Biofeedback Training after Successful Inverted Internal Limiting Membrane (ILM)-Flap Technique for High Myopic Macular Hole
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Objectives
2.2. Assessments
2.3. Surgical Technique
2.4. Biofeedback Strategy
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobayashi, H.; Kobayashi, K.; Okinami, S. Macular hole and myopic refraction. Br. J. Ophthalmol. 2002, 86, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Ripandelli, G.; Rossi, T.; Scarinci, F.; Scassa, C.; Parisi, V.; Stirpe, M. Macular vitreoretinal interface abnormalities in highly myopic eyes with posterior staphyloma. Retina 2012, 32, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Ikuno, Y.; Nishida, K. Retinoschisis: A predictive factor in vitrectomy for macular holes without retinal detachment in highly myopic eyes. Br. J. Ophthalmol. 2012, 96, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.T.; Pan, Q.T.; Zheng, J.W.; Zhang, Z.D. Foveal microstructure and visual outcomes of myopic macular hole surgery with or without the inverted internal limiting membrane flap technique. Br. J. Ophthalmol. 2019, 103, 1495–1502. [Google Scholar] [CrossRef]
- Bové, A.M.; Sabaté, S.; Gómez-Resa, M.; García-Arumí, J. Anatomical and Visual Outcomes of Inverted Internal Limiting Membrane Flap Technique versus Internal Limiting Membrane Peeling in Myopic Macular Hole without Retinal Detachment: A Preliminary Retrospective Study. Retina 2020, 40, 233–240. [Google Scholar] [CrossRef]
- Mete, M.; Alfano, A.; Guerriero, M.; Prigione, G.; Sartore, M.; Polito, A.; Pertile, G. Inverted Internal Limiting Membrane Flap Technique versus Complete Internal Limiting Membrane Removal in Myopic Macular Hole Surgery: A Comparative Study. Retina 2017, 37, 1923–1930. [Google Scholar] [CrossRef]
- Chatziralli, I.; Machairoudia, G.; Kazantzis, D.; Theodossiadis, G.; Theodossiadis, P. Inverted internal limiting membrane flap technique for myopic macular hole: A meta-analysis. Surv. Ophthalmol. 2021, 66, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Crossland, M.D.; Culham, L.E.; Kabanarou, S.A.; Rubin, G.S. Preferred retinal locus development in patients with macular disease. Ophthalmology 2005, 112, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Crossland, M.D.; Engel, S.A.; Legge, G.E. The Preferred Retinal Locus in Macular Disease: Toward a Consensus Definition. Retina 2011, 31, 2109–2114. [Google Scholar] [CrossRef]
