Influence of Physical Activity during Pregnancy on Type and Duration of Delivery, and Epidural Use: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Population
2.3. Intervention
2.4. Comparison
2.5. Outcome
2.6. Data Sources
- English: physical activity OR exercise OR training OR physical exercise OR fitness OR strength training OR physical intervention OR Pilates OR Yoga OR strengthening OR aerobic OR resistance training OR pelvic floor muscle training AND pregnancy OR maternal OR antenatal OR pregnant AND type of delivery OR mode of delivery OR duration of labor OR epidural OR anesthetic AND randomized clinical trial OR randomized controlled trial OR RCT OR Quasi experimental clinical trial.
- Spanish: actividad física O ejercicio O entrenamiento O ejercicio físico O fitness O entrenamiento de fuerza O intervención de actividad física O Pilates O Yoga O fortalecimiento O aeróbico O entrenamiento de resistencia O fortalecimiento del suelo pélvico Y embarazo O materno O antenatal O embarazada Y tipo de parto O modo de parto O duración del parto O epidural O anestesia Y ensayo clínico aleatorizado O ensayo controlado aleatorizado O ECA O cuasiexperimental.
2.7. Study Selection and Data Extraction
2.8. Quality of Evidence and Risk of Bias Assessments
2.9. Statistical Analysis
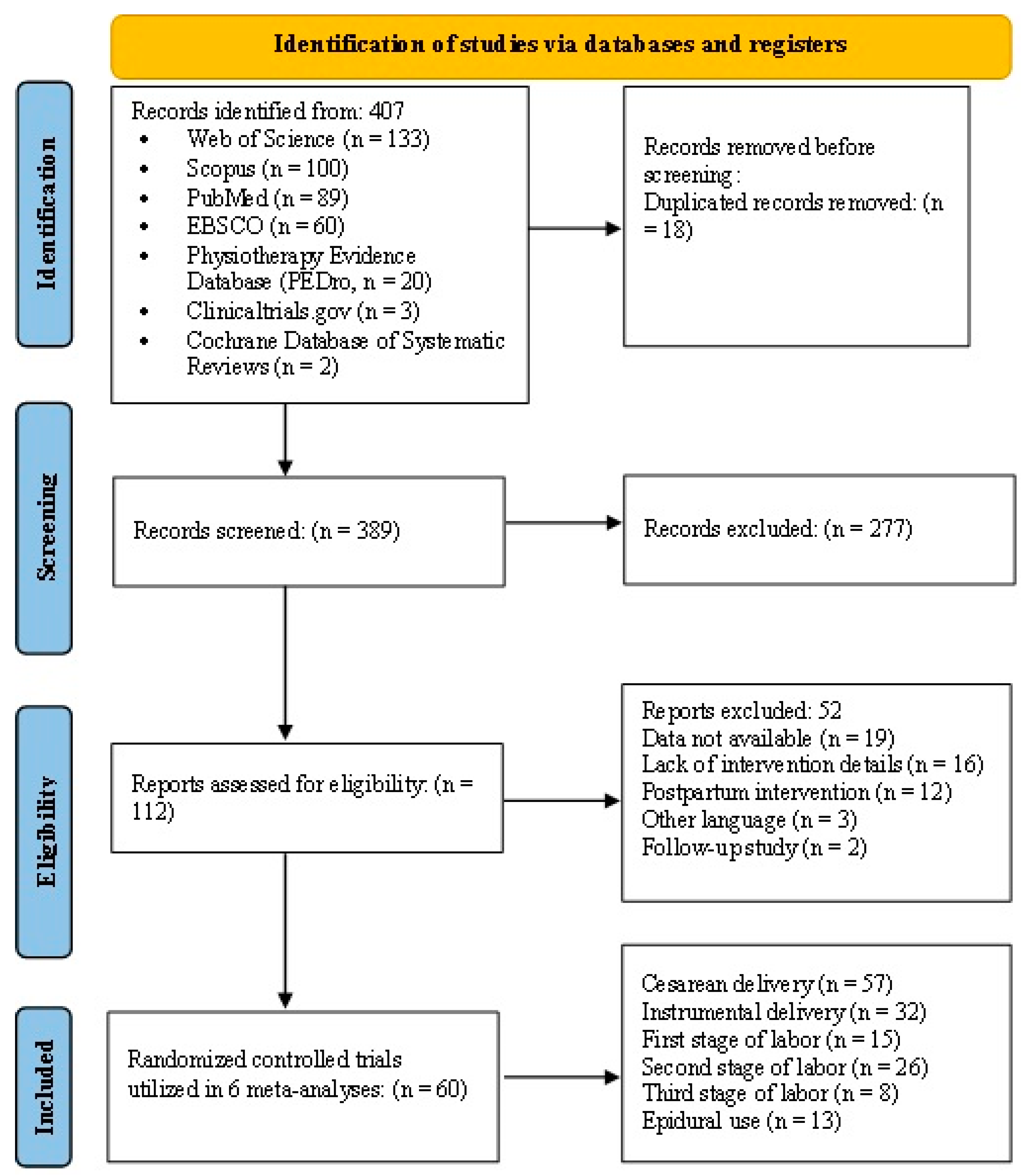
3. Results
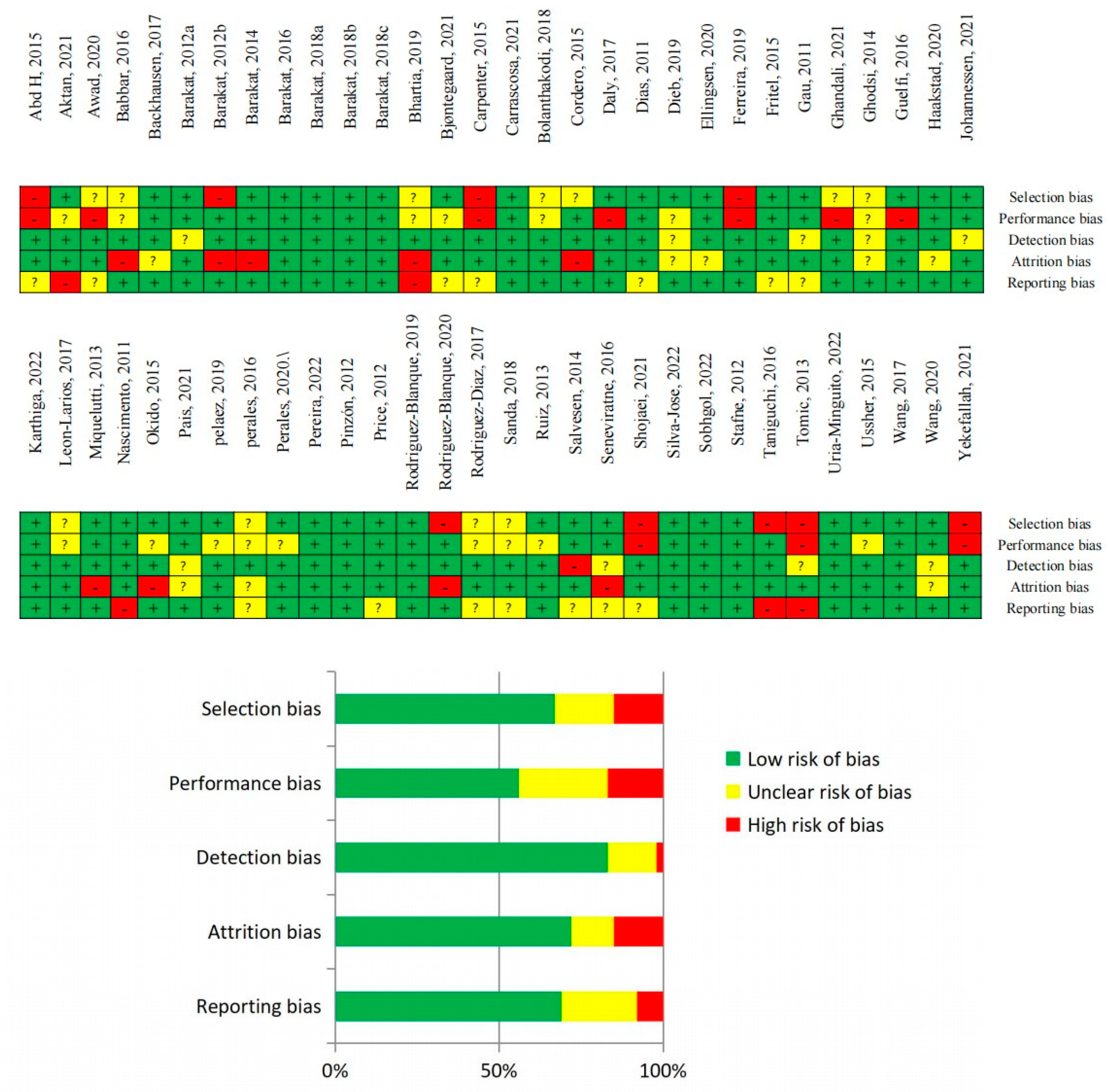
3.1. Certainty of Evidence and Risk of Bias
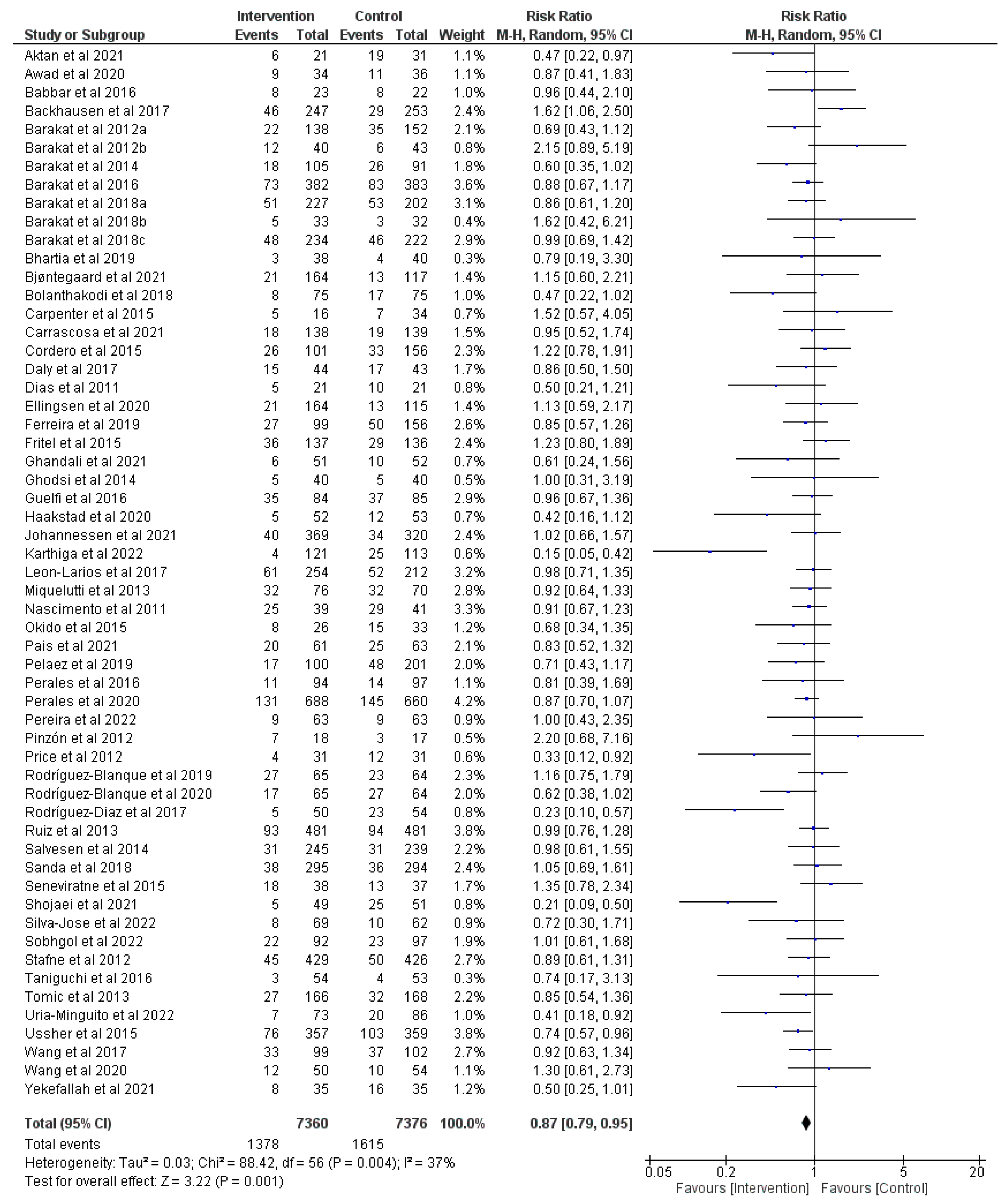
3.2. Effect of Prenatal Physical Activity on Cesarean Delivery
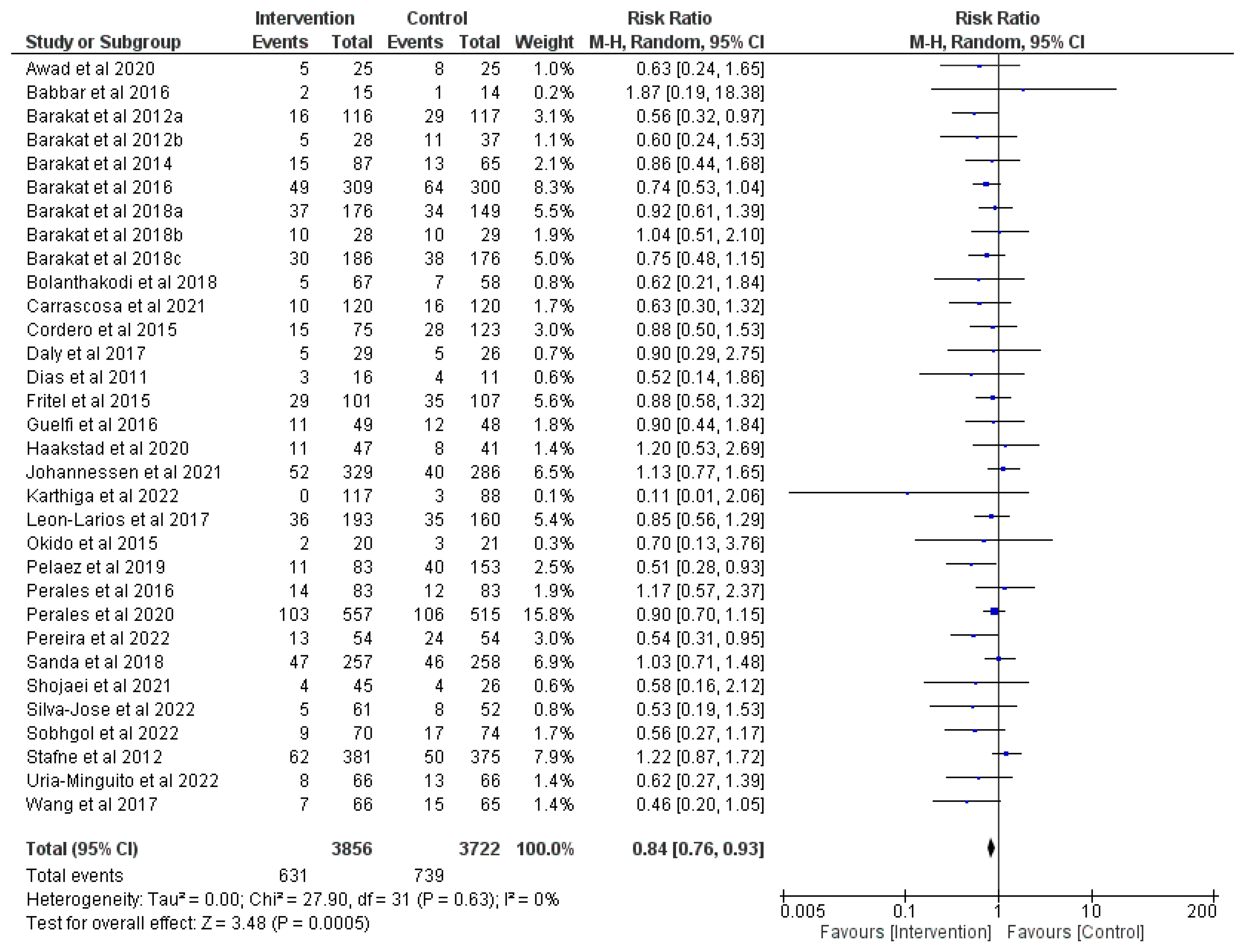
3.3. Effect of Prenatal Physical Activity on Instrumental Delivery
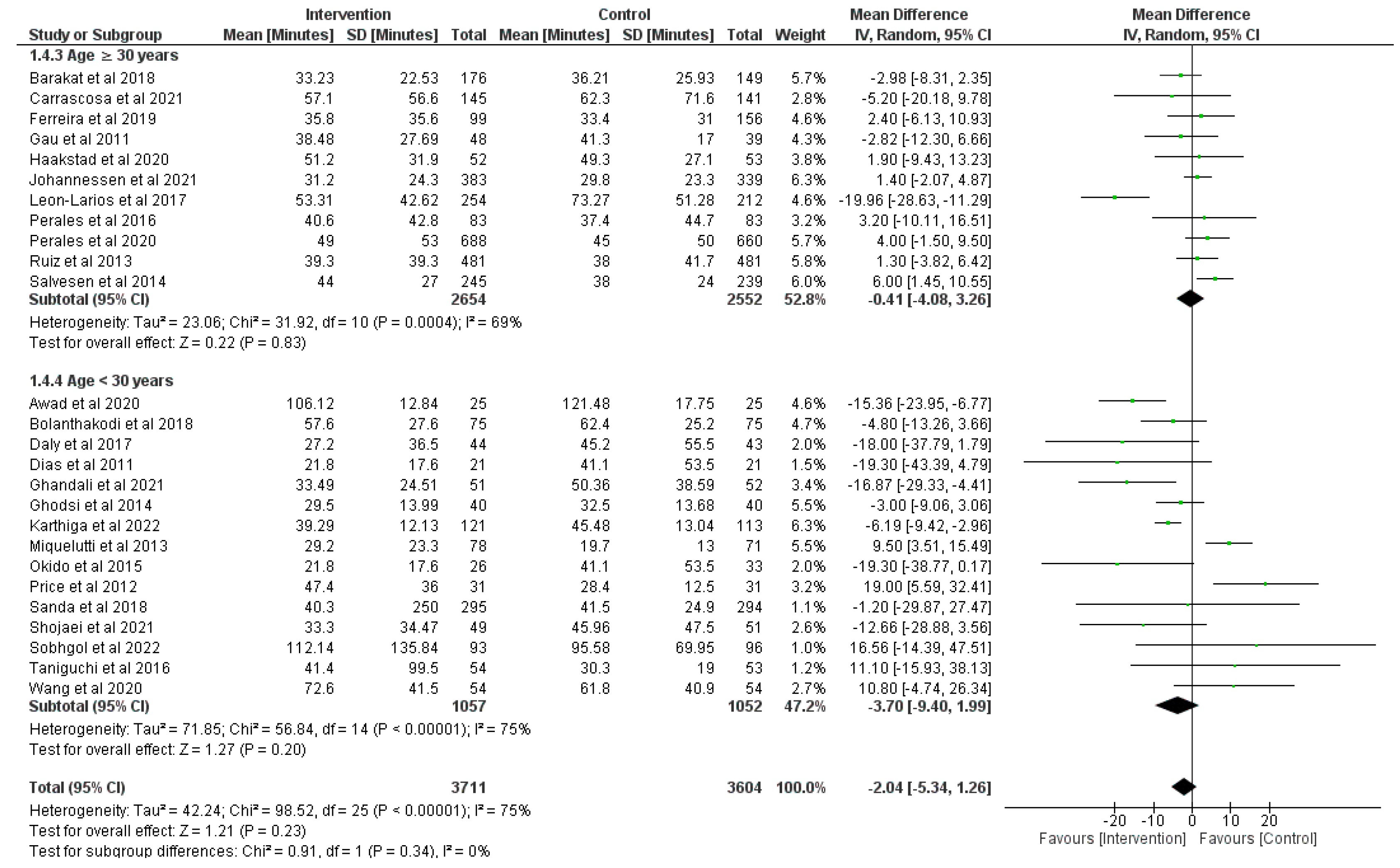
3.4. Effect of Physical Activity during Pregnancy on Duration of the First Stage of Labor
3.5. Effect of Physical Activity during Pregnancy on Duration of the Second Stage of Labor
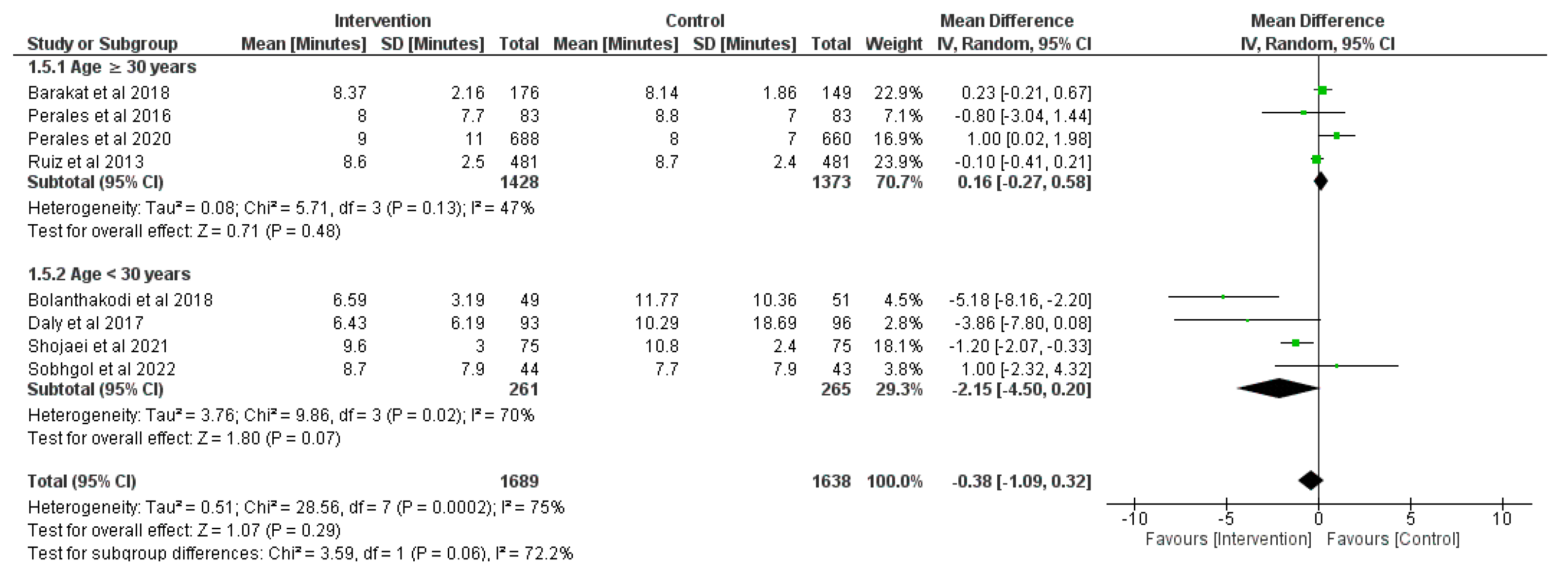
3.6. Effect of Physical Activity during Pregnancy on Duration of the Third Stage of Labor
3.7. Effect of Physical Activity during Pregnancy on Epidural Use
4. Discussion
Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burcher, P.; Gabriel, J.L.; Campo-Engelstein, L.; Kiley, K.C. The case against cesarean delivery on maternal request in labor. Obstet. Gynecol. 2013, 122, 684–687. [Google Scholar] [CrossRef]
- Halpern, S. SOGC Joint Policy Statement on Normal Childbirth. J. Obstet. Gynaecol. Can. 2009, 31, 602. [Google Scholar] [CrossRef]
- Betran, A.P.; Ye, J.; Moller, A.-B.; Souza, J.P.; Zhang, J. Trends and projections of caesarean section rates: Global and regional estimates. BMJ Global. Health 2021, 6, e005671. [Google Scholar] [CrossRef]
- Poyatos-León, R.; García-Hermoso, A.; Sanabria-Martínez, G.; Álvarez-Bueno, C.; Sánchez-López, M.; Martínez-Vizcaíno, V. Effects of exercise during pregnancy on mode of delivery: A meta-analysis. Acta Obstet. Gyn. Scan. 2015, 94, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, A.H.; Taylor, A.W.; Wilson, D.H.; Wilson, D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG Int. J. Obstet. Gynaecol. 2000, 107, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Sheiner, E.; Levy, A.; Feinstein, U.; Hallak, M.; Mazor, M. Risk factors and outcome of failure to progress during the first stage of labor: A population-based study. Acta Obstet. Gyn. Scan. 2002, 81, 222–226. [Google Scholar] [CrossRef]
- Khor, L.; Jeskins, G.; Cooper, G.; Paterson-Brown, S. National obstetric anaesthetic practice in the UK 1997/1998. Anesth 2000, 55, 1168–1172. [Google Scholar] [CrossRef]
- Anim-Somuah, M.; Smyth, R.; Howell, C. Epidural versus non-epidural or no analgesia in labour. Cochrane Database Syst. Rev. 2018, 5, CD000331. [Google Scholar] [PubMed]
- Awad, E.; Mobark, A.; Hamada, H.A.; Yousef, A.M.; El Nahas, E.M. Effect of antenatal exercises on bearing down in primiparous under epidural anesthesia during labor: A randomized controlled trial. Eur. Asian J. Biosci. 2020, 14, 1079–1085. [Google Scholar]
- Davenport, M.H.; Ruchat, S.-M.; Sobierajski, F.; Poitras, V.J.; Gray, C.E.; Yoo, C.; Skow, R.J.; Garcia, A.J.; Barrowman, N.; Meah, V.L.; et al. Impact of prenatal exercise on maternal harms, labour and delivery outcomes: A systematic review and meta-analysis. Br. J. Sports Med. 2019, 53, 99–107. [Google Scholar] [CrossRef]
- Domenjoz, I.; Kayser, B.; Boulvain, M. Effect of physical activity during pregnancy on mode of delivery. Am. J. Obstet. Gynecol. 2014, 211, e401–e411. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, L.; Wu, X.; Zhou, C. The Association between Physical Exercise during Pregnancy and Maternal and Neonatal Health Outcomes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Comput. Math Methods Med. 2022, 2022, 3462392. [Google Scholar] [CrossRef] [PubMed]
