Aberrant HPO Axis Alterations and Autoimmune Abnormalities in PCOS Patients with DOR: A Retrospective Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Patient Selection and Clinical Measurements
2.3. Biochemical Measurements
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Participants
3.2. Prevalence of DOR in Women with PCOS
3.3. Clinical, Endocrine and Metabolic Characteristics in Patients with PCOS According to FSH Levels
3.4. Associations of Basal FSH Levels with Endocrine and Metabolic Variables in PCOS Patients
3.5. The Independent Influence of BMI in Correlations between FSH and Metabolic Variables
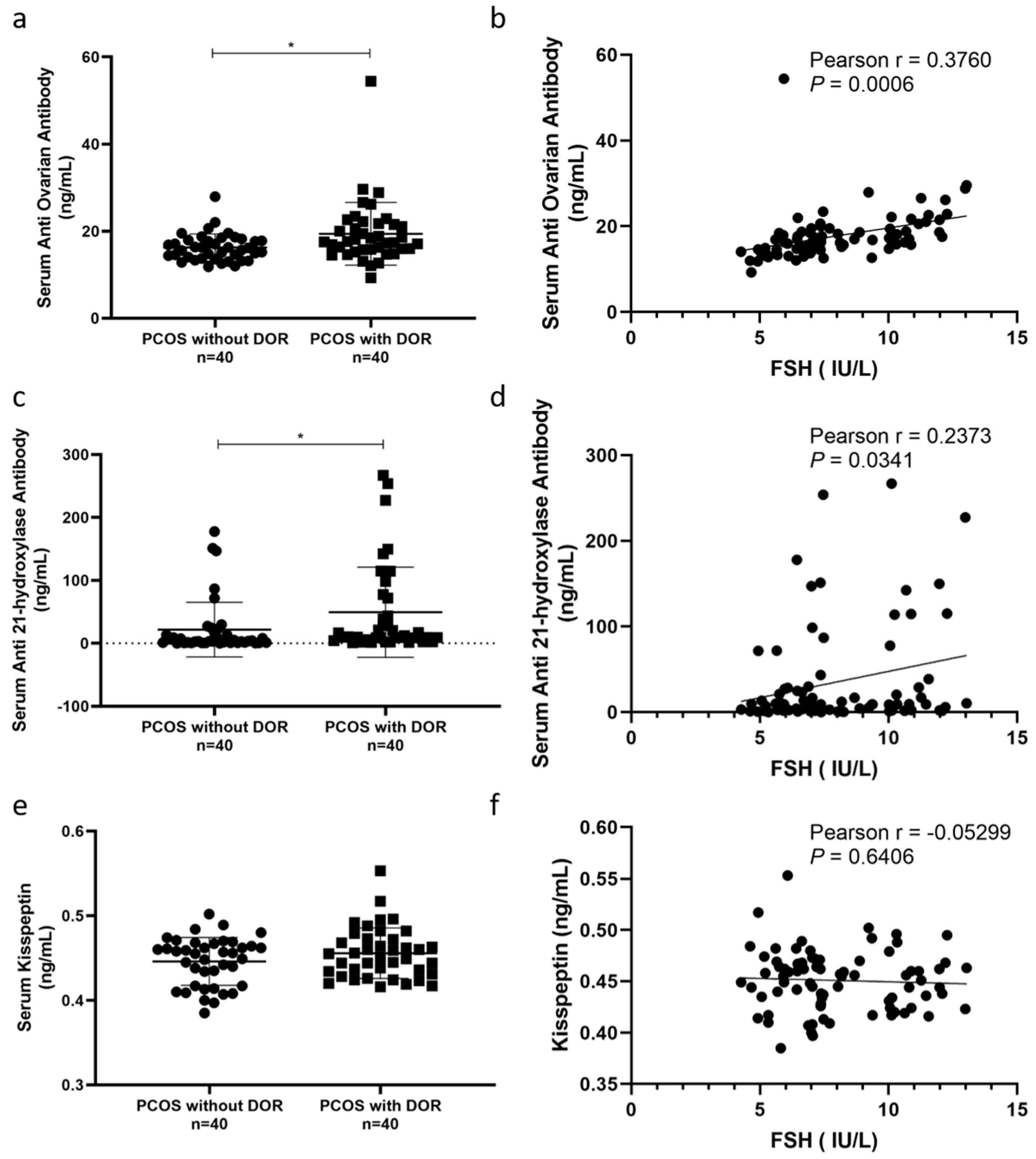
3.6. Autoimmune Abnormalities and Kisspeptin Levels in PCOS Patients with DOR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCOS | polycystic ovary syndrome |
| DOR | diminished ovarian reserve |
| HPO | hypothalamic–pituitary–ovarian |
| LH | luteinizing hormone |
| FSH | follicle-stimulating hormone |
| AMH | Anti-Müllerian hormone |
| AFC | antral follicle count |
| GnRH | gonadotropin-releasing hormone |
| BMI | body mass index |
| HOMA-IR | homeostasis model assessment insulin resistance |
| T | testosterone |
| AD | androstenedione |
| SHBG | sex hormone-binding globulin |
| FAI | free androgen index |
| WHR | waist–hip ratio |
| PRL | prolactin |
| TSH | thyroid-stimulating hormone |
| TG | triglycerides |
| TC | total cholesterol |
| LDL | low-density lipoprotein |
| HDL | high-density lipoprotein |
| GLU | fasting blood glucose |
| FIN | fasting insulin |
| AOAb | anti-ovarian antibody |
| 21-OHAb | anti-21-OH antibody |
References
- Mimouni, N.; Paiva, I.; Barbotin, A.L.; Timzoura, F.E.; Plassard, D.; Le Gras, S.; Ternier, G.; Pigny, P.; Catteau-Jonard, S.; Simon, V.; et al. Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab. 2021, 33, 513–530. [Google Scholar] [CrossRef]
- Skiba, M.A.; Islam, R.M.; Bell, R.J.; Davis, S.R. Understanding variation in prevalence estimates of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2018, 24, 694–709. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Ma, Y.; Xiao, J.; Luo, G.; Li, Y.; Wu, D. Multi-system reproductive metabolic disorder: Significance for the pathogenesis and therapy of polycystic ovary syndrome (pcos). Life Sci. 2019, 228, 167–175. [Google Scholar] [CrossRef]
- Mohammed, S.; Sundaram, V.; Adidam, V.C.; Zyuzikov, N. Polycystic ovary rat model exposure to 150 khz intermediate frequency: Hypothalamic-pituitary-ovarian axis at the receptor, cellular, tissue, and hormone levels. J. Ovarian Res. 2021, 14, 173. [Google Scholar] [CrossRef]
- Van der Ham, K.; Louwers, Y.V.; Laven, J. Cardiometabolic biomarkers in women with polycystic ovary syndrome. Fertil. Steril. 2022, 117, 887–896. [Google Scholar] [CrossRef]
