Advancing Precision Medicine in Myocarditis: Current Status and Future Perspectives in Endomyocardial Biopsy-Based Diagnostics and Therapeutic Approaches
Abstract
1. Introduction
2. Histological and Immunohistochemical Inflammation Diagnostics
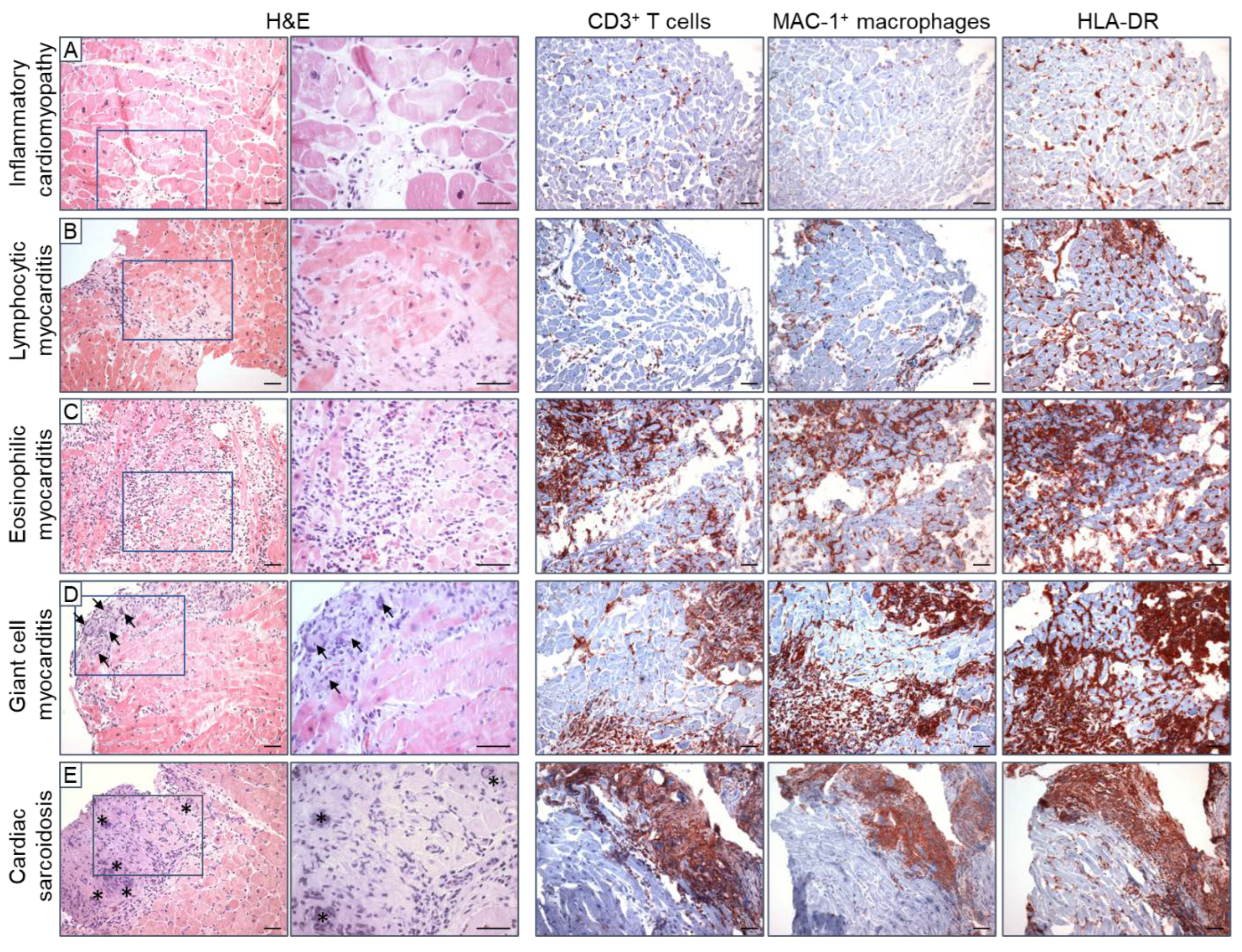
2.1. Histological EMB Evaluation—The Dallas Criteria
2.2. Immunohistochemical EMB Analysis
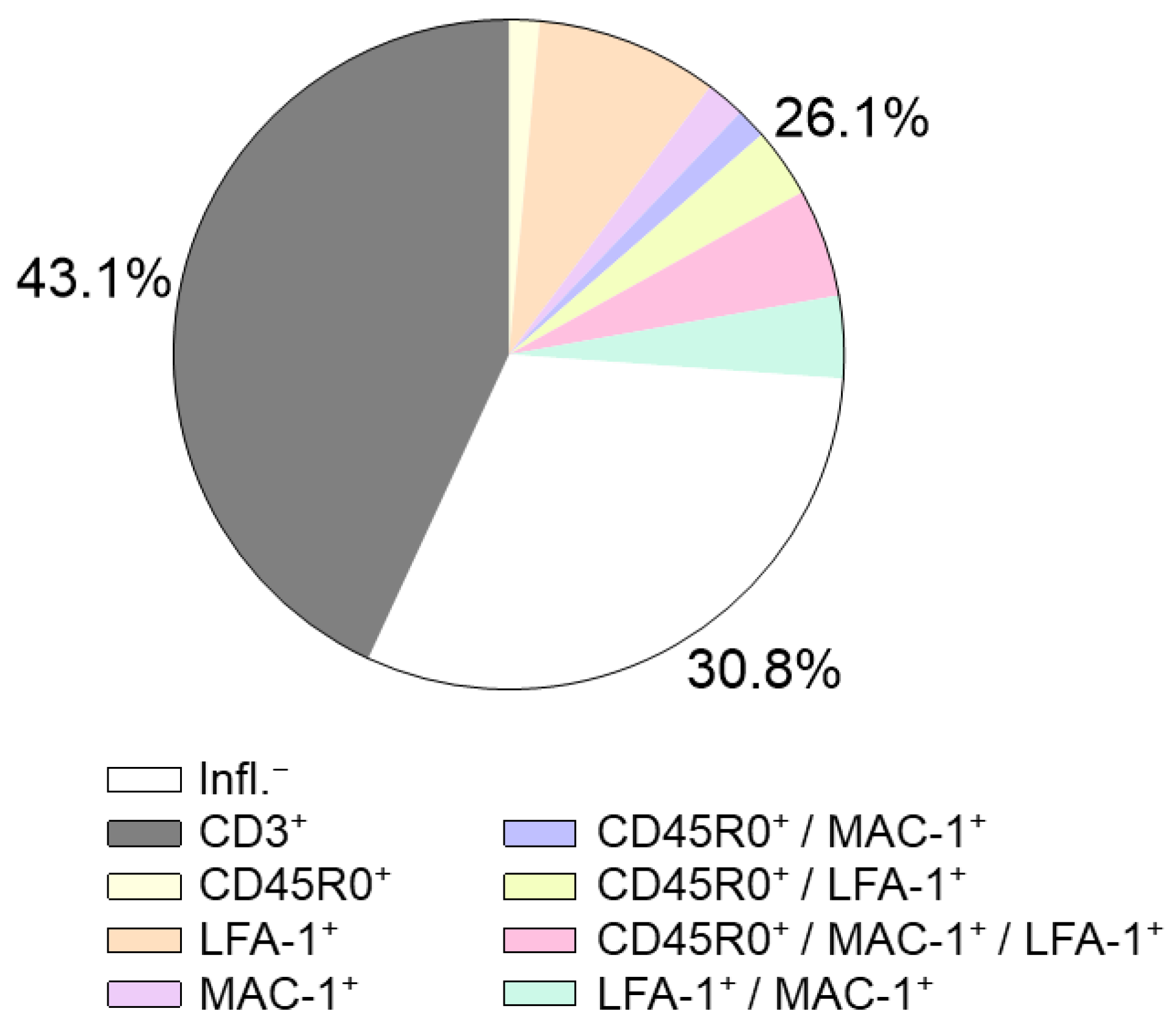
2.3. Prognostic Relevance of Immunohistochemical Markers
2.4. Further Immunohistochemical Markers
2.4.1. Lymphocytes
2.4.2. Monocytes and Macrophages
2.4.3. Cell Adhesion Molecules and the Human Leukocyte Antigen System
3. Virus Diagnostics
3.1. Enteroviruses and Adenoviruses
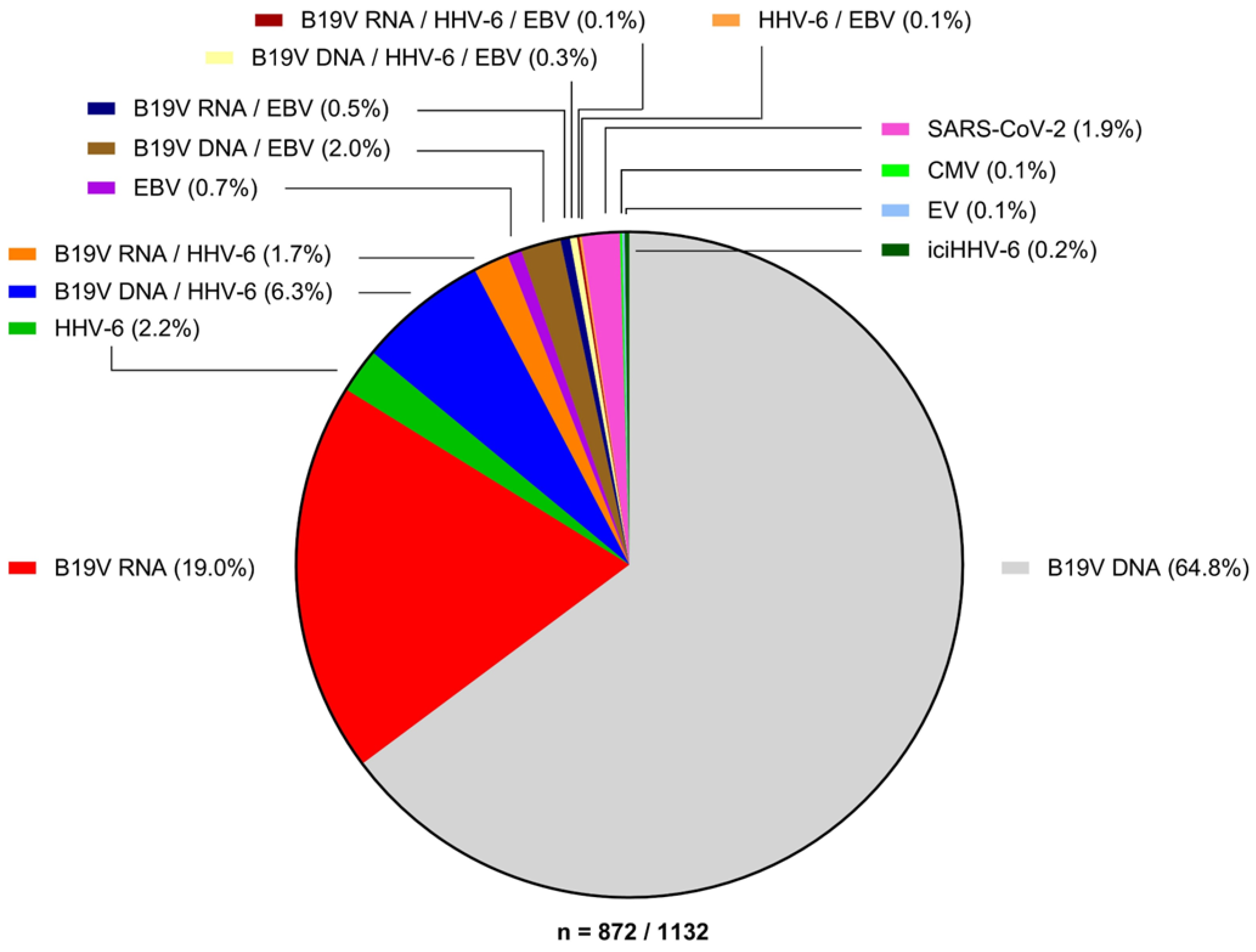
3.2. Parvovirus B19
3.3. Herpesviridae
3.4. SARS-CoV-2
3.5. Other Cardiotropic Viruses
3.6. Incidence of Viral Myocarditis
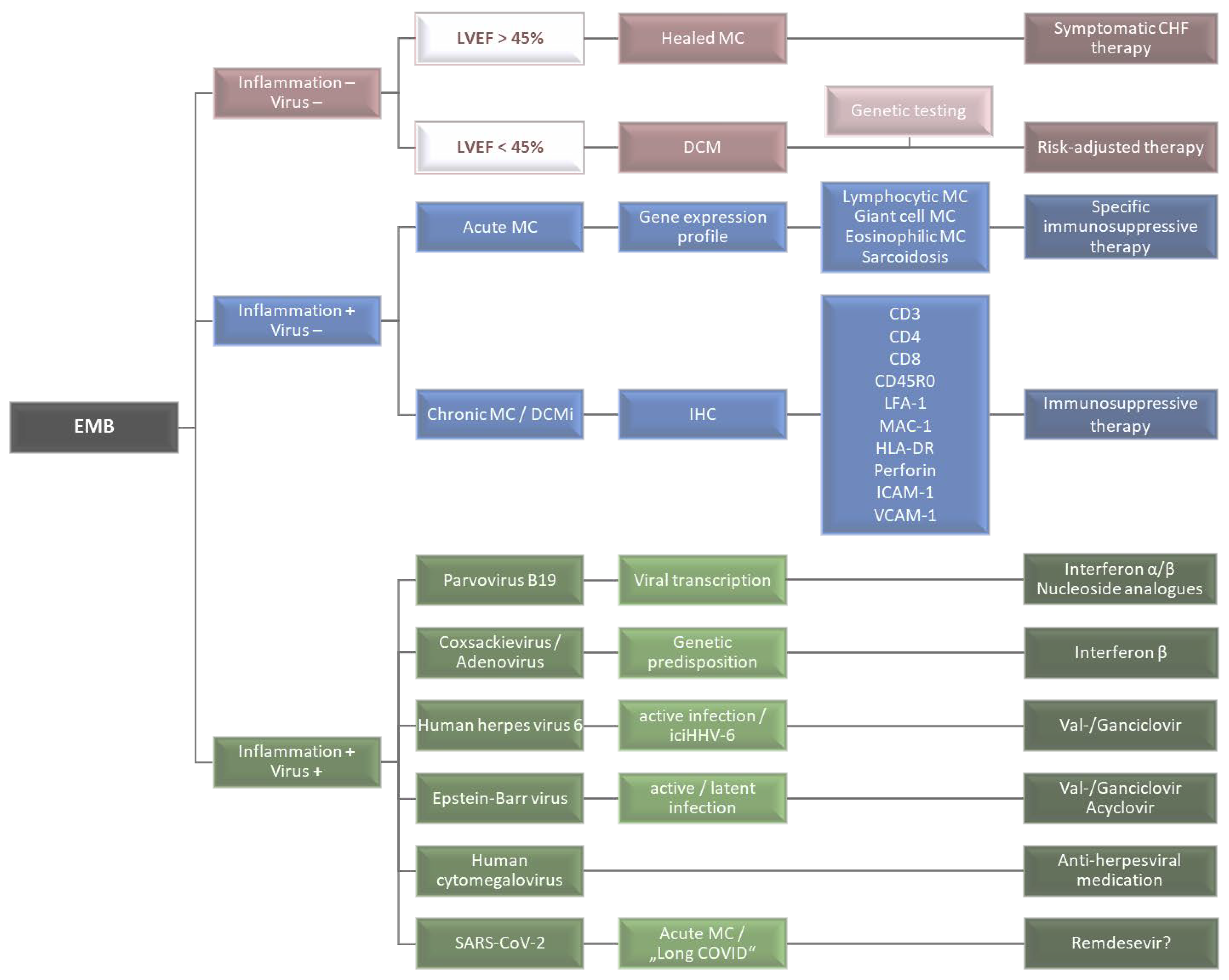
4. Advances in EMB Diagnostics
4.1. Gene Expression Profiling
4.2. miRNA Profiling
4.3. Next-Generation Sequencing in Pathogen Detection
4.4. Genetic Testing
5. Therapeutic Approaches
5.1. Immunosuppressive Therapy
5.2. Antiviral Therapy