- Crossland, M.D.; Crabb, D.P.; Rubin, G.S. Task-Specific Fixation Behavior in Macular Disease. Investig. Opthalmol. Vis. Sci. 2011, 52, 411–416. [Google Scholar] [CrossRef]
- Tarita-Nistor, L.; González, E.G.; Markowitz, S.N.; Steinbach, M.J. Fixation characteristics of patients with macular degeneration recorded with the MP-1 microperimeter. Retina 2008, 28, 125–133. [Google Scholar] [CrossRef]
- Bernard, J.B.; Chung, S.T.L. Visual Acuity Is Not the Best at the Preferred Retinal Locus in People with Macular Disease. Optom. Vis. Sci. 2018, 95, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.K.; Bedell, H.E. Functional changes at the preferred retinal locus in subjects with bilateral central vision loss. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 256, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Bellmann, C.; Feely, M.; Crossland, M.D.; Kabanarou, S.A.; Rubin, G.S. Fixation stability using central and pericentral fixation targets in patients with age-related macular degeneration. Ophthalmology 2004, 111, 2265–2270. [Google Scholar] [CrossRef] [PubMed]
- Culham, L.E.; Fitzke, F.W.; Timberlake, G.T.; Marshall, J. Assessment of fixation stability in normal subjects and patients using a scanning laser ophthalmoscope. Clin. Vis. Sci. 1993, 8, 551–561. [Google Scholar]
- Schönbach, E.M.; Ibrahim, M.A.; Strauss, R.W.; Birch, D.G.; Cideciyan, A.V.; Astrid Hahn, G.; Ho, A.; Kong, X.; Nasser, F.; Sunness, J.S.; et al. Progression of Stargardt Disease Study Group. Fixation location and stability using the MP-1 Microperimeter in Stargardt disease: ProgStar Report No. 3. Ophthalmol. Retin. 2017, 1, 68–76. [Google Scholar] [CrossRef]
- Amore, F.M.; Fasciani, R.; Silvestri, V.; Crossland, M.D.; de Waure, C.; Cruciani, F.; Reibaldi, A. Relationship between fixation stability measured with MP-1 and reading performance. Ophthalmic Physiol. Opt. 2013, 33, 611–617. [Google Scholar] [CrossRef]
- Shao, Q.; Xia, H.; Heussen, F.M.A.; Ouyang, Y.; Sun, X.; Fan, Y. Postoperative anatomical and functional outcomes of different stages of high myopia macular hole. BMC Ophthalmol. 2015, 15, 93. [Google Scholar] [CrossRef]
- Vingolo, E.M.; Salvatore, S.; Cavarretta, S. Low vision rehabilitation by means of MP-1 biofeed-back examination in patients with different macular diseases: A pilot study. Appl. Psychophysiol. Biofeedback 2009, 34, 127–133. [Google Scholar] [CrossRef]
