Fetal Cardiac Hemodynamic and Sonographic Anomalies in Maternal COVID-19 Infection Depending on Vaccination Status—Polish Multicenter Cohort Study
Abstract
1. Introduction
2. Material and Methods
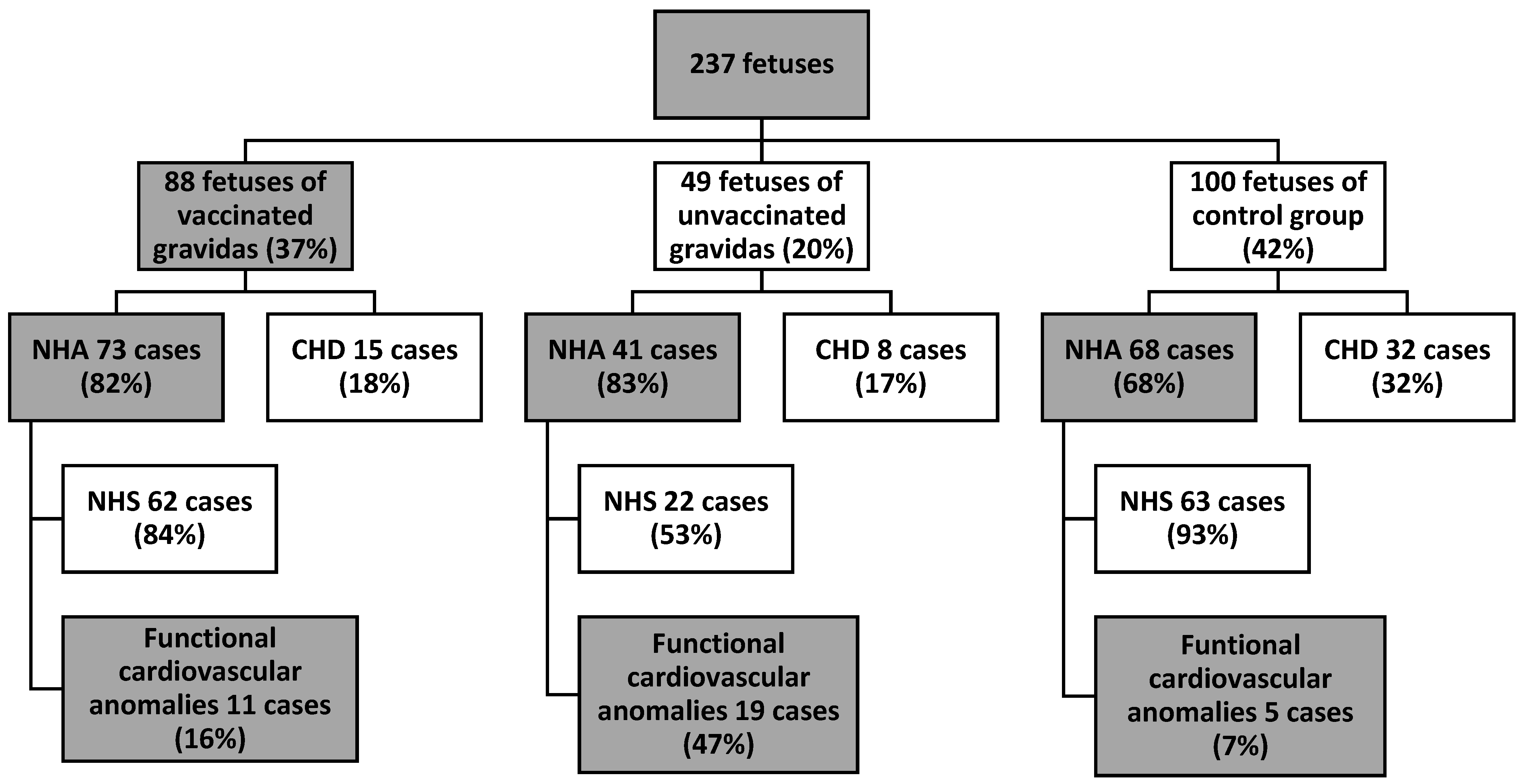
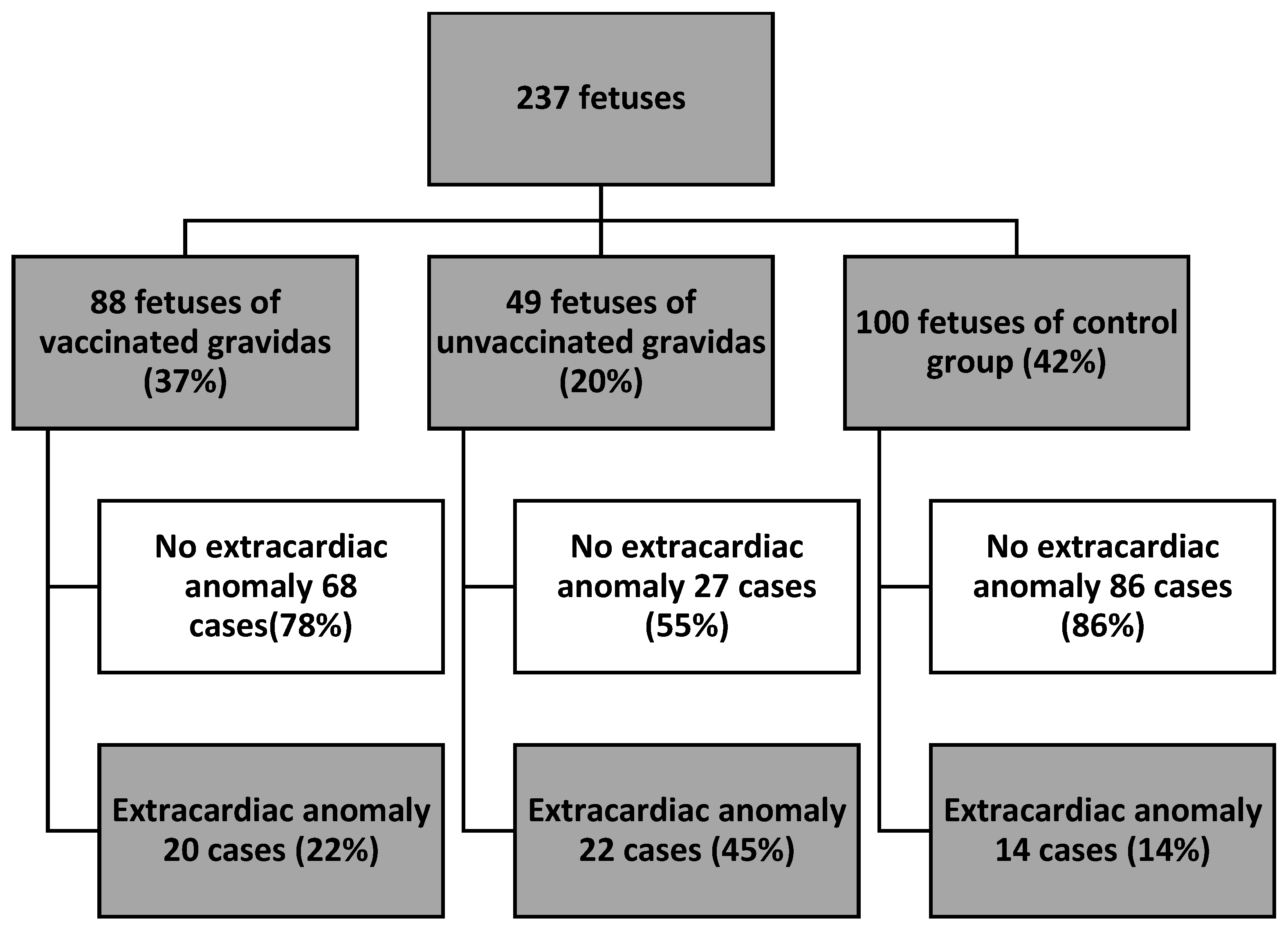
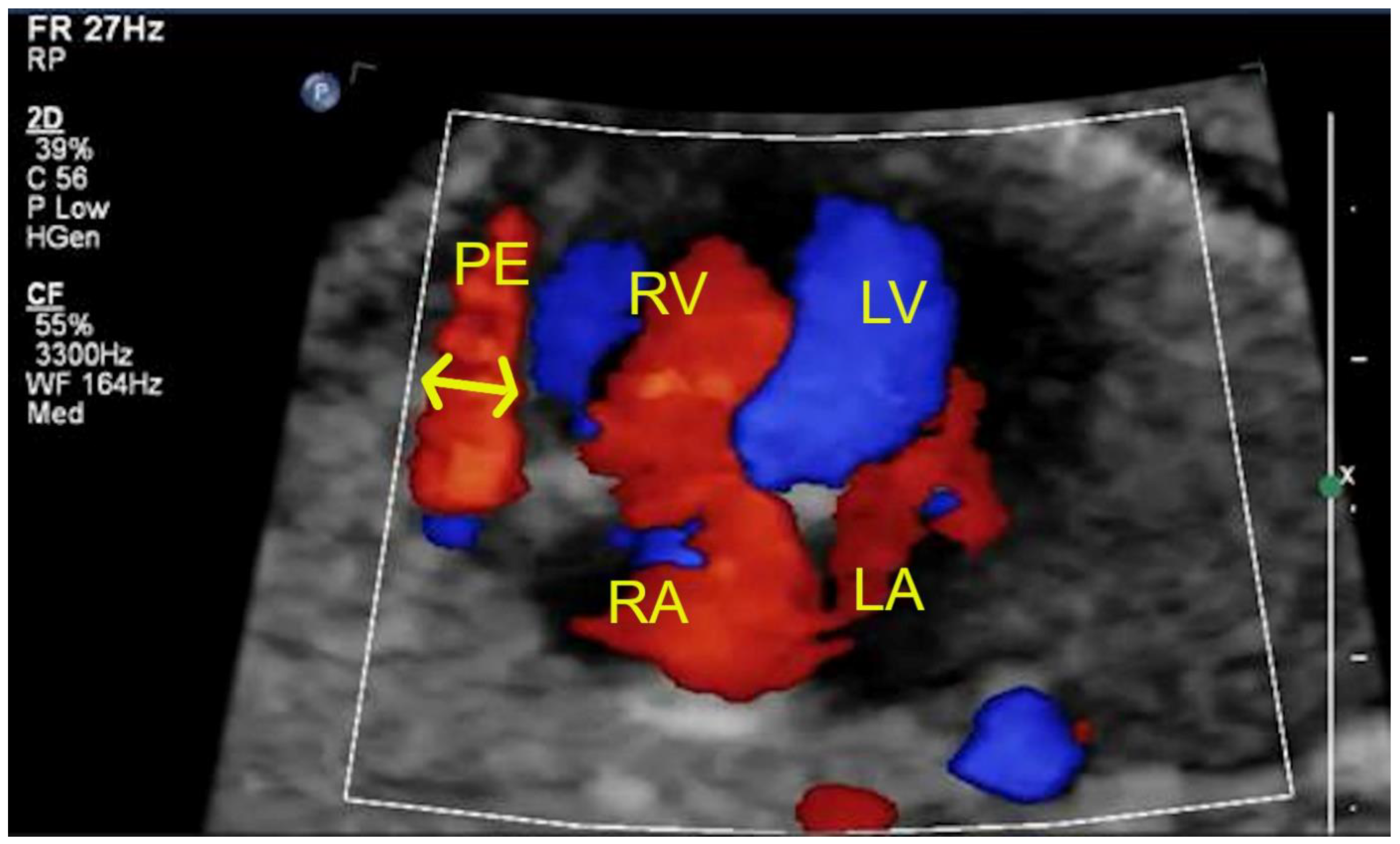
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Higgins, V.; Sohaei, D.; Diamandis, E.P.; Prassas, I. COVID-19: From an acute to chronic disease? Potential long-term health consequences. Crit. Rev. Clin. Lab. Sci. 2021, 58, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Pallarés, J.G.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Martínez, B.J.S.-A.; Bernal-Morel, E.; Courel-Ibáñez, J. Post-COVID-19 Syndrome and the Potential Benefits of Exercise. Int. J. Environ. Res. Public Health 2021, 18, 5329. [Google Scholar] [CrossRef]
- Wang, F.; Kream, R.M.; Stefano, G.B. Long-Term Respiratory and Neurological Sequelae of COVID-19. Med. Sci. Monit. 2020, 26, e928996. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Whitehead, C.L.; Walker, S.P. Consider pregnancy in COVID-19 therapeutic drug and vaccine trials. Lancet 2020, 395, e92. [Google Scholar] [CrossRef]
- Collier, A.Y.; McMahan, K.; Yu, J.; Tostanoski, L.H.; Aguayo, R.; Ansel, J.; Chandrashekar, A.; Patel, S.; Apraku Bondzie, E.; Sellers, D.; et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA 2021, 325, 2370–2380. [Google Scholar] [CrossRef]
- Vimercati, A.; Greco, P.; Loverro, G.; Lopalco, P.L.; Pansini, L.; Selvaggi, L. Maternal complications after caesarean section in HIV infected women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 90, 73–76. [Google Scholar] [CrossRef]