- Li, L.; Feng, Q.; Ye, M.; He, Y.; Yao, A.; Shi, K. Metabolic effect of obesity on polycystic ovary syndrome in adolescents: A meta-analysis. J. Obstet. Gynaecol. 2017, 37, 1036–1047. [Google Scholar] [CrossRef]
- Guleken, Z.; Bulut, H.; Bulut, B.; Depciuch, J. Assessment of the effect of endocrine abnormalities on biomacromolecules and lipids by ft-ir and biochemical assays as biomarker of metabolites in early polycystic ovary syndrome women. J. Pharm. Biomed. Anal. 2021, 204, 114250. [Google Scholar] [CrossRef]
- Cohen, J.; Chabbert-Buffet, N.; Darai, E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder--a plea for universal definitions. J. Assist. Reprod. Genet. 2015, 32, 1709–1712. [Google Scholar] [CrossRef]
- Lu, Q.; Shen, H.; Li, Y.; Zhang, C.; Wang, C.; Chen, X.; Liang, R.; Wei, L. Low testosterone levels in women with diminished ovarian reserve impair embryo implantation rate: A retrospective case-control study. J. Assist. Reprod. Genet. 2014, 31, 485–491. [Google Scholar] [CrossRef]
- Jin, J.; Ruan, X.; Hua, L.; Tian, X.; Li, Y.; Wang, L.; Mueck, A.O. Prevalence of diminished ovarian reserve in chinese women with polycystic ovary syndrome and sensitive diagnostic parameters. Gynecol. Endocrinol. 2017, 33, 694–697. [Google Scholar] [CrossRef]
- Wang, C.; Di, W.; Gu, Z. Endocrine and glycolipid metabolism characteristics of diminished ovarian reserve in chinese women with polycystic ovary syndrome. J. Int. Med. Res. 2020, 48, 1220712534. [Google Scholar] [CrossRef]
- Ferraretti, A.P.; La Marca, A.; Fauser, B.C.; Tarlatzis, B.; Nargund, G.; Gianaroli, L. Eshre consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: The bologna criteria. Hum. Reprod. 2011, 26, 1616–1624. [Google Scholar] [CrossRef]
- Devine, K.; Mumford, S.L.; Wu, M.; Decherney, A.H.; Hill, M.J.; Propst, A. Diminished ovarian reserve in the United-states assisted reproductive technology population: Diagnostic trends among 181,536 cycles from the society for assisted reproductive technology clinic outcomes reporting system. Fertil. Steril. 2015, 104, 612–619. [Google Scholar] [CrossRef]
- Li, F.; Lu, H.; Huang, Y.; Wang, X.; Zhang, Q.; Li, X.; Qiang, L.; Yang, Q. A systematic review and meta-analysis of the association between hashimoto’s thyroiditis and ovarian reserve. Int. Immunopharmacol. 2022, 108, 108670. [Google Scholar] [CrossRef]
- Pastore, L.M.; Christianson, M.S.; Stelling, J.; Kearns, W.G.; Segars, J.H. Reproductive ovarian testing and the alphabet soup of diagnoses: Dor, poi, pof, por, and for. J. Assist. Reprod. Genet. 2018, 35, 17–23. [Google Scholar] [CrossRef]
- Tal, R.; Seifer, D.B. Ovarian reserve testing: A user’s guide. Am. J. Obstet. Gynecol. 2017, 217, 129–140. [Google Scholar] [CrossRef]
