The Impact of Regional Maximum Tolerated Interlesion Distance on the Long-Term Ablation Outcomes in Ablation Index Guided Pulmonary Vein Isolation for Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Voltage Mapping Protocol
2.3. Ablation Index and Interlesion Distance Guided PVI
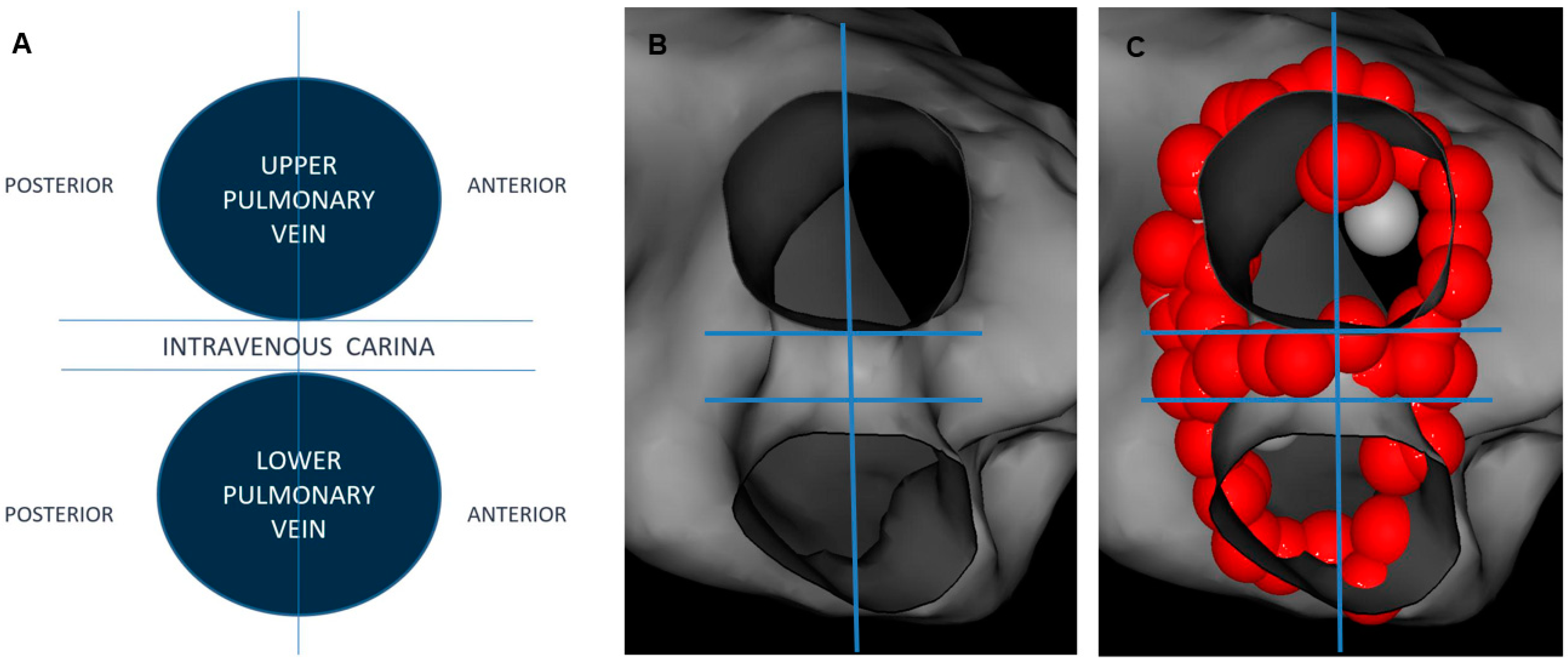
2.4. Postprocedural Assessment of Interlesion Distance
2.5. Follow-Up Strategy
2.6. Statistics
3. Results
3.1. Paroxysmal AF
3.2. Persistent AF
4. Discussion
5. Limitations
- (a)
- This was a non-randomized analysis. The next step should be to prospectively confirm the results, especially concerning maximum tolerated ILD at the posterior aspect of RUPV;
- (b)
- AFR could be explained, not only by the discontinuity of ablation lesions, but also by the lack of their transmurality, which was not assessed in this study;
- (c)
- We cannot exclude the fact that steerable sheaths may provide better spatial ablation catheter stability and translate into shorter expected ILD. Furthermore, the adopted ablation strategy in this study (high AI values combined with obligatory impedance drop) might have increased the lesion size compared to other ablation strategies and resulted in the high level of overlapping lesions. Therefore, the outcomes of this study should be interpreted in terms of applied workflow and the ablation catheter employed;
- (d)
- The assessment of ILD was performed postprocedurally. Therefore, ILD calculations included touch-up applications. Intraoperative ILD measurements between all ablation tags greatly prolong the procedure. This step was omitted for that reason. As a consequence, the ILD dataset with regard to achieving or not achieving FPI was missing;
- (e)
- The overall ablation success rate greatly depends on reliable AF recurrence detection. Intermittent rhythm monitoring modalities used in the study were short-term and discontinuous. We cannot rule out that the use of long-term and/or continuous ECG monitoring might have potentially decreased the ablation success rate if it had been applied.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.-H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017, 14, e275–e444. [Google Scholar] [CrossRef] [PubMed]
- Taghji, P.; El Haddad, M.; Phlips, T.; Wolf, M.; Knecht, S.; Vandekerckhove, Y.; Tavernier, R.; Nakagawa, H.; Duytschaever, M. Evaluation of a Strategy Aiming to Enclose the Pulmonary Veins With Contiguous and Optimized Radiofrequency Lesions in Paroxysmal Atrial Fibrillation: A Pilot Study. JACC Clin. Electrophysiol. 2018, 4, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Duytschaever, M.; Vijgen, J.; De Potter, T.; Scherr, D.; Van Herendael, H.; Knecht, S.; Kobza, R.; Berte, B.; Sandgaard, N.; Albenque, J.