Ankle Strategies for Step-Aside Movement during Straight Walking
Abstract
1. Introduction
- The PL would exhibit the greatest contraction during the left push phase of the step-aside movement, and all three ankle muscles would show increased contractions in the right loading phase compared to straight walking.
- The TA would have the most significant contraction to achieve left-toe clearance.
- 3.
- Mediolateral foot CoP (CoPx) displacement would increase medially during the left push phase of the step-aside movement. However, anteroposterior foot CoP (CoPy) displacement would be the same as in straight walking during both the left push and right loading phases [14].
- 4.
- The left forefoot GRF (F-GRF) and right heel GRF (H-GRF) would increase stepwise with rightward movement.
2. Methods
2.1. Participants
2.2. Experimental Procedure
2.3. Data Collection
2.3.1. Electromyography
2.3.2. Force and Center of Pressure
2.4. Statistical Analysis
2.4.1. Region of Interest
2.4.2. Multiple Linear Regression
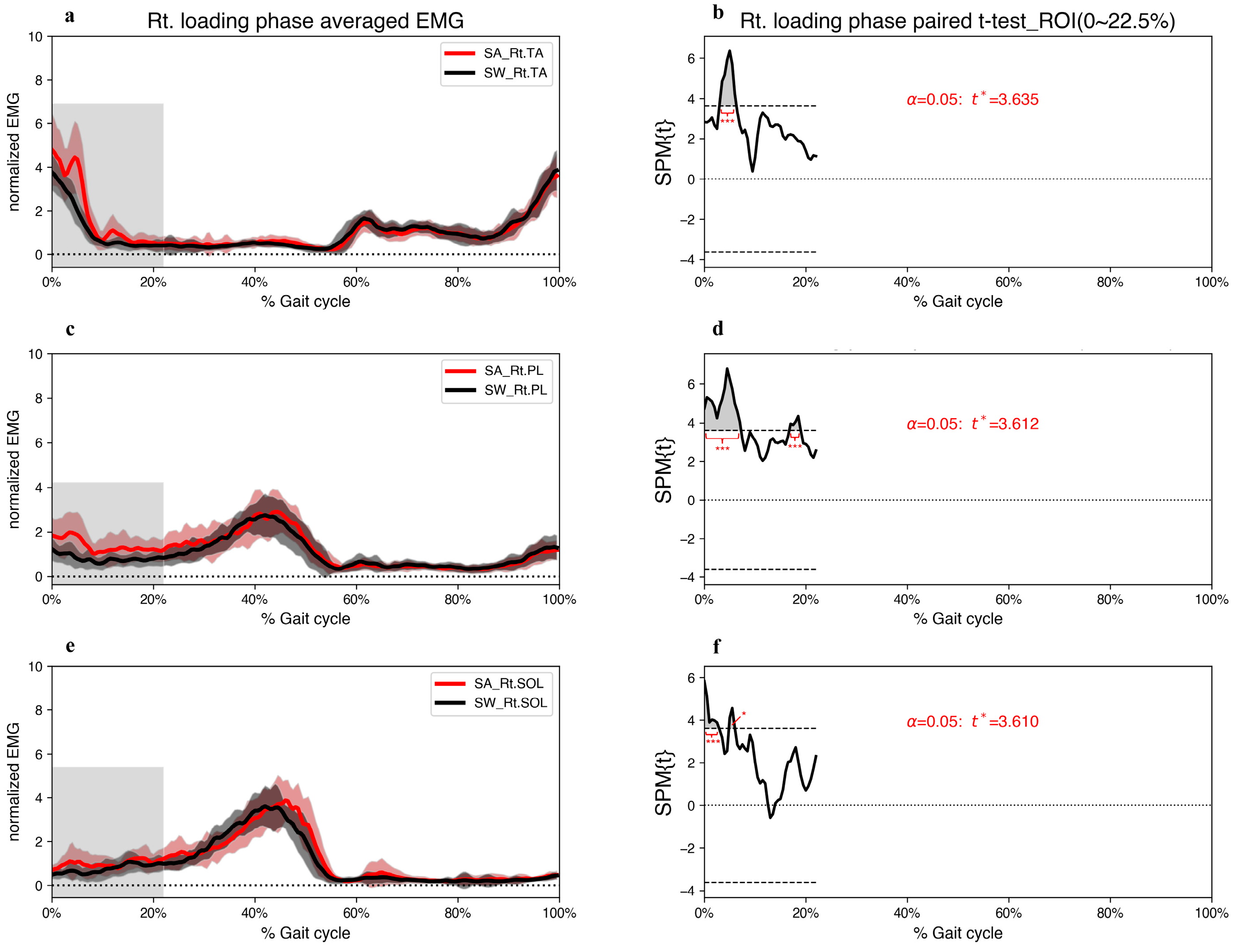
3. Results
Regression
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, L.; Cho, S. Ankle strategies for step-aside movement during quiet standing. PLoS ONE 2023, 18, e0281400. [Google Scholar] [CrossRef]
- Courtine, G.; Schieppati, M. Human walking along a curved path. II. Gait features and EMG patterns. Eur. J. Neurosci. 2003, 18, 191–205. [Google Scholar] [CrossRef]
- Haefeli, J.; Vögeli, S.; Michel, J.; Dietz, V. Preparation and performance of obstacle steps: Interaction between brain and spinal neuronal activity. Eur. J. Neurosci. 2011, 33, 338–348. [Google Scholar] [CrossRef]
- Grin, L.; Frank, J.; Allum, J.H. The effect of voluntary arm abduction on balance recovery following multidirectional stance perturbations. Exp. Brain Res. 2007, 178, 62–78. [Google Scholar] [CrossRef][Green Version]
- Michel, J.; Van Hedel, H.; Dietz, V. Obstacle stepping involves spinal anticipatory activity associated with quadrupedal limb coordination. Eur. J. Neurosci. 2008, 27, 1867–1875. [Google Scholar] [CrossRef]
- Hof, A.; Duysens, J. Responses of human ankle muscles to mediolateral balance perturbations during walking. Hum. Mov. Sci. 2018, 57, 69–82. [Google Scholar] [CrossRef]
- Hof, A.L.; van Bockel, R.M.; Schoppen, T.; Postema, K. Control of lateral balance in walking: Experimental findings in normal subjects and above-knee amputees. Gait Posture 2007, 25, 250–258. [Google Scholar] [CrossRef]
- Van Leeuwen, A.; Van Dieën, J.; Daffertshofer, A.; Bruijn, S. Ankle muscles drive mediolateral center of pressure control to ensure stable steady state gait. Sci. Rep. 2021, 11, 21481. [Google Scholar] [CrossRef]