- Gleicher, N.; Weghofer, A.; Barad, D.H. Anti-müllerian hormone (amh) defines, independent of age, low versus good live-birth chances in women with severely diminished ovarian reserve. Fertil. Steril. 2010, 94, 2824–2827. [Google Scholar] [CrossRef]
- Tremellen, K.; Zander-Fox, D. Serum anti-mullerian hormone assessment of ovarian reserve and polycystic ovary syndrome status over the reproductive lifespan. Aust. N. Z. J. Obstet. Gynaecol. 2015, 55, 384–389. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (pcos). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Haffner, S.M.; Miettinen, H.; Stern, M.P. The homeostasis model in the san antonio heart study. Diabetes Care 1997, 20, 1087–1092. [Google Scholar] [CrossRef]
- Dewailly, D.; Robin, G.; Peigne, M.; Decanter, C.; Pigny, P.; Catteau-Jonard, S. Interactions between androgens, fsh, anti-müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum. Reprod. Update 2016, 22, 709–724. [Google Scholar] [CrossRef]
- Barad, D. Hormonal effects in reproductive technology with focus on diminished ovarian reserve. Adv. Exp. Med. Biol. 2020, 1242, 13–36. [Google Scholar] [CrossRef]
- Pan, M.L.; Chen, L.R.; Tsao, H.M.; Chen, K.H. Polycystic ovarian syndrome and the risk of subsequent primary ovarian insufficiency: A nationwide population-based study. Menopause-J. N. Am. Menopause Soc. 2017, 24, 803–809. [Google Scholar] [CrossRef]
- Sen, A.; Kushnir, V.A.; Barad, D.H.; Gleicher, N. Endocrine autoimmune diseases and female infertility. Nat. Rev. Endocrinol. 2014, 10, 37–50. [Google Scholar] [CrossRef]
- Petríková, J.; Lazúrová, I. Ovarian failure and polycystic ovary syndrome. Autoimmun. Rev. 2012, 11, A471–A478. [Google Scholar] [CrossRef]
- Sattler, L.M.; Schniewind, H.A.; Minich, W.B.; Haudum, C.W.; Niklowitz, P.; Münzker, J.; Kovács, G.L.; Reinehr, T.; Obermayer-Pietsch, B.; Schomburg, L. Natural autoantibodies to the gonadotropin-releasing hormone receptor in polycystic ovarian syndrome. PLoS ONE 2021, 16, e249639. [Google Scholar] [CrossRef]
- Serin, A.N.; Birge, Ö.; Uysal, A.; Görar, S.; Tekeli, F. Hashimoto’s thyroiditis worsens ovaries in polycystic ovary syndrome patients compared to anti-müllerian hormone levels. BMC Endocr. Disord. 2021, 21, 44. [Google Scholar] [CrossRef]
- Adamska, A.; Bebkowska, A.; Krentowska, A.; Hryniewicka, J.; Adamski, M.; Leśniewska, M.; Polak, A.M.; Kowalska, I. Ovarian reserve and serum concentration of thyroid peroxidase antibodies in euthyroid women with different polycystic ovary syndrome phenotypes. Front. Endocrinol. 2020, 11, 440. [Google Scholar] [CrossRef]
- Janssen, O.E.; Mehlmauer, N.; Hahn, S.; Offner, A.H.; Gärtner, R. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur. J. Endocrinol. 2004, 150, 363–369. [Google Scholar] [CrossRef]