-P.; et al. Standardized pulmonary vein isolation workflow to enclose veins with contiguous lesions: The multicentre VISTAX trial. Europace 2020, 22, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.; Das, M.; Chaturvedi, V.; Asfour, I.K.; Daryanani, N.; Morgan, M.; Ronayne, C.; Shaw, M.; Snowdon, R.; Gupta, D. Prospective use of Ablation Index targets improves clinical outcomes following ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2017, 28, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Ramirez, I.D.; Baldenhofer, G.; Stangl, K.; Mont, L.; Althoff, T.F. Randomized study defining the optimum target interlesion distance in ablation index-guided atrial fibrillation ablation. Europace 2020, 22, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Theis, C.; Huber, C.; Kaiser, B.; Kaesemann, P.; Hui, F.; Pirozzolo, G.; Bekeredjian, R. Improved durable pulmonary vein isolation with shorter procedure times and lower energy levels using RF ablation with ablation index and a stringent lesion contiguity. Indian Pacing Electrophysiol. J. 2021, 21, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K.; Ejima, K.; Kataoka, S.; Kanai, M.; Higuchi, S.; Sh, S.H.; Shoda, M.; Hagiwara, N. Regional differences in the predictors of acute electrical reconnection following high-power pulmonary vein isolation for paroxysmal atrial fibrillation. J. Arrhythmia 2021, 37, 1260–1269. [Google Scholar] [CrossRef]
- Jankelson, L.; Dai, M.; Aizer, A.; Bernstein, S.; Park, D.S.; Holmes, D.; Chinitz, L.A.; Barbhaiya, C. Lesion Sequence and Catheter Spatial Stability Affect Lesion Quality Markers in Atrial Fibrillation Ablation. JACC Clin. Electrophysiol. 2021, 7, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Mitrzak, K.; Peller, M.; Krzowski, B.; Maciejewski, C.; Balsam, P.; Marchel, M.; Grabowski, M.; Lodziński, P. Safety and effectiveness of very-high-power, short-duration ablation in patients with atrial fibrillation: Preliminary results. Cardiol. J. 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kiedrowicz, R.M.; Wielusinski, M.; Wojtarowicz, A.; Kazmierczak, J. Predictors of the voltage derived left atrial fibrosis in patients with long-standing persistent atrial fibrillation. Cardiol. J. 2022, 29, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Takigawa, M.; Yamada, T.; Yoshida, Y.; Ishikawa, K.; Aoyama, Y.; Yamamoto, T.; Inoue, N.; Tatematsu, Y.; Nanasato, M.; Kato, K.; et al. The incidence and clinical significance of non-isolation of the pulmonary vein carina after encircling ipsilateral pulmonary veins isolation for paroxysmal atrial