- Chi, J.; Gong, W.; Gao, Q. Clinical characteristics and outcomes of pregnant women with COVID-19 and the risk of vertical transmission: A systematic review. Arch. Gynecol. Obstet. 2021, 303, 337–345. [Google Scholar] [CrossRef]
- Mirbeyk, M.; Saghazadeh, A.; Rezaei, N. A systematic review of pregnant women with COVID-19 and their neonates. Arch. Gynecol. Obstet. 2021, 304, 5–38. [Google Scholar] [CrossRef]
- Jafari, M.; Pormohammad, A.; Sheikh Neshin, S.A.; Ghorbani, S.; Bose, D.; Alimohammadi, S.; Basirjafari, S.; Mohammadi, M.; Rasmussen-Ivey, C.; Razizadeh, M.H.; et al. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: A systematic review and meta-analysis. Rev. Med. Virol. 2021, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kotabagi, P.; Fortune, L.; Essien, S.; Nauta, M.; Yoong, W. Anxiety and depression levels among pregnant women with COVID-19. Acta Obstet. Gynecol. Scand. 2020, 99, 953–954. [Google Scholar] [CrossRef]
- Kordjalik, P.; Radzymińska-Chruściel, B.; Słodki, M.; Włoch, A.; Szymkiewicz-Dangel, J.; Respondek-Liberska, M.; Tobotaet, Z. The Polish National Registry for Fetal Cardiac Pathology (www.orpkp.pl)—Selected data analysis for 2013 and 2014 and comparison with data from 2004 to 2012. Prenat. Cardiol. 2015, 5, 6–12. [Google Scholar]
- Bailão, L.A.; Osborne, N.G.; Rizzi, M.C.; Bonilla-Musoles, F.; Duarte, G.; Bailão, T.C. Ultrasound markers of fetal infection part 1: Viral infections. Ultrasound Q. 2005, 21, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Malinger, G.; Lev, D.; Lerman-Sagie, T. Imaging of fetal cytomegalovirus infection. Fetal Diagn. Ther. 2011, 29, 117–126. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Ville, Y. Fetal cytomegalovirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 38, 97–107. [Google Scholar] [CrossRef]