- Silva, C.A.; Yamakami, L.Y.; Aikawa, N.E.; Araujo, D.B.; Carvalho, J.F.; Bonfá, E. Autoimmune primary ovarian insufficiency. Autoimmun. Rev. 2014, 13, 427–430. [Google Scholar] [CrossRef]
- Reato, G.; Morlin, L.; Chen, S.; Furmaniak, J.; Smith, B.R.; Masiero, S.; Albergoni, M.P.; Cervato, S.; Zanchetta, R.; Betterle, C. Premature ovarian failure in patients with autoimmune addison’s disease: Clinical, genetic, and immunological evaluation. J. Clin. Endocrinol. Metab. 2011, 96, E1255–E1261. [Google Scholar] [CrossRef]
- Betterle, C.; Dal Pra, C.; Mantero, F.; Zanchetta, R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: Autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr. Rev. 2002, 23, 327–364. [Google Scholar] [CrossRef]
- Fénichel, P.; Gobert, B.; Carré, Y.; Barbarino-Monnier, P.; Hiéronimus, S. Polycystic ovary syndrome in autoimmune disease. Lancet 1999, 353, 2210. [Google Scholar] [CrossRef]
- Chen, C.W.; Huang, Y.L.; Tzeng, C.R.; Huang, R.L.; Chen, C.H. Idiopathic low ovarian reserve is associated with more frequent positive thyroid peroxidase antibodies. Thyroid 2017, 27, 1194–1200. [Google Scholar] [CrossRef]
- Hsieh, Y.T.; Ho, J. Thyroid autoimmunity is associated with higher risk of premature ovarian insufficiency-a nationwide health insurance research database study. Hum. Reprod. 2021, 36, 1621–1629. [Google Scholar] [CrossRef]
- Chen, Z.J.; Zhao, H.; He, L.; Shi, Y.; Qin, Y.; Shi, Y.; Li, Z.; You, L.; Zhao, J.; Liu, J.; et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat. Genet. 2011, 43, 55–59. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, H.; Shi, Y.; Cao, Y.; Yang, D.; Li, Z.; Zhang, B.; Liang, X.; Li, T.; Chen, J.; et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat. Genet. 2012, 44, 1020–1025. [Google Scholar] [CrossRef]
- Mcallister, J.M.; Legro, R.S.; Modi, B.P.; Strauss, J.R. Functional genomics of pcos: From gwas to molecular mechanisms. Trends Endocrinol. Metab. 2015, 26, 118–124. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The pathogenesis of polycystic ovary syndrome (pcos): The hypothesis of pcos as functional ovarian hyperandrogenism revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Risal, S.; Pei, Y.; Lu, H.; Manti, M.; Fornes, R.; Pui, H.P.; Zhao, Z.; Massart, J.; Ohlsson, C.; Lindgren, E.; et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat. Med. 2019, 25, 1894–1904. [Google Scholar] [CrossRef]
- Greene, A.D.; Patounakis, G.; Segars, J.H. Genetic associations with diminished ovarian reserve: A systematic review of the literature. J. Assist. Reprod. Genet. 2014, 31, 935–946. [Google Scholar] [CrossRef]
- Nilsson, E.; Zhang, B.; Skinner, M.K. Gene bionetworks that regulate ovarian primordial follicle assembly. BMC Genom. 2013, 14, 496. [Google Scholar] [CrossRef]