- Masoud, A.T.; AbdelGawad, M.M.; Elshamy, N.H.; Mohamed, O.M.; Hashem, Z.Y.; Abd Eltawab, A.K.; Samy, A.; Abbas, A.M. The effect of antenatal exercise on delivery outcomes: A systematic review and meta-analysis of randomized controlled trials. J. Gynecol. Obstet. Hum. 2020, 49, 101736. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- ACOG Committee on Obstetric Practice. “ACOG Committee Opinion. Number 267, January 2002.” Exercise during pregnancy and the postpartum period. Obstet. Gynecol. 2002, 99, 171–173. [Google Scholar]
- ACOG Committee on Obstetric Practice. “ACOG Committee Opinion. Number 650, December 2015.” Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet. Gynecol. 2015, 126, e135–e142. [Google Scholar]
- Granholm, A.; Alhazzani, W.; Møller, M.H. Use of the GRADE approach in systematic reviews and guidelines. Br. J. Anaesth. 2019, 123, 554–559. [Google Scholar] [CrossRef]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; pp. 205–228. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Hedges, L.V.; Tipton, E.; Johnson, M.C. Robust variance estimation in meta-regression with dependent effect size estimates. Res. Synth. Methods 2010, 1, 39–65. [Google Scholar] [CrossRef]
- Abd El fttah Ali, H. Effects of prenatal perineal massage and Kegel exercise on the episiotomy rate. IOSR-JNHS 2015, 4, 61–70. [Google Scholar]
- Aktan, B.; Kayikcioglu, F.; Akbayrak, T. The comparison of the effects of clinical Pilates exercises with and without childbirth training on pregnancy and birth results. Int. J. Clin. Pract. 2021, 75, e14516. [Google Scholar] [CrossRef]
- Babbar, S.; Hill, J.B.; Williams, K.B.; Pinon, M.; Chauhan, S.P.; Maulik, D. Acute feTal behavioral Response to prenatal Yoga: A single, blinded, randomized controlled trial (TRY yoga). Am. J. Obstet. Gynecol. 2016, 214, 399.e1–399.e8. [Google Scholar] [CrossRef] [PubMed]
- Backhausen, M.G.; Tabor, A.; Albert, H.; Rosthoj, S.; Damm, P.; Hegaard, H.K. The effects of an unsupervised water exercise program on low back pain and sick leave among healthy pregnant women—A randomised controlled trial. PLoS ONE 2017, 12, e0182114. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Cordero, Y.; Coteron, J.; Luaces, M.; Montejo, R. Exercise during pregnancy improves maternal glucose screen at 24-28 weeks: A randomised controlled trial. Br. J. Sports Med. 2012, 46, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Pelaez, M.; Lopez, C.; Montejo, R.; Coteron, J. Exercise during pregnancy reduces the rate of cesarean and instrumental deliveries: Results of a randomized controlled trial. J. Matern.-Fetal Neonatal Med. 2012, 25, 2372–2376. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Perales, M.; Bacchi, M.; Coteron, J.; Refoyo, I. A program of exercise throughout pregnancy. Is it safe to mother and newborn? Am. J. Health Promot. 2014, 29, 2–8. [Google Scholar] [CrossRef]
- Barakat, R.; Pelaez, M.; Cordero, Y.; Perales, M.; Lopez, C.; Coteron, J.; Mottola, M.F. Exercise during pregnancy protects against hypertension and macrosomia: Randomized clinical trial. Am. J. Obstet. Gynecol. 2016, 214, 649.e1–649.e8. [Google Scholar] [CrossRef]
- Barakat, R.; Franco, E.; Perales, M.; Lopez, C.; Mottola, M.F. Exercise during pregnancy is associated with a shorter duration of labor. A randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 224, 33–40. [Google Scholar] [CrossRef]
- Barakat, R.; Vargas, M.; Brik, M.; Fernandez, I.; Gil, J.; Coteron, J.; Santacruz, B. Does Exercise During Pregnancy Affect Placental Weight?: A Randomized Clinical Trial. Eval. Health Prof. 2018, 41, 400–414. [Google Scholar] [CrossRef]
- Barakat, R.; Refoyo, I.; Coteron, J.; Franco, E. Exercise during pregnancy has a preventative effect on excessive maternal weight gain and gestational diabetes. A randomized controlled trial. Braz. J. Phys. Ther. 2018, 23, 148–155. [Google Scholar] [CrossRef]
- Bhartia, N.; Jain, S.; Shankar, N.; Rajaram, S.; Gupta, M. Effects of antenatal yoga on maternal stress and clinical outcomes in north indian women: A randomised controlled trial. J. Indian Acad. Clin. Med. 2019, 20, 11. [Google Scholar]
- Bjontegaard, K.A.; Stafne, S.N.; Morkved, S.; Salvesen, K.A.; Evensen, K.A.I. Body mass index and physical activity in seven-year-old children whose mothers exercised during pregnancy: Follow-up of a multicentre randomised controlled trial. BMC Pediatr. 2021, 21, 496. [Google Scholar] [CrossRef] [PubMed]