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richardson, P.; McKenna, W.; Bristow, M.; Maisch, B.; Mautner, B.; O’Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Fairweather, D.L.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Prim. 2019, 5, 32. [Google Scholar] [CrossRef]
- Escher, F.; Kühl, U.; Lassner, D.; Schultheiss, H.-P. Cardiomyopathies-The special entity of myocarditis and inflammatory cardiomyopathy. J. Cardiol. Cardiovasc. Med. 2019, 4, 53–70. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Golpour, A.; Patriki, D.; Hanson, P.; McManus, B.; Heidecker, B. Epidemiological Impact of Myocarditis. J. Clin. Med. 2021, 10, 603. [Google Scholar] [CrossRef]
- Lampejo, T.; Durkin, S.M.; Bhatt, N.; Guttmann, O. Acute myocarditis: Aetiology, diagnosis and management. Clin. Med. 2021, 21, e505–e510. [Google Scholar] [CrossRef] [PubMed]
- Tymińska, A.; Ozierański, K.; Skwarek, A.; Kapłon-Cieślicka, A.; Baritussio, A.; Grabowski, M.; Marcolongo, R.; Caforio, A.L. Personalized Management of Myocarditis and Inflammatory Cardiomyopathy in Clinical Practice. J. Pers. Med. 2022, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T.J. Myocarditis. N. Engl. J. Med. 2009, 360, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Pilati, M.; Rebonato, M.; Formigari, R.; Butera, G. Endomyocardial