, J.; Brännström, M.; Landin-Wilhelmsen, K.; Dahlgren, E. Morbidity and mortality in PCOS: A prospective follow-up up to a mean age above 80 years. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 271, 195–203. [Google Scholar] [CrossRef]
- Mishra, S.R.; Chung, H.F.; Waller, M.; Mishra, G.D. Duration of estrogen exposure during reproductive years, age at menarche and age at menopause, and risk of cardiovascular disease events, all-cause and cardiovascular mortality: A systematic review and meta-analysis. BJOG 2021, 128, 809–821. [Google Scholar] [CrossRef]
- Mishra, S.R.; Waller, M.; Chung, H.F.; Mishra, G.D. Epidemiological studies of the association between reproductive lifespan characteristics and risk of Type 2 diabetes and hypertension: A systematic review. Maturitas 2022, 155, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Dapas, M.; Dunaif, A. Deconstructing a Syndrome: Genomic Insights into PCOS Causal Mechanisms and Classification. Endocr. Rev. 2022, 43, 927–965. [Google Scholar] [CrossRef]
- Cena, H.; Chiovato, L.; Nappi, R.E. Obesity, Polycystic Ovary Syndrome, and Infertility: A New Avenue for GLP-1 Receptor Agonists. J. Clin. Endocrinol. Metab. 2020, 105, e2695–e2709. [Google Scholar] [CrossRef] [PubMed]
- Nylander, M.; Frøssing, S.; Clausen, H.V.; Kistorp, C.; Faber, J.; Skouby, S.O. Effects of liraglutide on ovarian dysfunction in polycystic ovary syndrome: A randomized clinical trial. Reprod. Biomed. Online 2017, 35, 121–127. [Google Scholar] [CrossRef] [PubMed]
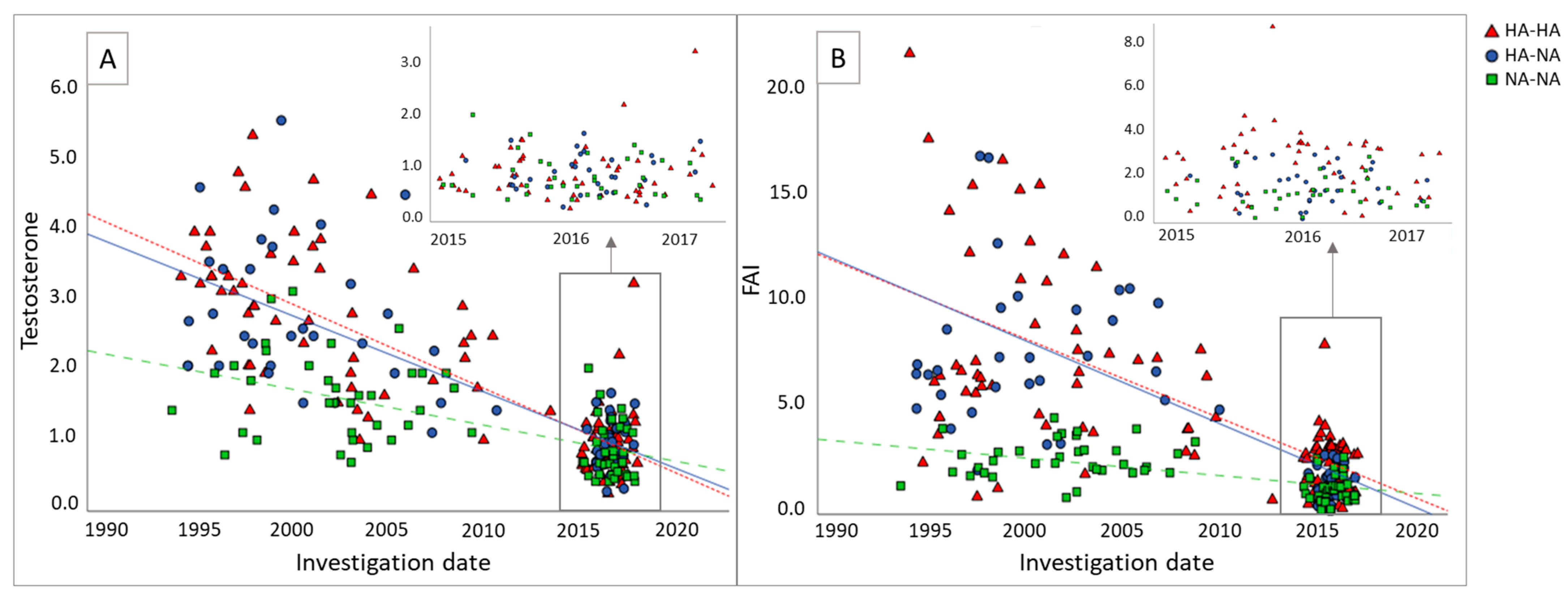
| HA-HA (n = 53) | HA-NA (n = 32) | NA-NA (n = 35) | p-Value | |
|---|---|---|---|---|
| Age (years) | 32.3 (27.4–37.1) | 30.6 (27.9–35.0) | 33.3 (30.7–36.8) | 0.14 |
| NE ethnicity | 42 (79.2) | 30 (93.8) | 30 (85.7) | 0.19 |
| Age at menarche (years) | 13.0 (12.0–15.0) | 13.0 (12.0–15.0) | 13.0 (12.3–15.0) | 0.72 |
| Amenorrhoea | 18 (34.0) | 4 (12.9) | 7 (20.0) | 0.18 |
| Oligomenorrhea | 34.0 (64.2) | 26 (83.9) | 28 (80.0) | |
| Regular cycle | 1 (1.9) | 1 (3.2) | 0 (0.0) | |
| BMI (kg/m2) | 27.5 (23.1–32.3) | 30.2 (24.0–33.9) | 22.4 (20.6–25.5) | <0.01 1,2 |
| Waist (cm) * | 94.0 (79.8–102.8) | 96.0 (82.3–110.3) | 75.0 (71.0–86.0) | <0.01 1,2 |