fibrillation: A pitfall of the double-Lasso technique. Europace 2012, 15, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.; Ahsan, S.; Honarbakhsh, S.; Lim, W.; Baca, M.; Graham, A.; Srinivasan, N.; Sawhney, V.; Sporton, S.; Schilling, R.J.; et al. A multicentered evaluation of ablation at higher power guided by ablation index: Establishing ablation targets for pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2019, 30, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Berte, B.; Hilfiker, G.; Moccetti, F.; Schefer, T.; Weberndörfer, V.; Cuculi, F.; Toggweiler, S.; Ruschitzka, F.; Kobza, R. Pulmonary vein isolation using ablation index vs. CLOSE protocol with a surround flow ablation catheter. Europace 2019, 22, 84–89. [Google Scholar] [CrossRef]
- Kiliszek, M.; Krzyżanowski, K.; Wierzbowski, R.; Winkler, A.; Smalc-Stasiak, M. The value of the ablation index in patients undergoing ablation for atrial fibrillation. Kardiologia Polska 2020, 78, 1015–1019. [Google Scholar] [CrossRef]
- Santoro, F.; Metzner, A.; Brunetti, N.D.; Heeger, C.-H.; Mathew, S.; Reissmann, B.; Lemeš, C.; Maurer, T.; Fink, T.; Rottner, L.; et al. Left atrial anterior line ablation using ablation index and inter-lesion distance measurement. Clin. Res. Cardiol. 2019, 108, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Makihara, Y.; Miyazaki, S.; Harama, T.; Obunai, K.; Watanabe, H.; Tada, H. Ablation Index Guided Left Atrial Posterior Wall Isolation. Int. Heart J. 2022, 63, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.-H.; Jiang, C.-Y. Pulmonary Vein Reconnection in Patients With and Without Atrial Fibrillation Recurrence After Ablation. JACC Clin. Electrophysiol. 2016, 2, 484–486. [Google Scholar] [CrossRef] [PubMed]


| PAF n = 130 | PsAF n = 130 | p | |
|---|---|---|---|
| Uninterrupted AF duration, months | - | 24 (12–36) | - |
| Age, years | 58 (53–62) | 66 (58–70) | <0.001 |
| Females, n (%) | 55 (42%) | 22 (17%) | <0.001 |
| Hypertension, n (%) | 82 (63%) | 101 (78%) | <0.001 |
| Chronic coronary syndrome, n (%) | 20 (15%) | 31 (24%) | 0.02 |
| Heart failure, n (%) | 3 (2%) | 33 (25%) | <0.001 |
| eGFR < 60 mL/min/1.73 m2, n (%) | 3 (2%) | 16 (12%) | <0.001 |
| Diabetes, n (%) | 17 (13%) | 29 (22%) | 0.03 |
| CHA2DS2–VASc score | 2 (1–3) | 3 (1–4) | <0.001 |
| Left ventricular ejection fraction, % | 60 (55–65) | 60 (55–65) | 0.8 |
| Left atrial antero-posterior diameter, mm | 42 (39–46) | 48 (43–55) | <0.001 |
| LCPV, n | 18 (14%) | 21 (16%) | 0.4 |
| RMPV, n | 0 | 0 | - |
| Perimeter of encirclement including intravenous carina, mm | |||
| LUPV | 78 (61–94) | 103 (93–119) | <0.001 |
| LCPV | 81 (63–92) | 109 (93–118)) | <0.001 |