- Bolanthakodi, C.; Raghunandan, C.; Saili, A.; Mondal, S.; Saxena, P. Prenatal Yoga: Effects on Alleviation of Labor Pain and Birth Outcomes. J. Altern. Complement. Med. 2018, 24, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.E.; Emery, S.J.; Uzun, O.; D’Silva, L.A.; Lewis, M.J. Influence of antenatal physical exercise on haemodynamics in pregnant women: A flexible randomisation approach. BMC Pregnancy Childbirth 2015, 15, 186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carrascosa, M.D.C.; Navas, A.; Artigues, C.; Ortas, S.; Portells, E.; Soler, A.; Bennasar-Veny, M.; Leiva, A.; Aquanatal, T. Effect of aerobic water exercise during pregnancy on epidural use and pain: A multi-centre, randomised, controlled trial. Midwifery 2021, 103, 103105. [Google Scholar] [CrossRef]
- Cordero, Y.; Mottola, M.F.; Vargas, J.; Blanco, M.; Barakat, R. Exercise Is Associated with a Reduction in Gestational Diabetes Mellitus. Med. Sci. Sports Exerc. 2015, 47, 1328–1333. [Google Scholar] [CrossRef]
- Daly, N.; Farren, M.; McKeating, A.; O’Kelly, R.; Stapleton, M.; Turner, M.J. A Medically Supervised Pregnancy Exercise Intervention in Obese Women: A Randomized Controlled Trial. Obstet. Gynecol. 2017, 130, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.A.; Driusso, P.; Aita, D.L.; Quintana, S.M.; Bø, K.; Ferreira, C.H. Effect of pelvic floor muscle training on labour and newborn outcomes: A randomized controlled trial. Braz. J. Phys. Ther. 2011, 15, 487–493. [Google Scholar] [CrossRef]
- Dieb, A.S.; Shoab, A.Y.; Nabil, H.; Gabr, A.; Abdallah, A.A.; Shaban, M.M.; Attia, A.H. Perineal massage and training reduce perineal trauma in pregnant women older than 35 years: A randomized controlled trial. Int. Urogynecol. J. 2020, 31, 613–619. [Google Scholar] [CrossRef]
- Ellingsen, M.S.; Pettersen, A.; Stafne, S.N.; Morkved, S.; Salvesen, K.A.; Evensen, K. Neurodevelopmental outcome in 7-year-old children is not affected by exercise during pregnancy: Follow up of a multicentre randomised controlled trial. BJOG 2020, 127, 508–517. [Google Scholar] [CrossRef]
- Ferreira, C.L.M.; Guerra, C.M.L.; Silva, A.I.T.J.; Rosário, H.R.V.d.; de Oliveira Pereira, M.B.F.L. Exercise in pregnancy: The impact of an intervention program in the duration of labor and mode of delivery. Rev. Bras. Ginecol. Obstet. 2019, 41, 68–75. [Google Scholar] [CrossRef]
- Fritel, X.; De Tayrac, R.; Bader, G.; Savary, D.; Gueye, A.; Deffieux, X.; Fernandez, H.; Richet, C.; Guilhot, J.; Fauconnier, A. Preventing urinary incontinence with supervised prenatal pelvic floor exercises: A randomized controlled trial. Obstet. Gynecol. 2015, 126, 370–377. [Google Scholar] [CrossRef]
- Gau, M.-L.; Chang, C.-Y.; Tian, S.-H.; Lin, K.-C. Effects of birth ball exercise on pain and self-efficacy during childbirth: A randomised controlled trial in Taiwan. Midwifery 2011, 27, e293–e300. [Google Scholar] [CrossRef] [PubMed]
- Ghandali, N.Y.; Iravani, M.; Habibi, A.; Cheraghian, B. The effectiveness of a Pilates exercise program during pregnancy on childbirth outcomes: A randomised controlled clinical trial. BMC Pregnancy Childbirth 2021, 21, 480. [Google Scholar] [CrossRef] [PubMed]
- Ghodsi, Z.; Asltoghiri, M. Effects of aerobic exercise training on maternal and neonatal outcome: A randomized controlled trial on pregnant women in Iran. J. Pak. Med. Assoc. 2014, 64, 1053–1056. [Google Scholar] [PubMed]
- Guelfi, K.J.; Ong, M.J.; Crisp, N.A.; Fournier, P.A.; Wallman, K.E.; Grove, J.R.; Doherty, D.A.; Newnham, J.P. Regular Exercise to Prevent the Recurrence of Gestational Diabetes Mellitus: A Randomized Controlled Trial. Obstet. Gynecol. 2016, 128, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Haakstad, L.A.; Bø, K. The marathon of labour—Does regular exercise training influence course of labour and mode of delivery? Secondary analysis from a randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 251, 8–13. [Google Scholar] [CrossRef]
- Johannessen, H.H.; Froshaug, B.E.; Lysaker, P.J.G.; Salvesen, K.A.; Lukasse, M.; Morkved, S.; Stafne, S.N. Regular antenatal exercise including pelvic floor muscle training reduces urinary incontinence 3 months postpartum-Follow up of a randomized controlled trial. Acta Obstet. Gynecol. Scand. 2021, 100, 294–301. [Google Scholar] [CrossRef]
- Karthiga, K.; Pal, G.K.; Dasari, P.; Nanda, N.; Velkumary, S.; Chinnakali, P.; Renugasundari, M.; Harichandrakumar, K.T. Effects of yoga on cardiometabolic risks and fetomaternal outcomes are associated with serum nitric oxide in gestational hypertension: A randomized control trial. Sci. Rep. 2022, 12, 11732. [Google Scholar] [CrossRef]