Biopsy in Pediatric Myocarditis and Dilated Cardiomyopathy: A Tool in Search for a Role. J. Cardiovasc. Dev. Dis. 2022, 9, 24. [Google Scholar] [CrossRef]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.-P.; Baumeier, C.; Aleshcheva, G.; Bock, C.-T.; Escher, F. Viral Myocarditis—From Pathophysiology to Treatment. J. Clin. Med. 2021, 10, 5240. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.-P.; Baumeier, C.; Pietsch, H.; Bock, C.-T.; Poller, W.; Escher, F. Cardiovascular consequences of viral infections: From COVID to other viral diseases. Cardiovasc. Res. 2021, 117, 2610–2623. [Google Scholar] [CrossRef] [PubMed]
- Angelini, A.; Calabrese, F.; Thiene, G. Histology and Immunohistology of Myocarditis BT-Inflammatory Cardiomyopathy (DCMi): Pathogenesis and Therapy; Schultheiss, H.-P., Noutsias, M., Eds.; Birkhäuser Basel: Basel, Switzerland, 2010; pp. 185–199. ISBN 978-3-7643-8352-7. [Google Scholar]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Lurz, P.; Eitel, I.; Adam, J.; Steiner, J.; Grothoff, M.; Desch, S.; Fuernau, G.; de Waha, S.; Sareban, M.; Luecke, C.; et al. Diagnostic Performance of CMR Imaging Compared With EMB in Patients With Suspected Myocarditis. JACC Cardiovasc. Imaging 2012, 5, 513–524. [Google Scholar] [CrossRef]