- Morales, M.U.; Saker, S.; Wilde, C.; Rubinstein, M.; Limoli, P.; Amoaku, W.M. Biofeedback fixation training method for improving eccentric vision in patients with loss of foveal function secondary to different maculopathies. Int. Ophthalmol. 2020, 40, 305–312. [Google Scholar] [CrossRef]
- Vingolo, E.M.; Salvatore, S.; Limoli, P.G. MP-1 biofeedback: Luminous pattern stimulus versus acoustic biofeedback in age related macular degeneration (AMD). Appl. Psychophysiol. Biofeedback 2013, 38, 11–16. [Google Scholar] [CrossRef]
- Amore, F.M.; Paliotta, S.; Silvestri, V.; Piscopo, P.; Turco, S.; Reibaldi, A. Biofeedback stimulation in patients with age-related macular degeneration: Comparison between 2 different methods. Can. J. Ophthalmol. 2013, 48, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Vingolo, E.M.; Fragiotta, S.; Domanico, D.; Limoli, P.G.; Nebbioso, M.; Spadea, L. Visual Recovery after Primary Retinal Detachment Surgery: Biofeedback Rehabilitative Strategy. J. Ophthalmol. 2016, 2016, 8092396. [Google Scholar] [CrossRef]
- Verdina, T.; Giacomelli, G.; Sodi, A.; Pennino, M.; Paggini, C.; Murro, V.; Virgili, G.; Menchini, U. Biofeedback rehabilitation of eccentric fixation in patients with Stargardt disease. Eur. J. Ophthalmol. 2013, 23, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Pan, X.; Xu, X.; Wang, R. A cortical model with multi-layers to study visual attentional modulation of neurons at the synaptic level. Cogn. Neurodynamics 2019, 13, 579–599. [Google Scholar] [CrossRef] [PubMed]
- Hall, N.J.; Colby, C.L. Remapping for visual stability. Philos. Trans. R Soc. Lond. Ser. B Biol. Sci. 2011, 366, 528–539. [Google Scholar] [CrossRef]
- Vingolo, E.M.; Cavarretta, S.; Domanico, D.; Parisi, F.; Malagola, R. Microperimetric biofeedback in AMD patients. Appl. Psychophysiol. Biofeedback 2007, 32, 185–189. [Google Scholar] [CrossRef]
- Ueda-Consolvo, T.; Otsuka, M.; Hayashi, Y.; Ishida, M.; Hayashi, A. Microperimetric Biofeedback Training Improved Visual Acuity after Successful Macular Hole Surgery. J. Ophthalmol. 2015, 2015, 572942. [Google Scholar] [CrossRef]