- Leon-Larios, F.; Corrales-Gutierrez, I.; Casado-Mejia, R.; Suarez-Serrano, C. Influence of a pelvic floor training programme to prevent perineal trauma: A quasi-randomised controlled trial. Midwifery 2017, 50, 72–77. [Google Scholar] [CrossRef]
- Miquelutti, M.A.; Cecatti, G.J.; Makuch, M.Y. Evaluation of a birth preparation program on lumbopelvic pain, urinary incontinence, anxiety and exercise: A randomized controlled trial. BMC Pregnancy Childbirth 2013, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, S.L.; Surita, F.G.; Parpinelli, M.A.; Siani, S.; Pinto e Silva, J.L. The effect of an antenatal physical exercise programme on maternal/perinatal outcomes and quality of life in overweight and obese pregnant women: A randomised clinical trial. BJOG 2011, 118, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Okido, M.M.; Valeri, F.L.; Martins, W.P.; Ferreira, C.H.; Duarte, G.; Cavalli, R.C. Assessment of foetal wellbeing in pregnant women subjected to pelvic floor muscle training: A controlled randomised study. Int. Urogynecol. J. 2015, 26, 1475–1481. [Google Scholar] [CrossRef]
- Pais, M.; Pai, M.V.; Kamath, A.; Bhat, R.; Bhat, P.; Joisa, G.H. A Randomized Controlled Trial on the Efficacy of Integrated Yoga on Pregnancy Outcome. Holist. Nurs. Pract. 2021, 35, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Gonzalez-Cerron, S.; Montejo, R.; Barakat, R. Protective effect of exercise in pregnant women including those who exceed weight gain recommendations: A randomized controlled trial. Mayo Clin. Proc. 2019, 94, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Perales, M.; Calabria, I.; Lopez, C.; Franco, E.; Coteron, J.; Barakat, R. Regular Exercise Throughout Pregnancy Is Associated With a Shorter First Stage of Labor. Am. J. Health Promot. 2016, 30, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Perales, M.; Valenzuela, P.L.; Barakat, R.; Cordero, Y.; Pelaez, M.; Lopez, C.; Ruilope, L.M.; Santos-Lozano, A.; Lucia, A. Gestational Exercise and Maternal and Child Health: Effects until Delivery and at Post-Natal Follow-up. J. Clin. Med. 2020, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.B.; Silva, R.; Ayres-de-Campos, D.; Clode, N. Physical exercise at term for enhancing the spontaneous onset of labor: A randomized clinical trial. J. Matern. Fetal. Neonatal. Med. 2022, 35, 775–779. [Google Scholar] [CrossRef]
- Pinzón, D.C.; Zamora, K.; Martínez, J.H.; Floréz-López, M.E.; Aguilar de Plata, A.C.; Mosquera, M.; Ramírez-Vélez, R. Type of delivery and gestational age is not affected by pregnant Latin-American women engaging in vigorous exercise. A secondary analysis of data from a controlled randomized trial. Rev. Salud Pública 2012, 14, 731–743. [Google Scholar]
- Price, B.B.; Amini, S.B.; Kappeler, K. Exercise in pregnancy: Effect on fitness and obstetric outcomes-a randomized trial. Med. Sci. Sports Exerc. 2012, 44, 2263–2269. [Google Scholar] [CrossRef]
- Rodriguez-Blanque, R.; Sanchez-Garcia, J.C.; Sanchez-Lopez, A.M.; Exposito-Ruiz, M.; Aguilar-Cordero, M.J. Randomized Clinical Trial of an Aquatic Physical Exercise Program During Pregnancy. J. Obstet. Gynecol. Neonatal. Nurs. 2019, 48, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Blanque, R.; Aguilar-Cordero, M.J.; Marin-Jimenez, A.E.; Nunez-Negrillo, A.M.; Sanchez-Lopez, A.M.; Sanchez-Garcia, J.C. Influence of a Water-Based Exercise Program in the Rate of Spontaneous Birth: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 795. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Díaz, L.; Ruiz-Frutos, C.; Vázquez-Lara, J.M.; Ramírez-Rodrigo, J.; Villaverde-Gutiérrez, C.; Torres-Luque, G. Effectiveness of a physical activity programme based on the Pilates method in pregnancy and labour. Enferm. Clin. 2017, 27, 271–277. [Google Scholar] [CrossRef]
- Ruiz, J.R.; Perales, M.; Pelaez, M.; Lopez, C.; Lucia, A.; Barakat, R. Supervised exercise–based intervention to prevent excessive gestational weight gain: A randomized controlled trial. Mayo Clin. Proc. 2013, 88, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Salvesen, K.Å.; Stafne, S.N.; Eggebø, T.M.; Mørkved, S. Does regular exercise in pregnancy influence duration of labor? A secondary analysis of a randomized controlled trial. Acta Obstet. Gynecol. Scand. 2014, 93, 73–79. [Google Scholar] [CrossRef]
- Sanda, B.; Vistad, I.; Sagedal, L.R.; Haakstad, L.A.H.; Lohne-Seiler, H.; Torstveit, M.K. What is the effect of physical activity on duration and mode of delivery? Secondary analysis from the Norwegian Fit for Delivery trial. Acta Obstet. Gyn. Scan. 2018, 97, 861–871. [Google Scholar] [CrossRef]