- Sylwestrzak, O.; Respondek-Liberska, M. Prenatal ultrasound evaluation in the current era of COVID-19—Looking only for major congenital defects or subtle sonographic and echocardiographic findings, as well? Prenat. Cardiol. 2020, 10, 50–56. [Google Scholar] [CrossRef]
- Floridia, M.; Mastroiacovo, P.; Tamburrini, E.; Tibaldi, C.; Todros, T.; Crepaldi, A.; Sansone, M.; Fiscon, M.; Liuzzi, G.; Guerra, B.; et al. Birth defects in a national cohort of pregnant women with HIV infection in Italy 2001–2011. BJOG 2013, 120, 1466–1476. [Google Scholar] [CrossRef]
- Słodki, M.; Respondek-Liberska, M. Fetal echocardiography: One of the most important tools in fetal diagnosis and assessing wellbeing. J. Clin. Ultrasound 2022, 50, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Sylwestrzak, O.; Strzelecka, I.; Słodki, M.; Respondek-Liberska, M. Fetal echocardiography is not only to detect congenital heart disease, but also to monitor, especially fetuses with different pathologies. Kardiologia Polska 2022, 80, 966–967. [Google Scholar] [CrossRef]
- WAPM (World Association of Perinatal Medicine) Working Group on COVID-19. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection. Ultrasound Obstet. Gynecol. 2021, 57, 232–241, Erratum in: Ultrasound Obstet. Gynecol. 2021, 58, 496. [Google Scholar] [CrossRef]
- Sahin, D.; Tanacan, A.; Erol, S.A.; Anuk, A.T.; Yetiskin, F.D.Y.; Keskin, H.L.; Ozcan, N.; Ozgu-Erdinc, A.S.; Eyi, E.G.Y.; Yucel, A.; et al. Updated experience of a tertiary pandemic center on 533 pregnant women with COVID-19 infection: A prospective cohort study from Turkey. Int. J. Gynaecol. Obstet. 2021, 152, 328–334. [Google Scholar] [CrossRef]