| LLPV | 77 (66–92) | 111 (98–123) | <0.001 |
| RUPV | 75 (63–91) | 109 (96–125) | <0.001 |
| RLPV | 72 (59–86) | 105 (90–118) | <0.001 |
| Total number of 6 mm RF tags, n | |||
| LUPV | 28 (23–34) | 34 (29–40) | <0.001 |
| LCPV | 32 (29–39) | 39 (31–46) | <0.001 |
| LLPV | 30 (22–35) | 33 (27–38) | <0.001 |
| RUPV | 26 (21–33) | 35 (29–41) | <0.001 |
| RLPV | 26 (22–33) | 32 (24–36) | <0.001 |
| Median interlesion distance, mm | |||
| LUPV | 4.4 (4.2–4.6) | 4.5 (4.4–4.7) | 0.8 |
| LCPV | 3.8 (3.7–4.1) | 3.9 (3.8–4.0) | 0.9 |
| LLPV | 4.4 (4.2–4.5) | 4.4 (4.2–4.5) | 0.8 |
| RUPV | 4.5 (4.3–4.6) | 4.6 (4.5–4.7) | 0.9 |
| RLPV | 4.3 (4.2–4.4) | 4.4 (4.3–4.5) | 0.8 |
| Dissociated PV activity following ablation, n | |||
| LUPV | 18 (14%) | 22 (17%) | 0.2 |
| LCPV | 30 (23%) | 35 (27%) | 0.6 |
| LLPV | 25 (19%) | 20 (15%) | 0.8 |
| RUPV | 66 (51%) | 56 (43%) | 0.5 |
| RLPV | 22 (17%) | 25 (19%) | 0.6 |
| First-pass PV isolation, n | |||
| LUPV | 43 (33%) | 39 (30%) | 0.5 |
| LCPV | 64 (49%) | 60 (46%) | 0.8 |
| LLPV | 32 (25%) | 35 (27%) | 0.6 |
| RUPV | 38 (29%) | 32 (25%) | 0.4 |
| RLPV | 42 (32%) | 35 (27%) | 0.1 |
| ILD | LCPV: Entire Encirclement | LCPV: Anterior Aspect | LCPV: Posterior Aspect | |||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | ||||
| 6.0–5.5 | 1 (1–3) | 1 (1–2) | 0.7 | 1 (1–1) | 1 (0–1) | 0.5 | 1 (1–1) | 1 (0–1) | 0.7 | |||
| 5.5–5.0 | 2 (1–4) | 1 (1–3) | 0.4 | 1 (1–2) | 1 (1–2) | 0.7 | 2 (1–4) | 1 (0–1) | 0.8 | |||
| 5.0–4.5 | 3 (2–4) | 4 (3–7) | 0.7 | 2 (1–3) | 2 (1–4) | 0.5 | 2 (1–4) | 2 (2–2) | 0.5 | |||
| 4.5–4.0 | 5 (3–8) | 4 (2–8) | 0.6 | 3 (2–6) | 2 (2–7) | 0.9 | 3 (1–6) | 3 (1–5) | 0.6 | |||
| ≤4.0 | 18 (13–24) | 19 (15–28) | 0.4 | 9 (6–18) | 9 (7–23) | 0.6 | 10 (8–25) | 11 (7–23) | 0.8 | |||
| ILD | LUPV: Entire Encirclement | LUPV: Anterior Aspect | LUPV: Posterior Aspect | LUPV: Intravenous Carina | ||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| 6.0–5.5 | 3 (2–4) | 2 (1–3) | 0.7 | 1 (1–2) | 1 (1–2) | 0.5 | 2 (1–5) | 1 (0–1) | 0.9 | 1 (1–3) | 1 (0–1) | 0.7 |
| 5.5–5.0 | 2 (1–3) | 1 (1–3) | 0.6 | 1 (0–1) | 0 (0–1) | 0.7 | 1 (1–3) | 1 (0–1) | 0.7 | 1 (1–2) | 0 (0–1) | 0.7 |
| 5.0–4.5 | 3 (1–4) | 3 (2–4) | 0.8 | 2 (1–4) | 2 (1–3) | 0.5 | 1 (1–3) | 2 (1–4) | 0.5 | 1 (0–1) | 1 (1–2) | 0.8 |
| 4.5–4.0 | 13 (7–19) | 12 (8–16) | 0.5 | 5 (3–11) | 7 (4–8) | 0.5 | 6 (2–9) | 7 (2–13) | 0.8 | 1 (1–3) | 1 (1–4) | 0.6 |
| ≤4.0 | 8 (5–15) | 9 (6–14) | 0.5 | 3 (1–6) | 5 (2–9) | 0.6 | 4 (3–5) | 4 (3–6) | 0.9 | 1 (0–1) | 1 (1–1) | 0.7 |
| ILD | LLPV: Entire Encirclement | LLPV: Anterior Aspect | LLPV: Posterior Aspect | LLPV: Intravenous Carina | ||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| 6.0–5.5 | 1 (1–3) | 1 (0–2) | 0.6 | 1 (0–1) | 0 (0–1) | 0.7 | 0 (0–1) | 1 (0–2) | 0.8 | 1 (1–4) | 1 (1–1) | 0.7 |