- Seneviratne, S.N.; Jiang, Y.; Derraik, J.; McCowan, L.; Parry, G.K.; Biggs, J.B.; Craigie, S.; Gusso, S.; Peres, G.; Rodrigues, R.O.; et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: A randomised controlled trial. BJOG 2016, 123, 588–597. [Google Scholar] [CrossRef]
- Shojaei, B.; Loripoor, M.; Sheikhfathollahi, M.; Aminzadeh, F. The effect of walking during late pregnancy on the outcomes of labor and delivery: A randomized clinical trial. J. Educ. Health Promt. 2021, 10, 277. [Google Scholar]
- Silva-Jose, C.; Sánchez-Polán, M.; Barakat, R.; Díaz-Blanco, Á.; Mottola, M.F.; Refoyo, I. A Virtual Exercise Program throughout Pregnancy during the COVID-19 Pandemic Modifies Maternal Weight Gain, Smoking Habits and Birth Weight—Randomized Clinical Trial. J. Clin. Med. 2022, 11, 4045. [Google Scholar] [CrossRef]
- Sobhgol, S.S.; Smith, C.A.; Thomson, R.; Dahlen, H.G. The effect of antenatal pelvic floor muscle exercise on sexual function and labour and birth outcomes: A randomised controlled trial. Women Birth 2022, 35, e607–e614. [Google Scholar] [CrossRef]
- Stafne, S.N.; Salvesen, K.A.; Romundstad, P.R.; Eggebo, T.M.; Carlsen, S.M.; Morkved, S. Regular exercise during pregnancy to prevent gestational diabetes: A randomized controlled trial. Obstet. Gynecol. 2012, 119, 29–36. [Google Scholar] [CrossRef]
- Taniguchi, C.; Sato, C. Home-based walking during pregnancy affects mood and birth outcomes among sedentary women: A randomized controlled trial. Int. J. Nurs. Pract. 2016, 22, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Tomic, V.; Sporis, G.; Tomic, J.; Milanovic, Z.; Zigmundovac-Klaic, D.; Pantelic, S. The effect of maternal exercise during pregnancy on abnormal fetal growth. Croat. Med. J. 2013, 54, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Uria-Minguito, A.; Silva-Jose, C.; Sanchez-Polan, M.; Diaz-Blanco, A.; Garcia-Benasach, F.; Carrero Martinez, V.; Alzola, I.; Barakat, R. The Effect of Online Supervised Exercise throughout Pregnancy on the Prevention of Gestational Diabetes in Healthy Pregnant Women during COVID-19 Pandemic: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 14104. [Google Scholar] [CrossRef] [PubMed]
- Ussher, M.; Lewis, S.; Aveyard, P.; Manyonda, I.; West, R.; Lewis, B.; Marcus, B.; Riaz, M.; Taylor, A.H.; Barton, P.; et al. The London Exercise And Pregnant smokers (LEAP) trial: A randomised controlled trial of physical activity for smoking cessation in pregnancy with an economic evaluation. Health Technol. Assess. 2015, 19, vii–xxiv. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Y.; Zhang, X.; Zhang, Y.; Xu, Q.; Sun, Y.; Su, S.; Zhang, L.; Liu, C.; Feng, Y.; et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. Obstet. Gynecol. 2017, 216, 340–351. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Luo, J.; Chen, Z.; Feng, S. Effect of app-based audio guidance pelvic floor muscle training on treatment of stress urinary incontinence in primiparas: A randomized controlled trial. Int. J. Nurs. Stud. 2020, 104, 103527. [Google Scholar] [CrossRef]
- Yekefallah, L.; Namdar, P.; Dehghankar, L.; Golestaneh, F.; Taheri, S.; Mohammadkhaniha, F. The effect of yoga on the delivery and neonatal outcomes in nulliparous pregnant women in Iran: A clinical trial study. BMC Pregnancy Childbirth 2021, 21, 351. [Google Scholar] [CrossRef]
- Laredo-Aguilera, J.A.; Gallardo-Bravo, M.; Rabanales-Sotos, J.A.; Cobo-Cuenca, A.I.; Carmona-Torres, J.M. Physical activity programs during pregnancy are effective for the control of gestational diabetes mellitus. Int. J. Environ. Res. Public Health 2020, 17, 6151. [Google Scholar] [CrossRef]
- Hamann, V.; Deruelle, P.; Enaux, C.; Deguen, S.; Kihal-Talantikite, W. Physical activity and gestational weight gain: A systematic review of observational studies. BMC Public Health 2022, 22, 1951. [Google Scholar] [CrossRef]
- Gregg, V.H.; Ferguson, J.E., 2nd. Exercise in Pregnancy. Clin. Sports Med. 2017, 36, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, J.M.; Heba, M.; Hutchinson, J. Stages of Labor; StatPearls: Tampa, FL, USA, 2023. [Google Scholar]


| Ref | Country | N | IG | CG | Physical Activity Intervention | Main Variables | Secondary Variables | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freq | Intens | Durat | Type | Superv | Time | Adh | |||||||
| Abd et al., 2015 [21] | Egypt | 180 | 110 | 70 | 7 | Low | 10–15 | Perineal massage | No | 4 w | - | Episiotomy Perineal tear | Type of delivery |
| 5 | Pelvic floor muscle training | ||||||||||||