- Sborgia, G.; Niro, A.; Tritto, T.; Albano, V.; Sborgia, L.; Sborgia, A.; Donghia, R.; Giancipoli, E.; Coassin, M.; Pastore, V.; et al. Microperimetric Biofeedback Training after Successful Inverted Flap Technique for Large Macular Hole. J. Clin. Med. 2020, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.; Vishal, M.; Babu, N.; Rajendran, A.; Ramasamy, K. Retrospective study of changes in ocular coherence tomography characteristics after failed macular hole surgery and outcomes of fluid-gas exchange for persistent macular hole. Indian J. Ophthalmol. 2018, 66, 1130–1135. [Google Scholar] [CrossRef]
- Chylack, L.T., Jr.; Wolfe, J.K.; Singer, D.M.; Leske, M.C.; Bullimore, M.A.; Bailey, I.L.; Friend, J.; McCarthy, D.; Wu, S.Y. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch. Ophthalmol. 1993, 111, 831–836. [Google Scholar] [CrossRef]
- Convento, E.; Barbaro, G. Technical insights in the interpretation of automatic microperimetry. In Perimetry and the Fundus: An Introduction to Microperimetry; Midena, E., Ed.; Slack Inc.: Thorofare, NJ, USA, 2007; pp. 229–237. [Google Scholar]
- Sborgia, G.; Niro, A.; Sborgia, A.; Albano, V.; Tritto, T.; Sborgia, L.; Pastore, V.; Donghia, R.; Giancipoli, E.; Recchimurzo, N.; et al. Inverted internal limiting membrane-flap technique for large macular hole: A microperimetric study. Int. J. Retin. Vitr. 2019, 5, 44. [Google Scholar] [CrossRef]
- Steinman, R.M. Effect of target size, luminance, and color on monocular fixation. J. Opt. Soc. Am. 1965, 55, 1158–1164. [Google Scholar] [CrossRef]
- Timberlake, G.T.; Sharma, M.K.; Grose, S.A.; Gobert, D.V.; Gauch, J.M.; Maino, J.H. Retinal location of the preferred retinal locus relative to the fovea in scanning laser ophthalmoscope images. Optom. Vis. Sci. 2005, 82, E177–E187. [Google Scholar] [CrossRef]
- Ozdemir, H.; Karacorlu, M.; Senturk, F.; Karacorlu, S.A.; Uysal, O. Retinal sensitivity and fixation changes 1 year after triamcinolone acetonide assisted internal limiting membrane peeling for macular hole surgery: A MP-1 microperimetric study. Acta Ophthalmol. 2010, 88, e222–e227. [Google Scholar] [CrossRef] [PubMed]
- Michalewska, Z.; Michalewski, J.; Adelman, R.A.; Nawrocki, J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology 2010, 117, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, U.L.; Frennesson, C.; Nilsson, S.E.G. Patients with AMD and a large absolute central scotoma can be trained successfully to use eccentric viewing, as demonstrated in a scanning laser ophthalmoscope. Vis. Res. 2003, 43, 1777–1787. [Google Scholar] [CrossRef]