- Goncu Ayhan, S.; Turgut, E.; Ozden Tokalioglu, E.; Oluklu, D.; Sakcak, B.; Uyan Hendem, D.; Tanacan, A.; Moraloglu Tekin, O.; Sahin, D. Post-COVID-19 fetal cardiac evaluation in moderate infection group of pregnant women. J. Clin. Ultrasound 2022, 50, 630–635. [Google Scholar] [CrossRef]
- Turgut, E.; Sakcak, B.; Uyan Hendem, D.; Oluklu, D.; Goncu Ayhan, S.; Sahin, D. Decreased fetal cardiac output in pregnant women with severe SARS-CoV-2 infection. Echocardiography 2022, 39, 803–810. [Google Scholar] [CrossRef]
- Mattos, S.; Chaves, M.; Freitas, C.; Severi, R.; Brissant, P.; Davino, D.; Souto-Maior, V. Fetal pericardial effusion after maternal COVID-19 vaccination: A fortuitous association? Prenat. Cardiol. 2022, 12, 20–24. [Google Scholar] [CrossRef]
- The Polish Society of Gynecologists and Obstetricians. Stanowisko PTGiP dotyczące szczepień kobiet ciężarnych przeciwko COVID19. 2021. Available online: https://www.ptgin.pl/aktualnosc/stanowisko-ptgip-dotyczace-szczepien-kobiet-ciezarnych-przeciwko-covid19 (accessed on 19 November 2022).
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. COVID-19 vaccine response in pregnant and lactating women: A cohort study. medRxiv 2021. Update in: Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar] [CrossRef]
- Garg, I.; Shekhar, R.; Sheikh, A.B.; Pal, S. COVID-19 Vaccine in Pregnant and Lactating Women: A Review of Existing Evidence and Practice Guidelines. Infect. Dis. Rep. 2021, 13, 685–699. [Google Scholar] [CrossRef]
- Skjefte, M.; Ngirbabul, M.; Akeju, O.; Escudero, D.; Hernandez-Diaz, S.; Wyszynski, D.F.; Wu, J.W. COVID-19 vaccine acceptance among pregnant women and mothers of young children: Results of a survey in 16 countries. Eur. J. Epidemiol. 2021, 36, 197–211. [Google Scholar] [CrossRef]