| Aktan et al., 2021 [22] | Turkey | 43 | 21 | 22 | 2 | Mod | 60 | Clinical Pilates exercise | Yes | 8 w | - | General anxiety, gestational weight gain | Type of delivery, birth weight |
| Awad et al., 2020 [9] | Egypt | 50 | 25 | 25 | 3 | Mod | 60 | Aerobic, pelvic floor exercises | Yes | 22 w | - | Duration of the second stage labor | Type of delivery and Apgar scores |
| 3 | 35 | No | |||||||||||
| Babbar et al., 2016 [23] | USA | 46 | 23 | 23 | 3 | Mod | 60 | Yoga | Yes | 8 w | 80% | Umbilical artery, type of delivery, birth weight | Gestational weight gain |
| Backhausen et al., 2017 [24] | Denmark | 516 | 258 | 258 | 2 | Low | 70 | Water exercises | No | 12 w | 76% | Low back pain, birth weight | Type of delivery |
| Barakat et al., 2012 [25] | Spain | 290 | 138 | 152 | 3 | Mod | 40–45 | Aerobic exercise | Yes | 28 w | - | Type of delivery | Gestational weight gain birth weight |
| Barakat et al., 2012 [26] | Spain | 83 | 40 | 43 | 3 | Low-Mod | 35–45 | Land aerobic and aquatic activity | Yes | 28 w | - | Gestational weight gain and gestational diabetes | Gestational age, type of delivery, birth weight and Apgar score |
| Barakat et al., 2014 [27] | Spain | 200 | 107 | 93 | 3 | Low-Mod | 55–60 | Aerobic exercise, pelvic floor muscle training | Yes | 28 w | 80% | Gestational age, gestational weight gain, type of delivery, gestational diabetes | Birth weight, head circumference |
| Barakat et al., 2016 [28] | Spain | 765 | 382 | 383 | 3 | Mod | 50–55 | Aerobic, strength, and flexibility exercise | Yes | 28 w | 80% | Hypertension | Type of delivery, gestational weight gain, birth weight |
| Barakat et al., 2018 [29] | Spain | 429 | 227 | 202 | 3 | Mod | 55–60 | Aerobic exercise | Yes | 28 w | 80% | Duration of labor | Type of delivery, use of epidural, birth weight |
| Barakat et al., 2018 [30] | Spain | 65 | 33 | 32 | 3 | Mod | 55–60 | Aerobic, pelvic floor strength, and flexibility exercises | Yes | 28 w | 85% | Placenta weight | Gestational age, type of delivery, birth weight |
| Barakat et al., 2018 [31] | Spain | 456 | 234 | 222 | 3 | Mod | 50–55 | Aerobic exercise | Yes | 28 w | - | Gestational weight gain | Gestational age, type of delivery, birth weight |
| Bhartia et al., 2019 [32] | India | 78 | 38 | 40 | 1 | Mod | 50 | Yoga | Yes | 12 w | - | Maternal Stress, type of delivery, birth weight | - |
| 2 | No | ||||||||||||
| Bjøntegaard et al., 2021 [33] | Norway | 281 | 164 | 117 | 1 | Mod-High | 60 | Aerobic, strength training and balance exercises | Yes | 12 w | - | Type of delivery, birth weight | Physical activity of children at age of seven |
| 2 | 45 | No | |||||||||||
| Bolanthakodi et al., 2018 [34] | India | 150 | 75 | 75 | 3 | Mod | 30 | Yoga | No | 9 w | - | Pain intensity, type of delivery, duration of delivery | Low birth weight, Preterm birth |
| Carpenter et al., 2015 [35] | UK | 50 | 16 | 34 | 1 | Low-Mod | 40 | Stationary cycling, pelvic floor exercises and water exercises | Yes | 18 w | - | Hemodynamic function | Type of delivery, birth weight |
| Carrascosa et al., 2021 [36] | Spain | 286 | 145 | 141 | 3 | Mod | 45 | Aquatic aerobic exercise | Yes | 20 w | - | Use of epidural analgesia during labor | Type of delivery, time of active labor, episiotomy |
| Cordero et al., 2015 [37] | Spain | 257 | 101 | 156 | 1–2 | Low | 50–60 | Aerobics in gym hall and aquatic activity | Yes | 26 w | 80% | Gestational Diabetes | Gestational weight gain, type of delivery, birth weight |
| Daly et al., 2017 [38] | Ireland | 88 | 44 | 44 | 3 | Mod | 50–60 | Aerobic, pelvic floor exercises | Yes | 26 w | - | Maternal fasting plasma glucose | Type of delivery, duration of labor, birth weight |
| Dias et al., 2011 [39] | Norway | 42 | 21 | 21 | 1 | Low | 30 | Pelvic floor muscle training | Yes | 16 w | 75% | Type of delivery, duration of labor, birth weight | Pelvic floor muscle strength |
| 6 | No | ||||||||||||
| Dieb et al., 2019 [40] | Egypt | 400 | 200 | 200 | 3 | Low | 5 | Pelvic floor muscle training | No | 4 w | - | Episiotomy, perineal tear, type of delivery | Duration of labor, fetal distress, episiotomy, birth weight |
| 3 | 10 | ||||||||||||
| Ellingsen et al., 2020 [41] | Norway | 279 | 164 | 115 | 1 | Mod | 60 | Aerobic and strength exercises | Yes | 12 w | - | Neurodevelopmental in 7-year-old children | Gestational age, birth weight, type of delivery |
| 2 | 45 | No | |||||||||||
| Ferreira et al., 2019 [42] | Portugal | 255 | 99 | 156 | 3 | Mod | 45–60 | Aerobic, strength, coordination and flexibility exercises | Yes | 24 w | - | Duration of labor, type of delivery | Episiotomy, perineal tear |