- De Giacinto, C.; Pastore, M.R.; Cirigliano, G.; Tognetto, D. Macular Hole in Myopic Eyes: A Narrative Review of the Current Surgical Techniques. J. Ophthalmol. 2019, 2019, 3230695. [Google Scholar] [CrossRef]
- Alkabes, M.; Pichi, F.; Nucci, P.; Massaro, D.; Dutra Medeiros, M.; Corcostegui, B.; Mateo, C. Anatomical and visual outcomes in high myopic macular hole (HM-MH) without retinal detachment: A review. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Graefes Arch. Klin. Exp. Ophthalmol. 2014, 252, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Feng, C.; Han, M.; He, J.; Zhang, R.; Yan, T.; Li, X.; Liu, Y.; Li, Y.; Wu, J. Inverted internal limiting membrane flap technique for retinal detachment due to macular holes in high myopia with axial length ≥ 30 mm. Sci. Rep. 2022, 12, 4258. [Google Scholar] [CrossRef]
- Theodossiadis, G.P.; Chatziralli, I.P.; Theodossiadis, P.G. Inverted internal limiting membrane insertion for macular hole–associated retinal detachment in high myopia. Am. J. Ophthalmol. 2016, 165, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Zaben, A.; Zapata, M.; Garcia-Arumi, J. Retinal sensitivity and choroidal thickness in high myopia. Retina 2015, 35, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhu, M.; Qu, X.; Xu, G.; Yu, Y.; Witt, R.E.; Wang, W. Reginal macular light sensitivity changes in myopic Chinese adults: An MP1 study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4451–4457. [Google Scholar] [CrossRef]
- Gella, L.; Raman, R.; Sharma, T. Evaluation of in vivo human retinal morphology and function in myopes. Curr. Eye Res. 2011, 36, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, A.; Gelso, A.; Di Nicola, M.; Scarinci, F.; Ripandelli, G.; Costagliola, C.; Rossi, T. Inverted ILM-flap techniques variants for macular hole surgery: Randomized clinical trial to compare retinal sensitivity and fixation stability. Sci. Rep. 2020, 10, 15832. [Google Scholar] [CrossRef]
- Chen, Y.J.; Huang, W.Y. Changes in retinal sensitivity following inverted internal limiting membrane flap technique for large macular holes. Taiwan J. Ophthalmol. 2021, 11, 273–279. [Google Scholar] [CrossRef]