| 5.5–5.0 | 1 (1–1) | 2 (1–2) | 0.5 | 1 (0–1) | 1 (1–2) | 0.7 | 1 (1–1) | 2 (1–3) | 0.5 | 1 (0–1) | 0 (0–1) | 0.8 |
| 5.0–4.5 | 1 (1–3) | 1 (0–1) | 0.7 | 0 (0–1) | 1 (0–2) | 0.8 | 1 (0–1) | 1 (1–2) | 0.7 | 1 (0–1) | 1 (0–2) | 0.7 |
| 4.5–4.0 | 16 (11–20) | 14 (11–18) | 0.5 | 8 (3–10) | 9 (3–12) | 0.7 | 8 (5–8) | 6 (5–7) | 0.6 | 2 (1–3) | 2 (1–3) | 0.5 |
| ≤4.0 | 12 (9–19) | 14 (11–17) | 0.4 | 7 (5–9) | 8 (6–10) | 0.7 | 6 (4–10) | 6 (4–10) | 0.6 | 1 (0–2) | 1 (1–2) | 0.7 |
| ILD | RUPV: Entire Encirclement | RUPV: Anterior Aspect | RUPV: Posterior Aspect | RUPV: Intravenous Carina | ||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| 6.0–5.5 | 3 (2–7) | 1 (0–2) | 0.2 | 0 (0–1) | 1 (0–1) | 0.8 | 3 (2–7) | 0 (0–1) | <0.001 | 0 (0–1) | 0 (0–1) | 0.8 |
| 5.5–5.0 | 2 (1–3) | 2 (1–4) | 0.8 | 1 (1–2) | 1 (0–1) | 0.9 | 0 (0–0) | 0 (0–1) | 0.8 | 1 (1–2) | 1 (1–1) | 0.8 |
| 5.0–4.5 | 5 (2–10) | 5 (2–9) | 0.8 | 5 (2–8) | 6 (3–10) | 0.6 | 0 (0–1) | 1 (0–1) | 0.7 | 1 (1–2) | 1 (0–2) | 0.7 |
| 4.5–4.0 | 8 (6–15) | 12 (8–14) | 0.5 | 6 (2–9) | 7 (2–12) | 0.8 | 3 (1–6) | 3 (1–7) | 0.8 | 2 (1–5) | 2 (2–5) | 0.6 |
| ≤4.0 | 6 (3–12) | 8 (3–14) | 0.5 | 1 (1–3) | 2 (1–5) | 0.7 | 3 (2–7) | 6 (3–10) | 0.6 | 2 (1–4) | 2 (1–4) | 0.9 |
| ILD | RLPV: Entire Encirclement | RLPV: Anterior Aspect | RLPV: Posterior Aspect | RLPV: Intravenous Carina | ||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| 6.0–5.5 | 1(1–3) | 2 (1–2) | 0.7 | 0 (0–1) | 1 (0–1) | 0.5 | 1 (0–1) | 0 (0–1) | 0.7 | 1 (1–2) | 0 (0–1) | 0.7 |
| 5.5–5.0 | 0 (0–1) | 1 (1–3) | 0.6 | 1 (0–1) | 1 (0–1) | 0.7 | 0 (0–1) | 1 (1–2) | 0.6 | 0 (0–1) | 1 (0–1) | 0.5 |
| 5.0–4.5 | 1 (1–3) | 2 (1–4) | 0.6 | 0 (0–2) | 1 (1–2) | 0.7 | 1 (1–1) | 1 (1–3) | 0.8 | 1 (0–1) | 2 (1–2) | 0.9 |
| 4.5–4.0 | 14 (9–17) | 15 (9–22) | 0.8 | 5 (2–10) | 5 (2–10) | 0.9 | 5 (2–9) | 5 (3–11) | 0.8 | 3 (1–5) | 2 (1–2) | 0.7 |
| ≤4.0 | 8 (6–11) | 7 (6–18) | 0.8 | 5 (2––7) | 4 (1–7) | 0.6 | 2 (1–4) | 3 (2–7) | 0.6 | 2 (1–3) | 2 (1–2) | 0.6 |
| ILD | LCPV: Entire Encirclement | LCPV: Anterior Aspect | LCPV: Posterior Aspect | |||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | ||||
| 6.0–5.5 | 2 (2–3) | 1 (0–2) | 0.4 | 1 (1–1) | 0 (0–1) | 0.2 | 0 (0–1) | 0 (0–1) | 0.9 | |||
| 5.5–5.0 | 2 (1–4) | 1 (0–3) | 0.3 | 1 (1–1) | 1 (0–2) | 0.5 | 1 (0–2) | 0 (0–1) | 0.3 | |||
| 5.0–4.5 | 4 (2–6) | 4 (3–6) | 0.7 | 2 (1–2) | 2 (1–4) | 0.5 | 2 (0–4) | 2 (2–2) | 0.4 | |||
| 4.5–4.0 | 2 (1–3) | 4 (3–5) | 0.2 | 2 (1–3) | 2 (2–3) | 0.9 | 0 (0–0) | 1 (1–2) | 0.2 | |||