- Hase, K.; Stein, R. Turning strategies during human walking. J. Neurophysiol. 1999, 81, 2914–2922. [Google Scholar] [CrossRef]
- Wikstrom, E.A.; Hass, C.J. Gait termination strategies differ between those with and without ankle instability. Clin. Biomech. 2012, 27, 619–624. [Google Scholar] [CrossRef]
- Hoogkamer, W.; Potocanac, Z.; Duysens, J. Quick foot placement adjustments during gait: Direction matters. Exp. Brain Res. 2015, 233, 3349–3357. [Google Scholar] [CrossRef]
- Kim, K.-J.; Uchiyama, E.; Kitaoka, H.B.; An, K.-N. An in vitro study of individual ankle muscle actions on the center of pressure. Gait Posture 2003, 17, 125–131. [Google Scholar] [CrossRef]
- Novacheck, T.F. The biomechanics of running. Gait Posture 1998, 7, 77–95. [Google Scholar] [CrossRef]
- Rose, J.; Gamble, J.G. Human Walking; Williams & Wilkins Baltimore: Baltimore, MD, USA, 1994; Volume 3. [Google Scholar]
- Tretriluxana, J.; Nanbancha, A.; Sinsurin, K.; Limroongreungrat, W.; Wang, H.-K. Neuromuscular control of the ankle during pre-landing in athletes with chronic ankle instability: Insights from statistical parametric mapping and muscle co-contraction analysis. Phys. Ther. Sport 2021, 47, 46–52. [Google Scholar] [CrossRef]
- Gibbons, C.T.; Amazeen, P.G.; Likens, A.D. Effects of foot placement on postural sway in the anteroposterior and mediolateral directions. Mot. Control. 2019, 23, 149–170. [Google Scholar] [CrossRef]
- Insperger, T.; Milton, J. Delay and Uncertainty in Human Balancing Tasks; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Franklin, D.W.; Osu, R.; Burdet, E.; Kawato, M.; Milner, T.E. Adaptation to stable and unstable dynamics achieved by combined impedance control and inverse dynamics model. J. Neurophysiol. 2003, 90, 3270–3282. [Google Scholar] [CrossRef]
- Heald, J.B.; Franklin, D.W.; Wolpert, D.M. Increasing muscle co-contraction speeds up internal model acquisition during dynamic motor learning. Sci. Rep. 2018, 8, 16355. [Google Scholar] [CrossRef]
- Babadi, S.; Vahdat, S.; Milner, T.E. Neural Substrates of Muscle Co-contraction during Dynamic Motor Adaptation. J. Neurosci. 2021, 41, 5667–5676. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European recommendations for surface electromyography. Roessingh Res. Dev. 1999, 8, 13–54. [Google Scholar]
- Rankin, B.L.; Buffo, S.K.; Dean, J.C. A neuromechanical strategy for mediolateral foot placement in walking humans. J. Neurophysiol. 2014, 112, 374–383. [Google Scholar] [CrossRef]
- Noraxon Ultium Insole Quick Start Guide. Available online: https://www.noraxon.com/noraxon-download/ultium-insoles-quick-start-guide (accessed on 2 August 2023).