- Palkovits, S.; Hirnschall, N.; Georgiev, S. Effect of Cataract Extraction on Retinal Sensitivity Measurements. Ophthalmic Res. 2021, 64, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Z.; Yu, Y.; Yang, X.; Qi, B.; Zhang, K.; Liu, W. Microstructural and microperimetric comparison of internal limiting membrane peeling and insertion in large idiopathic macular hole. BMC Ophthalmol. 2023, 23, 274. [Google Scholar] [CrossRef]
- Clark, A.F.; Balducci, N.; Pichi, F.; Veronese, C.; Morara, M.; Torrazza, C.; Ciardella, A.P. Swelling of the arcuate nerve fiber layer after internal limiting membrane peeling. Retina 2012, 32, 1608–1613. [Google Scholar] [CrossRef]
- Tarita-Nistor, L.; González, E.G.; Markowitz, S.N.; Steinbach, M.J. Plasticity of fixation in patients with central vision loss. Vis. Neurosci. 2009, 26, 487–494. [Google Scholar] [CrossRef]
- Tarita-Nistor, L.; Gonza´lez, E.G.; Mandelcorn, M.S.; Lillakas, L.; Steinbach, M.J. Fixation stability, fixation location, and visual acuity after successful macular hole surgery. Investig. Opthalmol. Vis. Sci. 2009, 50, 84–89. [Google Scholar] [CrossRef]
- Erbezci, M.; Ozturk, T. Preferred retinal locus locations in age-related macular degeneration. Retina 2018, 38, 2372–2378. [Google Scholar] [CrossRef] [PubMed]
- Petre, K.L.; Hazel, C.A.; Fine, E.M.; Rubin, G.S. Reading with eccentric fixation is faster in inferior visual field than in left visual field. Optom. Vis. Sci. 2000, 77, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Damkondwar, D.; Neriyanuri, S.; Sharma, T. Microperimetry biofeedback training in a patient with bilateral myopic macular degeneration with central scotoma. Indian J. Ophthalmol. 2015, 63, 534–536. [Google Scholar]
- Scuderi, G.; Verboschi, F.; Domanico, D.; Spadea, L. Fixation improvement through biofeedback rehabilitation in Stargardt disease. Case Rep. Med. 2016, 2016, 4264829. [Google Scholar] [CrossRef][Green Version]
- Bednar, J.A. Building a mechanistic model of the development and function of the primary visual cortex. J. Physiol. 2012, 106, 194–211. [Google Scholar] [CrossRef]
- Andrade, M.A.; Muro, E.M.; Morán, F. Simulation of plasticity in the adult visual cortex. Biol. Cybern. 2001, 84, 445–451. [Google Scholar] [CrossRef]
- Silvestri, V.; Turco, S.; Piscopo, P.; Guidobaldi, M.; Perna, F.; Sulfaro, M.; Amore, F. Biofeedback stimulation in the visually impaired: A systematic review of literature. Ophthalmic Physiol. Opt. 2021, 41, 342–364. [Google Scholar] [CrossRef]