- Yan, Y.; Gong, Z.; Zhang, L.; Li, Y.; Li, X.; Zhu, L.; Sun, L. Association of follicle-stimulating hormone receptor polymorphisms with ovarian response in chinese women: A prospective clinical study. PLoS ONE 2013, 8, e78138. [Google Scholar] [CrossRef]
- Valkenburg, O.; Uitterlinden, A.G.; Piersma, D.; Hofman, A.; Themmen, A.P.; de Jong, F.H.; Fauser, B.C.; Laven, J.S. Genetic polymorphisms of gnrh and gonadotrophic hormone receptors affect the phenotype of polycystic ovary syndrome. Hum. Reprod. 2009, 24, 2014–2022. [Google Scholar] [CrossRef]
- Nilsson, E.; Klukovich, R.; Sadler-Riggleman, I.; Beck, D.; Xie, Y.; Yan, W.; Skinner, M.K. Environmental toxicant induced epigenetic transgenerational inheritance of ovarian pathology and granulosa cell epigenome and transcriptome alterations: Ancestral origins of polycystic ovarian syndrome and primary ovarian insufiency. Epigenetics 2018, 13, 875–895. [Google Scholar] [CrossRef]
- Sharma, D.; Bhartiya, D. Dysfunctional ovarian stem cells due to neonatal endocrine disruption result in pcos and ovarian insufficiency in adult mice. Stem Cell Rev. Rep. 2022, 18, 2912–2927. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Wang, Z.; Zheng, N.; Yuan, F.; Li, B.; Li, X.; Deng, L.; Lin, M.; Chen, X.; et al. Enhanced glycolysis in granulosa cells promotes the activation of primordial follicles through mtor signaling. Cell Death Dis. 2022, 13, 87. [Google Scholar] [CrossRef]
- Dapas, M.; Lin, F.; Nadkarni, G.N.; Sisk, R.; Legro, R.S.; Urbanek, M.; Hayes, M.G.; Dunaif, A. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. 2020, 17, e1003132. [Google Scholar] [CrossRef]
- Anjali, G.; Kaur, S.; Lakra, R.; Taneja, J.; Kalsey, G.S.; Nagendra, A.; Shrivastav, T.G.; Devi, M.G.; Malhotra, N.; Kriplani, A.; et al. Fsh stimulates irs-2 expression in human granulosa cells through camp/sp1, an inoperative fsh action in pcos patients. Cell. Signal. 2015, 27, 2452–2466. [Google Scholar] [CrossRef]
- Chahal, N.; Geethadevi, A.; Kaur, S.; Lakra, R.; Nagendra, A.; Shrivastav, T.G.; De Pascali, F.; Reiter, E.; Crépieux, P.; Devi, M.G.; et al. Direct impact of gonadotropins on glucose uptake and storage in preovulatory granulosa cells: Implications in the pathogenesis of polycystic ovary syndrome. Metab-Clin. Exp. 2021, 115, 154458. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, H.; Li, W.; Chen, D.; Xu, Y.; Xu, A.; Ye, D. Targeting adipokines in polycystic ovary syndrome and related metabolic disorders: From experimental insights to clinical studies. Pharmacol. Ther. 2022, 240, 108284. [Google Scholar] [CrossRef]