| Hip (cm) * | 107.5 (98.0–114.5) | 111.5 (100.8–121.0) | 100.0 (89.0–109.0) | <0.01 2 |
| WHR | 0.9 (0.8–0.9) | 0.8 (0.8–1.0) | 0.8 (0.7–0.8) | <0.05 1,2 |
| Testosterone (nmol/L) | 2.79 (1.95–3.47) | 2.48 (2.06–3.50) | 1.64 (1.11–1.95) | <0.01 1,2 |
| FAI | 6.7 (4.2–11.3) | 6.4 (5.6–10.1) | 2.5 (2.0–3.1) | <0.01 1,2 |
| HA-HA (n = 53) | HA-NA (n = 32) | NA-NA (n = 35) | p-Value | Adjusted p-Value | |
|---|---|---|---|---|---|
| Age (years) | 46.9 (45.5–49.2) | 47.4 (46.9–49.7) | 47.6 (46.6–50.6) | 0.20 | 0.51 |
| BMI (kg/m2) | 30.1 (25.7–35.6) | 30.1 (26.1–34.2) | 24.1 (22.1–28.7) | <0.01 2,3 | - |
| Delta BMI (kg/m2) | 2.4 (0.7–5.5) | 2.1 (−2.6–5.0) | 2.0 (0.3–3.3) | 0.80 | - |
| Waist (cm) * | 98.0 (88.5–109.0) | 96.0 (84.0–107.0) | 89.0 (80.3–96.3) | <0.05 2,3 | 0.38 |
| Hip (cm) * | 101.0 (101.0–117.0) | 112.0 (103.0–117.0) | 104.5 (98.8–111.3) | 0.18 | 0.45 |
| Waist/Hip ratio * | 0.9 (0.9–1.0) | 0.9 (0.8–0.9) | 0.8 (0.8–0.9) | <0.01 2 | 0.25 |
| Physical activity (hours per week) * | 22.8 (13.9–30.0) | 20.5 (16.3–43.8) | 21.8 (13.3–29.3) | 0.79 | 0.82 |
| Systolic BP (mm Hg) | 130.0 (120.0–140.0) | 127.5 (120.0–140.0) | 120.0 (110.0–130.0) | <0.01 2,3 | 0.19 |
| Diastolic BP (mm Hg) | 85.0 (80.0–95.0) | 80.0 (75.0–90.0) | 80.0 (70.0–85.0) | <0.05 2 | 0.16 |
| Hypertension | 28 (53.8) | 17 (53.1) | 5 (14.3) | <0.01 2,3 | <0.05 2,3 |
| Antihypertensive medication | 12 (23.5) | 5 (15.6) | 1 (2.9) | <0.05 2 | 0.07 |
| Prevalent CVD | 1 (1.9) | 1 (3.1) | 1 (2.9) | 0.93 | 0.67 |
| Lipid lowering medication | 4 (7.5) | 3 (9.4) | 0 (0.0) | 0.20 | 0.30 |
| Total cholesterol (mmol/L) * | 5.2 (4.3–5.9) | 5.40 (4.2–6.2) | 5.2 (4.6–5.8) | 0.76 | 0.64 |
| HDL cholesterol (mmol/L) * | 1.4 (1.1–1.6) | 1.5 (1.2–1.7) | 1.82 (1.52–2.3) | <0.01 2,3 | <0.01 2 |
| LDL cholesterol (mmol/L) * | 3.2 (2.6–4.1) | 3.7 (2.5–4.2) | 2.99 (2.7–3.6) | 0.60 | 0.729 |
| Triglycerides (mmol/L) * | 1.0 (0.8–1.9) | 1.1 (0.9–1.5) | 0.85 (0.6–1.1) | <0.01 2,3 | 0.18 |
| Dyslipidemia * | 31 (60.8) | 21 (65.6) | 13 (38.2) | 0.05 | 0.16 |
| Androstenedione (nmol/L) | 2.9 (2.1–4.2) | 2.5 (2.0–3.0) | 2.6 (1.8–3.6) | 0.13 | 0.02 2 |
| Testosterone (nmol/L) | 1.1 (0.7–1.3) | 0.7 (0.5–1.0) | 0.7 (0.6–1.0) | <0.01 1,2 | <0.01 1,2 |