- Midena, E.; Pilotto, E.; Convento, E. Age-Related Macular Degeneration: Prevention of Blindness and Low-Vision Rehabilitation. In Rehabilitation Medicine for Elderly Patients. Practical Issues in Geriatrics; Masiero, S., Carraro, U., Eds.; Springer: Cham, Switzerland, 2018; pp. 293–298. [Google Scholar]
- Palmer, S.; Logan, D.; Nabili, S.; Dutton, G.N. Effective rehabilitation of reading by training in the technique of eccentric viewing: Evaluation of a 4-year programme of service delivery. Br. J. Ophthalmol. 2010, 94, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Murro, V.; Sodi, A.; Giacomelli, G.; Mucciolo, D.P.; Pennino, M.; Virgili, G.; Rizzo, S. Reading ability and quality of life in Stargardt disease. Eur. J. Ophthalmol. 2017, 27, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xia, H.; Liu, Y.; Wang, X.; Yuan, H.; Hou, Q.; Ge, Y.; Ding, Y.; Wang, Y.; Wang, C.; et al. Ellipsoid Zone and External Limiting Membrane-Related Parameters on Spectral Domain-Optical Coherence Tomography and Their Relationships with Visual Prognosis after Successful Macular Hole Surgery. Front. Med. 2021, 8, 779602. [Google Scholar] [CrossRef] [PubMed]
| Parameters * | Biofeedback Group (n = 12) | Control Group (n = 11) | p § |
|---|---|---|---|
| Gender (M) (%) | 10 (83.33) | 4 (36.36) | 0.04 ^ |
| Age (yrs) | 63.25 ± 8.79 | 68.00 ± 7.22 | 0.17 |
| AL (mm) | 26.72 ± 1.09 | 27.89 ± 2.40 | 0.04 |
| Hole size (µm) | 347.00 ± 115.84 | 404.73 ± 186.49 | 0.45 |
| Lens status (%) | 0.64 ^ | ||
| Phakic | 10 (83.33) | 8 (72.73) | |
| Pseudophakic | 2 (16.67) | 3 (27.27) | |
| RE in phakic eyes (D) | −8.35 ± 2.90 | −11.93 ± 7.80 | 0.35 |
| BCVA pre-surgical (logMAR) | 1.03 ± 0.11 | 1.15 ± 0.30 | 0.21 |
| Parameters * | Control Group | Biofeedback Group | p ^ | p ѱ | p ⸹ | p † | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 Months | 6 Months | 12 Months | Baseline | 3 Months | 6 Months | 12 Months | |||||
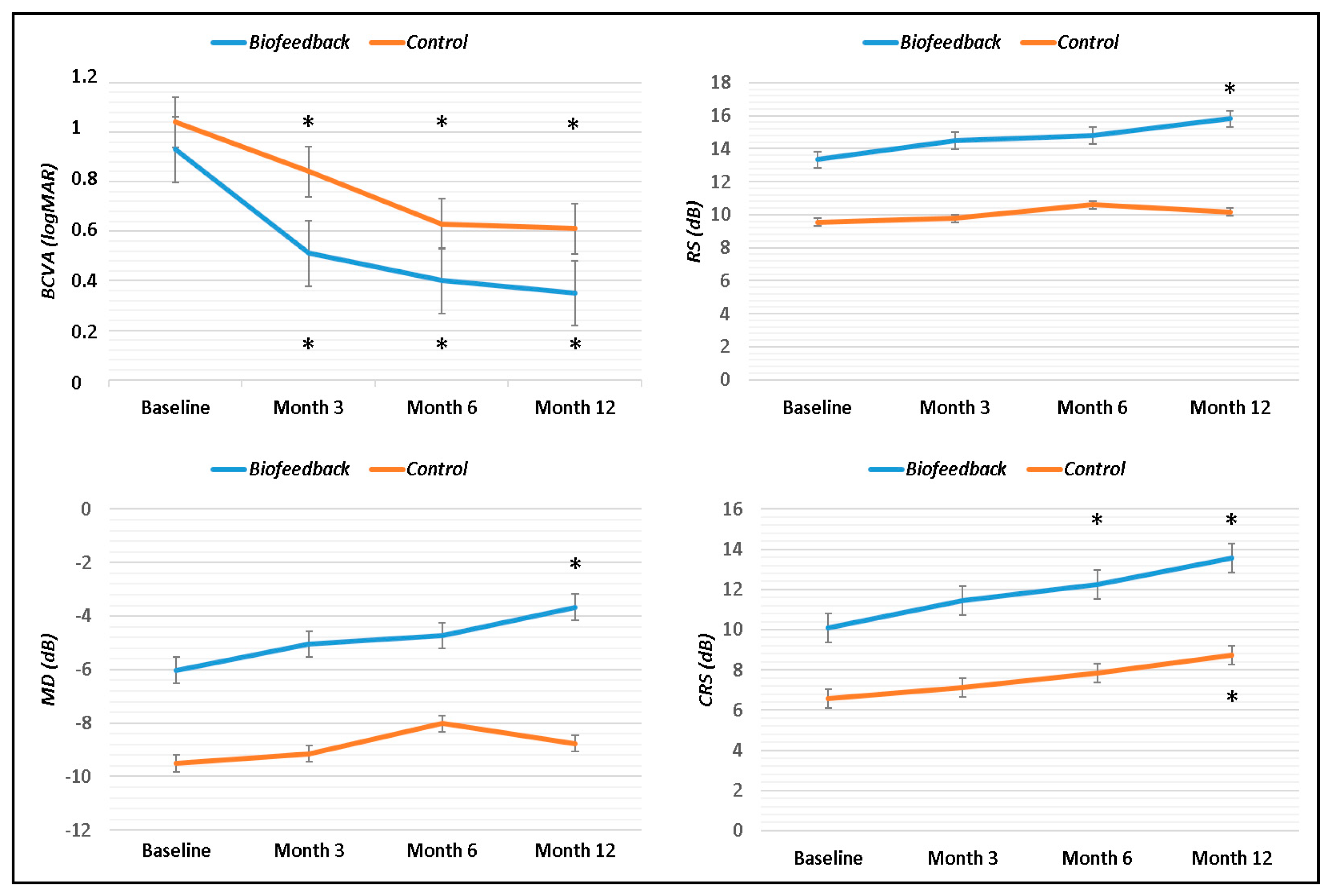
| BCVA (logMAR) | 1.04 ± 0.22 | 0.83 ± 0.34 | 0.63 ± 0.31 | 0.61 ± 0.35 | 0.92 ± 0.19 | 0.51 ± 0.24 | 0.40 ± 0.23 | 0.35 ± 0.22 | 0.20 | 0.01 | 0.22 | 0.09 |
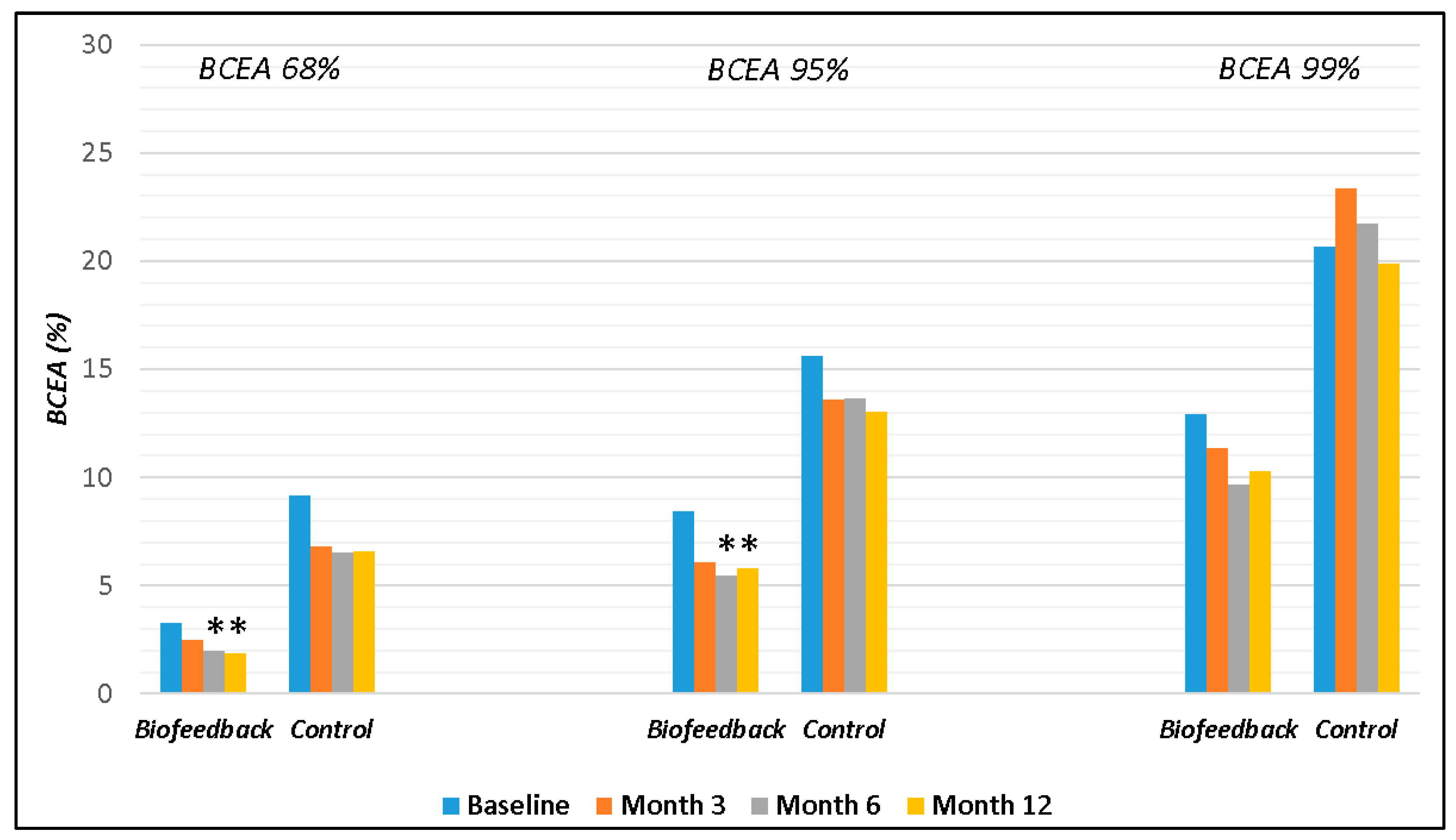
| BCEA 68% | 9.20 ± 8.78 | 6.79 ± 6.68 | 6.54 ± 7.32 | 6.61 ± 7.44 | 3.27 ± 2.61 | 2.51 ± 2.14 | 1.98 ± 2.07 | 1.85 ± 2.15 | 0.05 | 0.008 | 0.002 | 0.0003 |