- Guleken, Z.; Bulut, H.; Bulut, B.; Paja, W.; Orzechowska, B.; Parlinska-Wojtan, M.; Depciuch, J. Identification of polycystic ovary syndrome from blood serum using hormone levels via raman spectroscopy and multivariate analysis. Spectroc. Acta Pt. A-Mol. Biomol. Spectr. 2022, 273, 121029. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, M.; Deng, M.; Zhang, H.; Lin, Z.; Fei, X.; Zhang, H. Clomiphene citrate mild stimulation improved follicular development outcomes in pcos women with high luteinizing hormone and poor ovarian response: A case report. Medicine (Baltimore) 2022, 101, e31323. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Lei, Y.L.; Deng, Y.; Ma, R.L.; Ding, X.S.; Xue, W.; Sun, A.J. Treatment progress in diminished ovarian reserve: Western and chinese medicine. Chin. J. Integr. Med. 2022, 29, 361–367. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Wei, N.; Tai, Z.; Yu, C.; Xu, Z. Research progress on the effect of epilepsy and antiseizure medications on pcos through hpo axis. Front. Endocrinol. 2021, 12, 787854. [Google Scholar] [CrossRef]
- Dapas, M.; Dunaif, A. Deconstructing a syndrome: Genomic insights into pcos causal mechanisms and classification. Endocr. Rev. 2022, 43, 927–965. [Google Scholar] [CrossRef]
- Meczekalski, B.; Katulski, K.; Podfigurna-Stopa, A.; Czyzyk, A.; Genazzani, A.D. Spontaneous endogenous pulsatile release of kisspeptin is temporally coupled with luteinizing hormone in healthy women. Fertil. Steril. 2016, 105, 1345–1350. [Google Scholar] [CrossRef]
| PCOS without DOR (n = 1757) | PCOS with DOR (n = 550) | p-Value | Reference Value | |
|---|---|---|---|---|
| Age, years | 25.95 ± 5.16 | 25.93 ± 5.14 | 0.907 | N/A |
| BMI, kg/m2 | 24.23 ± 4.89 | 22.77 ± 4.67 | <0.0001 | 18.5–23.9 Overweight: 24–27.9 Obese: ≥28 |
| Waist/hip ratio | 0.83 ± 0.06 | 0.81 ± 0.07 | <0.0001 | 0.67–0.8 |
| LH, IU/L | 10.76 ± 6.84 | 15.66 ± 16.94 | <0.0001 | 2.12–10.89 |
| FSH, IU/L | 6.62 ± 1.48 | 6.58 ± 3.09 | 0.686 | 3.85–8.78 |
| E2, pmol/L | 163.60 ± 61.29 | 460.38 ± 344.52 | <0.0001 | 55.7–469.2 |
| Prolactin, μg/L | 12.92 ± 6.72 | 14.29 ± 7.64 | <0.0001 | 3.34–26.72 |
| T, nmol/L | 2.28 ± 0.89 | 2.33 ± 0.85 | 0.256 | 0.35–2.6 |
| AD, ng/mL | 3.50 ± 1.28 | 3.63 ± 1.49 | 0.727 | 0.3–3.3 |
| SHBG, nmol/L | 34.38 ± 22.91 | 45.18 ± 27.60 | <0.0001 | 18.2–135.5 |
| FAI | 10.29 ± 9.55 | 7.66 ± 6.58 | <0.0001 | N/A |
| LH/FSH | 1.63 ± 0.99 | 2.32 ± 1.67 | <0.0001 | N/A |
| FSH/LH | 0.88 ± 0.57 | 1.00 ± 1.58 | 0.090 | N/A |
| AMH, ng/mL | 8.22 ± 4.09 | 7.27 ± 3.56 | 0.030 | 2–6.8 |
| AFC † | 36.05 ± 13.24 (n = 919) | 29.98 ± 10.11 (n = 255) | <0.0001 | |
| GLU, mmol/L | 5.14 ± 0.72 | 5.05 ± 0.69 | 0.018 | 3.9–6.1 |
| FINS, mIU/L | 10.20 ± 10.20 | 8.24 ± 6.94 | <0.0001 | 1.9–23 |
| HOMA-IR | 2.42 ± 2.84 | 1.91 ± 1.88 | <0.0001 | N/A |
| TSH, mIU/L | 2.39 ± 2.06 | 2.24 ± 1.43 | 0.086 | 0.27–4.20 |
| TC, mmol/L | 4.67 ± 1.30 | 4.66 ± 0.83 | 0.873 | <5.72 |