- Orpyx Medical Technologies (Calgary, Canada). Orpyx LogR: Validation of Plantar Pressure Measurement Performance; White paper, Orpyx Medical Technologies: Calgary, AB, Canada, 2017. [Google Scholar]
- Pataky, T.C. One-dimensional statistical parametric mapping in Python. Comput. Methods Biomech. Biomed. Eng. 2012, 15, 295–301. [Google Scholar] [CrossRef]
- Pataky, T.C.; Robinson, M.A.; Vanrenterghem, J. Region-of-interest analyses of one-dimensional biomechanical trajectories: Bridging 0D and 1D theory, augmenting statistical power. PeerJ 2016, 4, e2652. [Google Scholar] [CrossRef]
- Pataky, T.C. Generalized n-dimensional biomechanical field analysis using statistical parametric mapping. J. Biomech. 2010, 43, 1976–1982. [Google Scholar] [CrossRef]
- Cao, J.; Worsley, K.J. The detection of local shape changes via the geometry of Hotelling’s T2 fields. Ann. Statist. 1999, 27, 925–942. [Google Scholar] [CrossRef]
- Pataky, T.C.; Robinson, M.A.; Vanrenterghem, J. Vector field statistical analysis of kinematic and force trajectories. J. Biomech. 2013, 46, 2394–2401. [Google Scholar] [CrossRef]
- Adler, R.J.; Taylor, J.E. Random Fields and Geometry; Springer: Berlin/Heidelberg, Germany, 2007; Volume 80. [Google Scholar]
- Pataky, T.C. RFT1D: Smooth one-dimensional random field upcrossing probabilities in Python. J. Stat. Softw. 2016, 71, 1–22. [Google Scholar] [CrossRef]
- Pataky, T.C.; Vanrenterghem, J.; Robinson, M.A. The probability of false positives in zero-dimensional analyses of one-dimensional kinematic, force and EMG trajectories. J. Biomech. 2016, 49, 1468–1476. [Google Scholar] [CrossRef]
- Yiou, E.; Caderby, T.; Delafontaine, A.; Fourcade, P.; Honeine, J.-L. Balance control during gait initiation: State-of-the-art and research perspectives. World J. Orthop. 2017, 8, 815. [Google Scholar] [CrossRef]
- Bavdek, R.; Zdolšek, A.; Strojnik, V.; Dolenec, A. Peroneal muscle activity during different types of walking. J. Foot Ankle Res. 2018, 11, 50. [Google Scholar] [CrossRef]
- Martin, P.E.; Marsh, A.P. Step length and frequency effects on ground reaction forces during walking. J. Biomech. 1992, 25, 1237–1239. [Google Scholar] [CrossRef]





| Dependent Variables (Lt. Push Phase) | Independent Variables (Non-Multicollinearity) | Standardized Regression Coefficient (β) | Coefficient of Determination (R2) | p Value | |
|---|---|---|---|---|---|
| Step-aside movement | CoPx | PL | 0.465 | 0.216 | <0.001 |
| CoPy | PL | 0.549 | 0.355 | <0.001 | |
| TA | −0.215 | 0.011 | |||
| F-GRF | PL | 0.635 | 0.890 | <0.001 | |
| CoPx | 0.423 | <0.001 | |||
| TA | −0.168 | <0.001 | |||
| Straight walking | CoPx | TA | −0.604 | 0.364 | <0.001 |
| CoPy | TA | −0.311 | 0.243 | 0.002 | |
| PL | 0.288 | 0.003 | |||
| F-GRF | CoPy | 0.291 | 0.760 | <0.001 | |
| SOL | 0.715 | <0.001 | |||
| Dependent Variables (Rt. Loading Phase) | Independent Variables (Non-Multicollinearity) | Standardized Regression Coefficient (β) | Coefficient of Determination (R2) | p Value | |
| Step-aside movement | H-GRF | CoPy | −0.450 | 0.202 | 0.002 |
| Straight walking | H-GRF | CoPx | 1.467 | 0.898 | <0.001 |
| CoPy | −1.033 | <0.001 | |||
| PL | 0.208 | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Cho, S. Ankle Strategies for Step-Aside Movement during Straight Walking. J. Clin. Med. 2023, 12, 5215. https://doi.org/10.3390/jcm12165215
Xie L, Cho S. Ankle Strategies for Step-Aside Movement during Straight Walking. Journal of Clinical Medicine. 2023; 12(16):5215. https://doi.org/10.3390/jcm12165215
Chicago/Turabian StyleXie, Lingchao, and Sanghyun Cho. 2023. "Ankle Strategies for Step-Aside Movement during Straight Walking" Journal of Clinical Medicine 12, no. 16: 5215. https://doi.org/10.3390/jcm12165215
APA StyleXie, L., & Cho, S. (2023). Ankle Strategies for Step-Aside Movement during Straight Walking. Journal of Clinical Medicine, 12(16), 5215. https://doi.org/10.3390/jcm12165215


.png)