| BCEA 95% | 15.60 ± 13.42 | 13.58 ± 9.67 | 13.66 ± 10.89 | 13.02 ± 9.81 | 8.44 ± 5.86 | 6.08 ± 5.58 | 5.47 ± 5.59 | 5.80 ± 5.79 | 0.15 | 0.007 | 0.01 | 0.006 |
| BCEA 99% | 20.68 ± 12.24 | 23.34 ± 11.28 | 21.73 ± 11.89 | 19.86 ± 10.2 | 12.91 ± 9.35 | 11.37 ± 10.17 | 9.67 ± 9.56 | 10.26 ± 9.92 | 0.10 | 0.003 | 0.001 | 0.004 |
| RS (dB) | 9.54 ± 5.10 | 9.77 ± 4.59 | 10.61 ± 4.82 | 10.16 ± 4.23 | 13.32 ± 4.02 | 14.48 ± 2.60 | 14.80 ± 2.71 | 15.80 ± 2.59 | 0.11 | 0.02 | 0.04 | 0.0007 |
| MD (dB) | −9.50 ± 4.73 | −9.15 ± 4.17 | −8.03 ± 4.64 | −8.76 ± 4.34 | −6.02 ± 4.05 | −5.05 ± 2.64 | −4.73 ± 2.81 | −3.67 ± 2.72 | 0.09 | 0.01 | 0.09 | 0.003 |
| CRS (dB) | 6.56 ± 4.06 | 7.12 ± 4.35 | 7.83 ± 4.52 | 8.73 ± 4.45 | 10.07 ± 4.88 | 11.45 ± 4.81 | 12.26 ± 3.89 | 13.55 ± 4.20 | 0.11 | 0.02 | 0.02 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sborgia, A.; Niro, A.; Pastore, V.; Albano, V.; Boscia, G.; Piepoli, M.; Di Pardo, C.; Accurso Tagano, L.; Zerbinati, M.; Landini, L.; et al. Biofeedback Training after Successful Inverted Internal Limiting Membrane (ILM)-Flap Technique for High Myopic Macular Hole. J. Clin. Med. 2023, 12, 5188. https://doi.org/10.3390/jcm12165188
Sborgia A, Niro A, Pastore V, Albano V, Boscia G, Piepoli M, Di Pardo C, Accurso Tagano L, Zerbinati M, Landini L, et al. Biofeedback Training after Successful Inverted Internal Limiting Membrane (ILM)-Flap Technique for High Myopic Macular Hole. Journal of Clinical Medicine. 2023; 12(16):5188. https://doi.org/10.3390/jcm12165188
Chicago/Turabian StyleSborgia, Alessandra, Alfredo Niro, Valentina Pastore, Valeria Albano, Giacomo Boscia, Marina Piepoli, Camilla Di Pardo, Lorenzo Accurso Tagano, Marta Zerbinati, Luca Landini, and et al. 2023. "Biofeedback Training after Successful Inverted Internal Limiting Membrane (ILM)-Flap Technique for High Myopic Macular Hole" Journal of Clinical Medicine 12, no. 16: 5188. https://doi.org/10.3390/jcm12165188
APA StyleSborgia, A., Niro, A., Pastore, V., Albano, V., Boscia, G., Piepoli, M., Di Pardo, C., Accurso Tagano, L., Zerbinati, M., Landini, L., Pignataro, M. G., Petruzzella, G., Donghia, R., Alqahtani, A. S., Coassin, M., Dell’Omo, R., Boscia, F., Alessio, G., & Sborgia, G. (2023). Biofeedback Training after Successful Inverted Internal Limiting Membrane (ILM)-Flap Technique for High Myopic Macular Hole. Journal of Clinical Medicine, 12(16), 5188. https://doi.org/10.3390/jcm12165188