| TG, mmol/L | 1.18 ± 0.77 | 1.01 ± 0.59 | <0.0001 | <1.7 |
| HDL-C, mmol/L | 1.36 ± 0.29 | 1.43 ± 0.29 | 0.015 | 0.9–2.00 |
| LDL-C, mmol/L | 2.74 ± 0.77 | 2.66 ± 0.74 | 0.059 | <3.4 |
| Pregnancy | 0.37 ± 0.80 | 0.34 ± 0.79 | 0.417 | |
| Birth | 0.19 ± 0.46 | 0.15 ± 0.40 | 0.091 | |
| abortion | 0.18 ± 0.53 | 0.19 ± 0.55 | 0.839 |
| PCOS without DOR (n = 1387) | PCOS with DOR (n = 455) | p-Value | |||
|---|---|---|---|---|---|
| Subtypes | Counts | Rate (%) | Counts | Rate (%) | |
| HA + O + PCO | 1024 | 73.82 | 321 | 70.55 | <0.01 |
| HA + O | 66 | 4.76 | 27 | 5.93 | 0.320 |
| HA + PCO | 70 | 5.05 | 27 | 5.93 | 0.462 |
| O + PCO | 227 | 16.37 | 80 | 17.59 | 0.546 |
| FSH < 3.85 (n = 161) | FSH 3.85–8.78 (n = 1874) | FSH 8.78–10 (n = 166) | FSH > 10 (n = 106) | p-Value | |
|---|---|---|---|---|---|
| Age, years | 26.08 ± 5.44 | 25.89 ± 5.19 | 26.35 ± 4.81 | 26.03 ± 4.62 | 0.707 |
| BMI, kg/m2 | 24.12 ± 4.41 a | 24.03 ± 4.89 a | 23.26 ± 4.98 ab | 21.77 ± 4.63 b | <0.0001 |
| Waist/hip ratio | 0.82 ± 0.06 ab | 0.83 ± 0.07 a | 0.81 ± 0.06 ab | 0.80 ± 0.07 b | 0.0005 |
| LH, IU/L | 5.28 ± 3.37 a | 11.24 ± 7.48 a | 15.91 ± 10.86 b | 27.88 ± 28.65 c | <0.0001 |
| FSH, IU/L | 2.66 ± 0.77 a | 6.44 ± 1.18 b | 9.33 ± 0.35 c | 11.34 ± 1.41 c | <0.0001 |
| E2, pmol/L | 566.96 ± 436.92 a | 204.72 ± 146.68 b | 202.99 ± 152.61 b | 349.52 ± 385.13 c | <0.0001 |
| Prolactin, μg/L | 17.03 ± 8.35 a | 12.95 ± 6.75 bc | 12.00 ± 5.71 b | 14.75 ± 8.30 ac | <0.0001 |
| T, nmol/L | 2.05 ± 0.73 a | 2.30 ± 0.90 b | 2.41 ± 0.90 b | 2.40 ± 0.75 b | 0.0008 |
| AD, ng/mL | 3.34 ± 1.25 | 3.52 ± 1.33 | 3.62 ± 1.40 | 3.87 ± 1.51 | 0.957 |
| SHBG, nmol/L | 42.84 ± 32.56 ab | 35.51 ± 23.34 b | 41.01 ± 24.84 ab | 47.61 ± 26.83 a | <0.0001 |
| FAI | 7.90 ± 7.55 a | 10.03 ± 9.35 b | 8.40 ± 6.94 ab | 7.58 ± 6.96 a | 0.0003 |
| LH/FSH | 2.02 ± 1.28 ac | 1.75 ± 1.14 b | 1.70 ± 1.16 bc | 2.36 ± 2.16 ad | 0.002 |
| FSH/LH | 0.96 ± 1.60 | 0.92 ± 0.87 | 0.84 ± 0.56 | 0.78 ± 0.68 | 0.280 |
| AMH | 7.74 ± 4.63 | 7.98 ± 4.04 | 8.69 ± 5.08 | 7.45 ± 4.38 | 0.789 |
| GLU, mmol/L | 5.06 ± 0.58 | 5.13 ± 0.73 | 5.08 ± 0.76 | 5.04 ± 0.59 | 0.226 |
| FINS, mIU/L | 9.82 ± 7.91 a | 10.05 ± 9.35 a | 7.96 ± 5.33 ab | 6.79 ± 4.99 b | <0.0001 |
| HOMA-IR | 2.26 ± 2.03 a | 2.38 ± 2.82 ab | 1.88 ± 1.70 bd | 1.55 ± 1.26 cd | <0.0001 |
| TSH, mIU/L | 2.46 ± 1.97 | 2.35 ± 2.00 | 2.29 ± 1.34 | 2.46 ± 1.30 | 0.981 |
| TC, mmol/L | 4.58 ± 0.84 | 4.65 ± 1.27 | 4.78 ± 0.86 | 4.80 ± 0.94 | 0.330 |
| TG, mmol/L | 1.03 ± 0.56 | 1.16 ± 0.74 | 1.11 ± 0.91 | 1.06 ± 0.63 | 0.127 |
| HDL-C, mmol/L | 1.39 ± 0.30 | 1.36 ± 0.29 | 1.40 ± 0.33 | 1.46 ± 0.31 | 0.278 |
| LDL-C, mmol/L | 2.69 ± 0.77 | 2.71 ± 0.77 | 2.83 ± 0.74 | 2.70 ± 0.78 | 0.295 |
| r | ||
|---|---|---|
| all PCOS (n = 2307) | PCOS with DOR (n = 550) | |