- Hassan, K.; Doubell, A.; Kyriakakis, C.; Joubert, L.; Robbertse, P.-P.; Van Zyl, G.; Zaharie, D.; Herbst, P. Comparing the findings and diagnostic sensitivity of cardiovascular magnetic resonance in biopsy confirmed acute myocarditis with infarct-like vs. heart failure presentation. J. Cardiovasc. Magn. Reson. 2022, 24, 69. [Google Scholar] [CrossRef]
- Francone, M. Role of Cardiac Magnetic Resonance in the Evaluation of Dilated Cardiomyopathy: Diagnostic Contribution and Prognostic Significance. ISRN Radiol. 2014, 2014, 365404. [Google Scholar] [CrossRef]
- Bière, L.; Piriou, N.; Ernande, L.; Rouzet, F.; Lairez, O. Imaging of myocarditis and inflammatory cardiomyopathies. Arch. Cardiovasc. Dis. 2019, 112, 630–641. [Google Scholar] [CrossRef]
- Bustin, A.; Witschey, W.R.T.; van Heeswijk, R.B.; Cochet, H.; Stuber, M. Magnetic resonance myocardial T1ρ mapping. J. Cardiovasc. Magn. Reson. 2023, 25, 34. [Google Scholar] [CrossRef]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy. Circ. Heart Fail. 2020, 13, e007405. [Google Scholar] [CrossRef]