| Fritel et al., 2015 [43] | France | 282 | 140 | 142 | 1 | Low | 20–30 | Pelvic floor training | Yes | 8 w | - | Urinary incontinence | Type of delivery, birth weight |
| Gau et al., 2011 [44] | China | 87 | 48 | 39 | 3 | Low | 20 | Ball exercise | No | 8 w | - | Childbirth pain | Duration of labor |
| Ghandali et al., 2021 [45] | Iran | 103 | 51 | 52 | 2 | Low-Mod | 35 | Pilates exercise | Yes | 8 | - | Type of delivery, episiotomy, duration of labor | Maternal satisfaction with childbirth process |
| Ghodsi et al., 2014 [46] | Iran | 80 | 40 | 40 | 3 | Low | 15 | Stationary cycling | No | 15 w | - | Gestational weight gain, type of delivery, perineal tear | Pregnancy length, first and second stage of labor, Apgar score |
| Guelfi et al., 2016 [47] | Australia | 172 | 85 | 87 | 3 | Mod | 20–60 | Stationary cycling program | Yes | 14 w | - | Gestational diabetes | Type of delivery, birth weight |
| Haakstad et al., 2020 [48] | Norway | 105 | 52 | 53 | 2 | Mod | 60 | Aerobic dance and strength training | Yes | 12 w | 80% | Birth weight | Gestational age, type of delivery |
| 1 | 30 | No | |||||||||||
| Johannessen et al., 2021 [49] | Norway | 722 | 383 | 339 | 1 | Mod | 55–70 | Aerobic, strength and pelvic floor exercises | Yes | 12 w | - | Urinary incontinence at 3 months postpartum | Type of delivery, episiotomy, epidural, duration of labor, birth weight |
| 2 | 45 | No | |||||||||||
| Karthiga et al., 2022 [50] | India | 234 | 121 | 113 | 7 | Mod | 60 | Yoga | No | 20 w | - | Gestational hypertension, preeclampsia, premature delivery | Type of delivery, duration of labor, birth weight |
| León-Larios et al., 2017 [51] | Spain | 466 | 254 | 212 | 5 | Low | 18–23 | Perineal massage and pelvic floor exercises | No | 6 w | - | Perineal tear and episiotomy | Type of delivery, duration of labor, birth weight and epidural |
| Miquelutti et al., 2013 [52] | Brazil | 149 | 78 | 71 | 7 | Low | 10–30 | Aerobic and pelvic floor muscle exercises | No | 14 w | - | Urinary incontinence, lumbopelvic pain and anxiety | Type of delivery, duration of labor |
| Nascimento et al., 2011 [53] | Brazil | 80 | 39 | 41 | 1 | Low-Mod | 40 | Aerobic exercise and walking | Yes | 17 w | 62.5% | Scoring women on meeting the intervention goals | Gestational weight gain, birth weight, macrosomia, and low birth weight |
| 5 | No | ||||||||||||
| Okido et al., 2015 [54] | Brazil | 59 | 26 | 33 | 7 | Low | 20 | Pelvic floor muscle training | No | 16 w | - | PI of the uterine artery, type of delivery, duration of delivery, birth weight | Episiotomy, urinary incontinence |
| Pais et al., 2021 [55] | India | 124 | 61 | 63 | 7 | Low | 45 | Yoga | No | 20 w | - | Preeclampsia and gestational diabetes | Gestational age, duration of labor, type of delivery, birth weight |
| Pelaez et al., 2019 [56] | Spain | 345 | 230 | 115 | 3 | Low-Mod | 60–65 | Aerobic and resistance training | Yes | 24 w | 80% | Gestational weight gain | Gestational diabetes, macrosomia, type of delivery |
| Perales et al., 2016 [57] | Spain | 166 | 83 | 83 | 3 | Low-Mod | 55–60 | Aerobic, strength exercises, pelvic floor muscle training | Yes | 28 w | - | Duration of labor, gestational age, gestational weight gain, type of delivery, birth weigh | Birth size, head circumference, Apgar score |
| Perales et al., 2020 [58] | Spain | 1348 | 668 | 660 | 3 | Low-Mod | 50–55 | Aerobic and pelvic floor exercises | Yes | 30 | 95% | Gestational weight gain, hypertension and diabetes | Type of delivery, birth weight, gestational age |
| Pereira et al., 2022 [59] | Portugal | 126 | 63 | 63 | 3 | Low-Mod | 30 | Walking | Yes | 3 w | - | Rate of labor induction | Type of delivery, birth weight |
| Pinzón et al., 2012 [60] | Colombia | 64 | 31 | 33 | 3 | Low-Mod | 60 | Aerobic and stretching exercises | Yes | 12 w | - | Gestational age, gestational weight gain, type of delivery | Birth weight, birth size, head circumference, Apgar score |
| Price et al., 2012 [61] | USA | 62 | 31 | 31 | 3 | Mod | 45–60 | Aerobic exercise and walk briskly | Yes | 23 w | - | Gestational weight gain. duration of labor, birth weight, postpartum recovery | Length of first and second stage of labor, type of delivery, gestational diabetes |
| 1 | 30–60 | No | |||||||||||
| Rodríguez-Blanque et al., 2019 [62] | Spain | 129 | 65 | 64 | 3 | Mod | 60 | Aquatic physical exercise | Yes | 17 w | - | Laceration and episiotomy rates | Type of delivery, birth weight and anesthesia |
| Rodríguez-Blanque et al., 2020 [63] | Spain | 129 | 65 | 64 | 3 | Mod | 60 | Aquatic physical exercise | Yes | 17 w | - | Gestational weight gain, type of delivery | Birth weight, Apgar score |