| FAI | 2.7 (1.4–3.2) | 1.8 (0.9–2.3) | 1.2 (0.8–1.5) | <0.01 1,2 | <0.01 1,2 |
| NT-pro-BNP (>15 pmol/L) | 5.0 (3.0–9.0) | 6.0 (3.0–12.0) | 6.0 (4.0–8.5) | 0.81 | 0.72 |
| Insulin (pmol/L) | 92.0 (52.0–161.0) | 95.0 (46.0–146.0) | 66.0 (45.0–95.0) | <0.05 2 | 0.34 |
| Glucose (mmol/L) | 5.5 (5.1–5.9) | 5.1 (4.8–5.6) | 5.30 (4.9–5.5) | 0.13 | 0.47 |
| Diabetes | 7 (13.2) | 2 (6.3) | 1 (2.9) | 0.20 | 0.24 |
| Metabolic syndrome (NCEP definition) * | 16 (37.2) | 10 (35.7) | 3 (8.6) | <0.05 2,3 | 0.12 |
| HA-HA | HA-NA | NA-NA | p-Value | Adjusted p-Value | |
|---|---|---|---|---|---|
| Mean CIMT (um) | n = 47 0.6 (0.6–0.7) | n = 28 0.6 (0.6–0.7) | n = 33 0.6 (0.6–0.7) | 0.93 | 0.82 |
| CACs | n = 32 | n = 18 | n = 18 | 0.32 | 0.19 |
| No CAC (0 AU) | 25 (78.1) | 15 (83.3) | 17 (94.4) | ||
| Any CAC (0–400 AU) | 7 (21.9) | 3 (16.7) | 1 (5.6) | ||
| Presence of coronary plaque | n = 31 5 (15.6) | 0 (0.0) | n = 18 3 (16.7) | 0.23 | 0.94 |
| Relevant CVD | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Ham, K.; Koster, M.P.H.; Velthuis, B.K.; Budde, R.P.J.; Fauser, B.C.J.M.; Laven, J.S.E.; Louwers, Y.V. Change in Androgenic Status and Cardiometabolic Profile of Middle-Aged Women with Polycystic Ovary Syndrome. J. Clin. Med. 2023, 12, 5226. https://doi.org/10.3390/jcm12165226
van der Ham K, Koster MPH, Velthuis BK, Budde RPJ, Fauser BCJM, Laven JSE, Louwers YV. Change in Androgenic Status and Cardiometabolic Profile of Middle-Aged Women with Polycystic Ovary Syndrome. Journal of Clinical Medicine. 2023; 12(16):5226. https://doi.org/10.3390/jcm12165226
Chicago/Turabian Stylevan der Ham, Kim, Maria P. H. Koster, Birgitta K. Velthuis, Ricardo P. J. Budde, Bart C. J. M. Fauser, Joop S. E. Laven, and Yvonne V. Louwers. 2023. "Change in Androgenic Status and Cardiometabolic Profile of Middle-Aged Women with Polycystic Ovary Syndrome" Journal of Clinical Medicine 12, no. 16: 5226. https://doi.org/10.3390/jcm12165226
APA Stylevan der Ham, K., Koster, M. P. H., Velthuis, B. K., Budde, R. P. J., Fauser, B. C. J. M., Laven, J. S. E., & Louwers, Y. V. (2023). Change in Androgenic Status and Cardiometabolic Profile of Middle-Aged Women with Polycystic Ovary Syndrome. Journal of Clinical Medicine, 12(16), 5226. https://doi.org/10.3390/jcm12165226