| ≤4.0 | 27 (22–34) | 32 (28–38) | 0.3 | 16 (14–20) | 18 (16–23) | 0.2 | 17 (15–21) | 21 (17–23) | 0.2 | |||
| ILD | LUPV: Entire Encirclement | LUPV: Anterior Aspect | LUPV: Posterior Aspect | LUPV: Intravenous Carina | ||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| 6.0–5.5 | 2 (1–3) | 2 (1–4) | 0.5 | 0 (0–1) | 1 (0–2) | 0.2 | 0 (0–1) | 0 (0–1) | 0.8 | 1 (1–3) | 1 (0–1) | 0.6 |
| 5.5–5.0 | 2 (1–3) | 2 (1–3) | 0.5 | 0 (0–1) | 0 (0–2) | 0.7 | 1 (0–1) | 1 (0–1) | 0.9 | 0 (0–1) | 0 (0–1) | 0.8 |
| 5.0–4.5 | 3 (1–4) | 3 (2–4) | 0.6 | 2 (0–2) | 1 (0–2) | 0.3 | 1 (0–1) | 1 (0–2) | 0.8 | 1 (0–1) | 1 (0–2) | 0.8 |
| 4.5–4.0 | 15 (12–18) | 13 (12–15) | 0.3 | 8 (5–9) | 7 (5–8) | 0.5 | 1 (0–2) | 1 (0–2) | 0.7 | 5 (4–6) | 6 (4–7) | 0.5 |
| ≤4.0 | 11 (7–21) | 12 (8–19) | 0.4 | 4 (3–7) | 6 (4–8) | 0.2 | 4 (3–5) | 4 (3–6) | 0.8 | 1 (0–1) | 1 (0–1) | 0.9 |
| ILD | LLPV: Entire Encirclement | LLPV: Anterior Aspect | LLPV: Posterior Aspect | LLPV: Intravenous Carina | ||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| 6.0–5.5 | 1 (0–3) | 1 (0–2) | 0.8 | 0 (0–1) | 0 (0–1) | 0.7 | 0 (0–1) | 0 (0–1) | 0.8 | 1 (1–3) | 1 (1–1) | 0.6 |
| 5.5–5.0 | 1 (0–2) | 1 (0–2) | 0.7 | 0 (0–1) | 0 (0–1) | 0.9 | 0 (0–1) | 0 (0–1) | 0.8 | 0 (0–1) | 0 (0–1) | 0.9 |
| 5.0–4.5 | 1 (0–3) | 2 (0–4) | 0.5 | 0 (0–1) | 1 (0–2) | 0.7 | 1 (0–1) | 1 (0–2) | 0.7 | 1 (0–1) | 1 (0–2) | 0.7 |
| 4.5–4.0 | 10 (8–13) | 12 (11–14) | 0.6 | 4 (3–7) | 5 (3–8) | 0.8 | 6 (5–8) | 6 (5–7) | 0.6 | 2 (1–3) | 3 (1–3) | 0.6 |
| ≤4.0 | 16 (13–19) | 14 (11–17) | 0.5 | 7 (5–9) | 8 (6–10) | 0.7 | 7 (4–8) | 7 (5–9) | 0.5 | 2 (1–2) | 2 (1–2) | 0.6 |
| ILD | RUPV: Entire Encirclement | RUPV: Anterior Aspect | RUPV: Posterior Aspect | RUPV: Intravenous Carina | ||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| 6.0–5.5 | 6 (2–7) | 2 (0–2) | 0.01 | 1 (0–1) | 0 (0–1) | 0.4 | 4 (2–6) | 0 (0–1) | <0.001 | 1 (1–2) | 1 (1–2) | 0.7 |
| 5.5–5.0 | 1 (1–3) | 2 (1–3) | 0.5 | 1 (0–2) | 1 (0–1) | 0.5 | 0 (0–0) | 1 (0–1) | 0.7 | 0 (0–1) | 1 (0–1) | 0.8 |
| 5.0–4.5 | 8 (4–10) | 9 (6–12) | 0.4 | 1 (0–3) | 1 (0–2) | 0.5 | 0 (0–1) | 1 (0–2) | 0.7 | 1 (0–2) | 1 (0–2) | 0.7 |
| 4.5–4.0 | 8 (6–15) | 12 (11–14) | 0.3 | 6 (1–3) | 7 (1–2) | 0.6 | 1 (0–2) | 2 (1–2) | 0.6 | 3 (1–4) | 4 (2–6) | 0.4 |
| ≤4.0 | 12 (6–14) | 10 (8–16) | 0.5 | 3 (1–3) | 4 (1–5) | 0.4 | 10 (7–12) | 8 (6–10) | 0.5 | 2 (1–3) | 2 (1–2) | 0.2 |
| ILD | RLPV: Entire Encirclement | RLPV: Anterior Aspect | RLPV: Posterior Aspect | RLPV: Intravenous Carina | ||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| 6.0–5.5 | 1(1–3) | 2 (1–3) | 0.6 | 0 (0–1) | 1 (0–1) | 0.6 | 1 (1–1) | 1 (0–1) | 0.7 | 1 (1–2) | 1 (0–2) | 0.7 |