- Sutton, D.; D’Alton, M.; Zhang, Y.; Kahe, K.; Cepin, A.; Goffman, D.; Staniczenko, A.; Yates, H.; Burgansky, A.; Coletta, J.; et al. COVID-19 vaccine acceptance among pregnant, breastfeeding, and nonpregnant reproductive-aged women. Am. J. Obstet. Gynecol. MFM 2021, 3, 100403. [Google Scholar] [CrossRef]
- Gravett, M.G.; Witkin, S.S.; Haluska, G.J.; Edwards, J.L.; Cook, M.J.; Novy, M.J. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am. J. Obstet. Gynecol. 1994, 171, 1660–1667. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [PubMed]
| Which Pregnancy? | Number of Gravidas |
|---|---|
| 1 | 93 |
| 2 | 82 |
| 3 | 32 |
| 4 | 17 |
| 5 | 7 |
| 6 | 2 |
| 8 | 2 |
| Functional Cardiovascular Anomaly | Group V (n = 72) | Group UV (n = 42) | Control Group (n = 68) |
|---|---|---|---|
| Tricuspid insufficiency (in color and in spectral Doppler) | 2 | 8 | 2 |
| Pericardial effusion (>2 mm) | 1 | 4 | - |
| Myocardial hypertrophy (Septal thickness > 4.5 mm) | 3 | 1 | 1 |
| Bright spot | 4 | 4 | - |
| Mild cardiomegaly | 2 | - | - |
| Abnormal foramen ovale blood flow (bidirectional or left–right) | 2 | - | - |
| Reversal flow in ductus arteriosus | - | 1 | 1 |
| Arrhythmia (PAC) | - | 1 | 1 |
| Total: | 14 (18%) | 19 (47%) | 5 (8%) |
| Extracardiac Anomaly | Group V (n = 88) | Group UV (n = 49) | Control Group (n = 100) |
|---|---|---|---|
| Thick placenta (>50 mm) with abnormal 3D Doppler vascularity | 8 | 4 | 6 |
| Umbilical cord around the fetal neck | 4 | 6 | 2 |
| Oligohydramnios | 3 | 4 | 4 |
| Polihydramnios | 3 | 2 | 4 |
| Pyelectasis | 3 | 5 | 2 |
| Hiperechogenic bowels | 3 | - | 3 |
| Renal cyst | 1 | - | - |
| Lung cyst | 1 | - | - |