- McCarthy, R.E.; Boehmer, J.P.; Hruban, R.H.; Hutchins, G.M.; Kasper, E.K.; Hare, J.M.; Baughman, K.L. Long-Term Outcome of Fulminant Myocarditis as Compared with Acute (Nonfulminant) Myocarditis. N. Engl. J. Med. 2000, 342, 690–695. [Google Scholar] [CrossRef]
- Aretz, H.T. Myocarditis: The Dallas criteria. Hum. Pathol. 1987, 18, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Grogan, M.; Redfield, M.M.; Bailey, K.R.; Reeder, G.S.; Gersh, B.J.; Edwards, W.D.; Rodeheffer, R.J. Long-term outcome of patients with biopsy-proved myocarditis: Comparison with idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 1995, 26, 80–84. [Google Scholar] [CrossRef]
- Chow, L.H.; Radio, S.J.; Sears, T.D.; Mcmanus, B.M. Insensitivity of right ventricular endomyocardial biopsy in the diagnosis of myocarditis. J. Am. Coll. Cardiol. 1989, 14, 915–920. [Google Scholar] [CrossRef]
- Baughman, K.L. Diagnosis of Myocarditis. Circulation 2006, 113, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Shanes, J.G.; Ghali, J.; Billingham, M.E.; Ferrans, V.J.; Fenoglio, J.J.; Edwards, W.D.; Tsai, C.C.; Saffitz, J.E.; Isner, J.; Furner, S. Interobserver variability in the pathologic interpretation of endomyocardial biopsy results. Circulation 1987, 75, 401–405. [Google Scholar] [CrossRef] [PubMed]