| 5.5–5.0 | 1 (0–2) | 2 (1–3) | 0.5 | 0 (0–1) | 1 (0–1) | 0.6 | 1 (0–1) | 1 (0–2) | 0.6 | 0 (0–1) | 1 (0–1) | 0.4 |
| 5.0–4.5 | 2 (1–4) | 3 (1–4) | 0.6 | 1 (1–2) | 1 (1–2) | 0.7 | 1 (0–1) | 2 (1–3) | 0.8 | 1 (0–2) | 1 (1–2) | 0.6 |
| 4.5–4.0 | 15 (11–18) | 16 (11–20) | 0.7 | 7 (3–9) | 6 (4–9) | 0.4 | 6 (4–9) | 6 (3–9) | 0.8 | 3 (1–4) | 2 (1–2) | 0.7 |
| ≤4.0 | 11 (7–12) | 10 (6–18) | 0.8 | 5 (2––8) | 5 (3–8) | 0.6 | 3 (1–4) | 4 (2–5) | 0.6 | 2 (1–4) | 2 (1–3) | 0.5 |
| LCPV: Entire Encirclement | LCPV: Anterior Aspect | LCPV: Posterior Aspect | ||||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | ||||
| ILD, mm | 3.8 (3.6–4.0) | 3.8 (3.6–3.9) | 0.8 | 3.9 (3.8–4.0) | 3.9 (3.7–4.0) | 0.7 | 3.9 (3.7–4.0) | 3.8 (3.6–3.9) | 0.5 | |||
| LUPV: Entire Encirclement | LUPV: Anterior Aspect | LUPV: Posterior Aspect | LUPV: Intravenous Carina | |||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| ILD, mm | 4.5 (4.4–4.7) | 4.4 (4.3–4.6) | 0.7 | 4.5 (4.4–4.7) | 4.4 (4.3–4.5) | 0.6 | 4.4 (4.4–4.6) | 4.5 (4.4–4.7) | 0.8 | 4.5 (4.3–4.5) | 4.5 (4.4–4.6) | 0.8 |
| LLPV: Entire Encirclement | LLPV: Anterior Aspect | LLPV: Posterior Aspect | LLPV: Intravenous Carina | |||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| ILD, mm | 4.4 (4.1–4.5) | 4.4 (4.2–4.6) | 0.8 | 4.5 (4.3–4.6) | 4.4 (4.3–4.6) | 0.7 | 4.3 (4.1–4.5) | 4.3 (4.2–.4.4) | 0.7 | 4.5 (4.2–4.7) | 4.4 (4.2–4.6) | 0.6 |
| RUPV: Entire Encirclement | RUPV: Anterior Aspect | RUPV: Posterior Aspect | RUPV: Intravenous Carina | |||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| ILD, mm | 4.5 (4.3–4.7) | 4.5 (4.4–4.7) | 0.7 | 4.5 (4.3–4.7) | 4.6 (4.4–4.6) | 0.7 | 4.5 (4.3–4.6) | 4.6 (4.3–4.5) | 0.6 | 4.5 (4.3–4.6) | 4.4 (4.2–4.6) | 0.5 |
| RLPV: Entire Encirclement | RLPV: Anterior Aspect | RLPV: Posterior Aspect | RLPV: Intravenous Carina | |||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| ILD, mm | 4.3 (4.1–4.5) | 4.3 (4.1–4.5) | 0.8 | 4.4 (4.2–4.7) | 4.4 (4.2–4.5) | 0.7 | 4.3 (4.1–4.6) | 4.4 (4.2–4.6) | 0.7 | 4.4 (4.3–.4.5) | 4.4 (4.3–4.6) | 0.7 |
| LCPV: Entire Encirclement | LCPV: Anterior Aspect | LCPV: Posterior Aspect | ||||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | ||||
| ILD, mm | 3.9 (3.7–4.0) | 3.8 (3.6–4.0) | 0.8 | 3.9 (3.8–4.0) | 3.9 (3.7–4.0) | 0.7 | 3.9 (3.7–4.0) | 3.8 (3.6–3.9) | 0.5 | |||
| LUPV: Entire Encirclement | LUPV: Anterior Aspect | LUPV: Posterior Aspect | LUPV: Intravenous Carina | |||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| ILD, mm | 4.5 (4.4–4.7) | 4.5 (4.3–4.6) | 0.6 | 4.5 (4.4–4.6) | 4.4 (4.3–4.5) | 0.7 | 4.5 (4.4–4.6) | 4.5 (4.4–4.7) | 0.8 | 4.4 (4.3–4.5) | 4.5 (4.4–4.6) | 0.7 |