| Hiperechogenic lungs | 1 | - | - |
| Bowels distension | 2 | 2 | - |
| Hydrops testis | 1 | 2 | - |
| Mild ventriculomegaly | - | - | 1 |
| Visible bronchogram | - | - | 1 |
| Vacuolised placenta | - | 1 | - |
| Total: | 30 | 26 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzelecka, I.; Sylwestrzak, O.; Murlewska, J.; Węgrzynowski, J.; Leszczyńska, K.; Preis, K.; Respondek-Liberska, M. Fetal Cardiac Hemodynamic and Sonographic Anomalies in Maternal COVID-19 Infection Depending on Vaccination Status—Polish Multicenter Cohort Study. J. Clin. Med. 2023, 12, 5186. https://doi.org/10.3390/jcm12165186
Strzelecka I, Sylwestrzak O, Murlewska J, Węgrzynowski J, Leszczyńska K, Preis K, Respondek-Liberska M. Fetal Cardiac Hemodynamic and Sonographic Anomalies in Maternal COVID-19 Infection Depending on Vaccination Status—Polish Multicenter Cohort Study. Journal of Clinical Medicine. 2023; 12(16):5186. https://doi.org/10.3390/jcm12165186
Chicago/Turabian StyleStrzelecka, Iwona, Oskar Sylwestrzak, Julia Murlewska, Jerzy Węgrzynowski, Katarzyna Leszczyńska, Krzysztof Preis, and Maria Respondek-Liberska. 2023. "Fetal Cardiac Hemodynamic and Sonographic Anomalies in Maternal COVID-19 Infection Depending on Vaccination Status—Polish Multicenter Cohort Study" Journal of Clinical Medicine 12, no. 16: 5186. https://doi.org/10.3390/jcm12165186
APA StyleStrzelecka, I., Sylwestrzak, O., Murlewska, J., Węgrzynowski, J., Leszczyńska, K., Preis, K., & Respondek-Liberska, M. (2023). Fetal Cardiac Hemodynamic and Sonographic Anomalies in Maternal COVID-19 Infection Depending on Vaccination Status—Polish Multicenter Cohort Study. Journal of Clinical Medicine, 12(16), 5186. https://doi.org/10.3390/jcm12165186