| Age, years | 0.271 | −0.021 |
| BMI, kg/m2 | −0.102 **** | −0.154 *** |
| Waist/hip ratio | −0.085 * | −0.145 * |
| LH, IU/L | 0.438 **** | 0.521 **** |
| E2, pmol/L | −0.159 **** | −0.251 **** |
| Prolactin, μg/L | −0.091 **** | −0.084 |
| T, nmol/L | 0.096 **** | 0.140 ** |
| AD, ng/mL | 0.018 | 0.108 * |
| SHBG, nmol/L | 0.008 ** | 0.094 |
| FAI | −0.046 | −0.008 |
| AMH | 0.07634 | 0.08875 |
| GLU, mmol/L | −0.019 | 0.006 |
| FINS, mIU/L | −0.095 **** | −0.127 ** |
| HOMA-IR | −0.080 *** | −0.108 * |
| TC, mmol/L | 0.030 | 0.083 |
| TG, mmol/L | 0.036 | 0.028 |
| HDL-C, mmol/L | 0.031 | 0.050 |
| LDL-C, mmol/L | 0.007 | −0.002 |
| Parameters | β1 | β2 | Multiple R | R Squared | Adjusted R Squared |
|---|---|---|---|---|---|
| FSH | BMI | ||||
| Waist/hip ratio | −0.0004 | 0.008 **** | 0.578 | 0.334 | 0.332 |
| LH, IU/L | 2.211 **** | −0.227 **** | 0.447 | 0.200 | 0.200 |
| E2, pmol/L | −19.080 **** | −4.303 **** | 0.189 | 0.036 | 0.035 |
| Prolactin, μg/L | −0.319 **** | -0.046 | 0.093 | 0.009 | 0.008 |
| T, nmol/L | 0.0442 **** | 0.009 * | 0.106 | 0.011 | 0.010 |
| AD, ng/mL | 0.076 | −0.002 | 0.016 | 0.0003 | −0.001 |
| SHBG, nmol/L | 0.082 | −2.329 **** | 0.470 | 0.221 | 0.220 |
| FAI | −0.007 | 0.665 **** | 0.367 | 0.134 | 0.133 |
| GLU, mmol/L | 0.003 | 0.042 **** | 0.281 | 0.079 | 0.078 |
| FINS, mIU/L | −0.202 * | 0.998 **** | 0.519 | 0.269 | 0.268 |
| HOMA-IR | −0.041 | 0.261 **** | 0.484 | 0.235 | 0.240 |
| TC, mmol/L | 0.025 | 0.026 **** | 0.109 | 0.012 | 0.011 |
| TG, mmol/L | 0.001 | 0.057 **** | 0.379 | 0.144 | 0.143 |
| HDL-C, mmol/L | −0.002 | −0.027 **** | 0.452 | 0.204 | 0.201 |
| LDL-C, mmol/L | 0.014 | 0.0456 **** | 0.290 | 0.084 | 0.083 |
| TSH, mIU/L | 0.0120 | 0.0445 | 0.034 | 0.001 | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, X.; He, Z.; Bao, Z.; Di, W.; Gu, Z. Aberrant HPO Axis Alterations and Autoimmune Abnormalities in PCOS Patients with DOR: A Retrospective Analysis. J. Clin. Med. 2023, 12, 5212. https://doi.org/10.3390/jcm12165212
Geng X, He Z, Bao Z, Di W, Gu Z. Aberrant HPO Axis Alterations and Autoimmune Abnormalities in PCOS Patients with DOR: A Retrospective Analysis. Journal of Clinical Medicine. 2023; 12(16):5212. https://doi.org/10.3390/jcm12165212
Chicago/Turabian StyleGeng, Xueying, Zhihong He, Zhouzhou Bao, Wen Di, and Zhuowei Gu. 2023. "Aberrant HPO Axis Alterations and Autoimmune Abnormalities in PCOS Patients with DOR: A Retrospective Analysis" Journal of Clinical Medicine 12, no. 16: 5212. https://doi.org/10.3390/jcm12165212
APA StyleGeng, X., He, Z., Bao, Z., Di, W., & Gu, Z. (2023). Aberrant HPO Axis Alterations and Autoimmune Abnormalities in PCOS Patients with DOR: A Retrospective Analysis. Journal of Clinical Medicine, 12(16), 5212. https://doi.org/10.3390/jcm12165212