| LLPV: Entire Encirclement | LLPV: Anterior Aspect | LLPV: Posterior Aspect | LLPV: Intravenous Carina | |||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| ILD, mm | 4.3 (4.2–4.5) | 4.4 (4.3–4.5) | 0.5 | 4.4 (4.3–4.5) | 4.4 (4.3–4.5) | 0.7 | 4.4 (4.3–4.5) | 4.3 (4.2–4.4) | 0.3 | 4.5 (4.4–4.7) | 4.4 (4.3–4.5) | 0.4 |
| RUPV: Entire Encirclement | RUPV: Anterior Aspect | RUPV: Posterior Aspect | RUPV: Intravenous Carina | |||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| ILD, mm | 4.6 (4.4–4.7) | 4.5 (4.4–4.6) | 0.6 | 4.6 (4.4–4.7) | 4.5 (4.4–4.6) | 0.7 | 4.6 (4.5–4.7) | 4.4 (4.3–4.5) | 0.4 | 4.5 (4.3–4.6) | 4.5 (4.4–4.6) | 0.6 |
| RLPV: Entire Encirclement | RLPV: Anterior Aspect | RLPV: Posterior Aspect | RLPV: Intravenous Carina | |||||||||
| AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | AFR (+) | AFR (−) | p-Value | |
| ILD, mm | 4.4 (4.3–4.6) | 4.3 (4.2–4.5) | 0.6 | 4.3 (4.2–4.6) | 4.4 (4.2–4.5) | 0.5 | 4.4 (4.2–4.6) | 4.4 (4.2–4.5) | 0.7 | 4.4 (4.3–.4.5) | 4.3 (4.3–4.5) | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiedrowicz, R.M.; Wielusinski, M.; Krasnik, W.; Jankowska, O.; Zakrzewski, S.; Duda, L.; Peregud-Pogorzelska, M.; Kladna, A.; Kazmierczak, J. The Impact of Regional Maximum Tolerated Interlesion Distance on the Long-Term Ablation Outcomes in Ablation Index Guided Pulmonary Vein Isolation for Atrial Fibrillation. J. Clin. Med. 2023, 12, 5056. https://doi.org/10.3390/jcm12155056
Kiedrowicz RM, Wielusinski M, Krasnik W, Jankowska O, Zakrzewski S, Duda L, Peregud-Pogorzelska M, Kladna A, Kazmierczak J. The Impact of Regional Maximum Tolerated Interlesion Distance on the Long-Term Ablation Outcomes in Ablation Index Guided Pulmonary Vein Isolation for Atrial Fibrillation. Journal of Clinical Medicine. 2023; 12(15):5056. https://doi.org/10.3390/jcm12155056
Chicago/Turabian StyleKiedrowicz, Radoslaw M., Maciej Wielusinski, Wojciech Krasnik, Olga Jankowska, Szymon Zakrzewski, Lukasz Duda, Małgorzata Peregud-Pogorzelska, Aleksandra Kladna, and Jaroslaw Kazmierczak. 2023. "The Impact of Regional Maximum Tolerated Interlesion Distance on the Long-Term Ablation Outcomes in Ablation Index Guided Pulmonary Vein Isolation for Atrial Fibrillation" Journal of Clinical Medicine 12, no. 15: 5056. https://doi.org/10.3390/jcm12155056
APA StyleKiedrowicz, R. M., Wielusinski, M., Krasnik, W., Jankowska, O., Zakrzewski, S., Duda, L., Peregud-Pogorzelska, M., Kladna, A., & Kazmierczak, J. (2023). The Impact of Regional Maximum Tolerated Interlesion Distance on the Long-Term Ablation Outcomes in Ablation Index Guided Pulmonary Vein Isolation for Atrial Fibrillation. Journal of Clinical Medicine, 12(15), 5056. https://doi.org/10.3390/jcm12155056