| Rodríguez-Diaz et al., 2017 [64] | Spain | 100 | 50 | 50 | 2 | Mod | 40–45 | Pilates | Yes | 8 w | 90% | Gestational weight gain, blood pressure, strength, flexibility, and spinal curvature | Type of delivery, episiotomy analgesia and birth weight |
| Ruiz et al., 2013 [65] | Spain | 962 | 481 | 481 | 3 | Low-Mod | 50–55 | Aerobic and resistance exercises | Yes | 28 w | 97% | Gestational weight gain | Birth weight, duration of labor |
| Salvesen et al., 2014 [66] | Sweden | 855 | 427 | 426 | 1 | Low-Mod | 55–70 | Aerobic, strength and pelvic floor exercise | Yes | 12 w | - | Gestational diabetes | Urinary and anal incontinence, lumbopelvic pain, and duration of labor |
| 2 | 45 | No | |||||||||||
| Sanda et al., 2018 [67] | Norway | 589 | 295 | 294 | 3 | Mod | 60 | Aerobic exercises | Yes | 22 w | - | Gestational age, duration of labor, type of delivery | - |
| 2 | 30 | No | |||||||||||
| Seneviratne et al., 2015 [68] | New Zealand | 75 | 38 | 37 | 3–5 | Mod | 15–30 | Stationary cycling program | No | 16 w | 33% | Birth weight, type of delivery | Gestational weight gain, gestational age |
| Shojaei et al., 2021 [69] | Iran | 100 | 49 | 51 | 4 | Mod | 40 | Walking | No | 4 w | - | Duration of labor | - |
| Silva-Jose et al., 2022 [70] | Spain | 157 | 78 | 79 | 3 | Mod | 55–60 | Aerobic, strength and pelvic floor exercises | Yes | 28 w | 80% | Gestational weight gain | Type of delivery, birth weight |
| Sobhgol et al., 2022 [71] | Australia | 200 | 100 | 100 | 1–2 | Low | 10 | Pelvic floor muscle exercises | No | 16 w | 50% | Female Sexual Function | Type of delivery, perineal tear, episiotomy, duration of labor, birth weight |
| Stafne et al., 2012 [72] | Norway | 761 | 396 | 365 | 1 | Mod-High | 60 | Aerobic, strength, and pelvic floor exercises | Yes | 12 w | - | Gestational diabetes, insulin resistance | Birth weight, gestational age, Apgar scores |
| 3 | 45 | No | |||||||||||
| Taniguchi et al., 2016 [73] | Japan | 118 | 60 | 58 | 3 | Mod | 30 | Walk briskly | Yes | 6 w | 80% | Duration of labor; type of delivery, birth weight | - |
| Tomic et al., 2013 [74] | Croatia | 334 | 166 | 168 | 3 | Low-Mod | 50 | Aerobic exercise | Yes | 28 w | 80% | Macrosomia birth weight, gestational weight gain | Preeclampsia, gestational diabetes, type of delivery |
| Uria-Minguito et al., 2022 [75] | Spain | 203 | 102 | 101 | 3 | Mod | 50–60 | Aerobic, strength, and pelvic floor exercises | Yes | 28 w | - | Gestational diabetes | Gestational weight gain, type of delivery, birth weight |
| Ussher et al., 2015 [76] | UK | 789 | 394 | 395 | 3–4 | Low | 20 | Exercise on a treadmill | Yes | 6 w | - | Continuous smoking abstinence | Gestational age, preterm birth, type of delivery, birth weight |
| Wang et al., 2017 [77] | China | 226 | 112 | 114 | 3 | Mod | 45–60 | Stationary cycling program | Yes | 24 w | 75% | Gestational diabetes | Gestational weight gain, birth weight, macrosomia |
| Wang et.al., 2020 [78] | China | 108 | 54 | 54 | 7 | Low | 30 | Pelvic floor muscle training | No | 12 w | - | Stress urinary incontinence, episiotomy | Duration of labor and type of delivery |
| Yekefallah et al., 2021 [79] | Iran | 70 | 35 | 35 | 2 | Low -Mod | 75 | Yoga | Yes | 11 w | - | Episiotomy, perineal tear, type of delivery | Birth weight, gestational age, duration of labor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Ruchat, S.-M.; Silva-Jose, C.; Gil-Ares, J.; Barakat, R.; Sánchez-Polán, M. Influence of Physical Activity during Pregnancy on Type and Duration of Delivery, and Epidural Use: Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 5139. https://doi.org/10.3390/jcm12155139
Zhang D, Ruchat S-M, Silva-Jose C, Gil-Ares J, Barakat R, Sánchez-Polán M. Influence of Physical Activity during Pregnancy on Type and Duration of Delivery, and Epidural Use: Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(15):5139. https://doi.org/10.3390/jcm12155139
Chicago/Turabian StyleZhang, Dingfeng, Stephanie-May Ruchat, Cristina Silva-Jose, Javier Gil-Ares, Rubén Barakat, and Miguel Sánchez-Polán. 2023. "Influence of Physical Activity during Pregnancy on Type and Duration of Delivery, and Epidural Use: Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 15: 5139. https://doi.org/10.3390/jcm12155139
APA StyleZhang, D., Ruchat, S.-M., Silva-Jose, C., Gil-Ares, J., Barakat, R., & Sánchez-Polán, M. (2023). Influence of Physical Activity during Pregnancy on Type and Duration of Delivery, and Epidural Use: Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(15), 5139. https://doi.org/10.3390/jcm12155139