- HAUCK, A.J.; KEARNEY, D.L.; EDWARDS, W.D. Evaluation of Postmortem Endomyocardial Biopsy Specimens From 38 Patients With Lymphocytic Myocarditis: Implications for Role of Sampling Error. Mayo Clin. Proc. 1989, 64, 1235–1245. [Google Scholar] [CrossRef]
- Leone, O.; Veinot, J.P.; Angelini, A.; Baandrup, U.T.; Basso, C.; Berry, G.; Bruneval, P.; Burke, M.; Butany, J.; Calabrese, F.; et al. 2011 Consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 2012, 21, 245–274. [Google Scholar] [CrossRef]
- Kuhl, U.; Noutsias, M.; Seeberg, B.; Schultheiss, H.P. Immunohistological evidence for a chronic intramyocardial inflammatory process in dilated cardiomyopathy. Heart 1996, 75, 295–300. [Google Scholar] [CrossRef]
- Noutsias, M.; Pauschinger, M.; Schultheiss, H.; Kühlabdf, U. Phenotypic characterization of infiltrates in dilated cardiomyopathy-diagnostic significance of T-lymphocytes and macrophages in inflammatory cardiomyopathy. Med. Sci. Monit. 2002, 8, 487. [Google Scholar]
- Ammirati, E.; Buono, A.; Moroni, F.; Gigli, L.; Power, J.R.; Ciabatti, M.; Garascia, A.; Adler, E.D.; Pieroni, M. State-of-the-Art of Endomyocardial Biopsy on Acute Myocarditis and Chronic Inflammatory Cardiomyopathy. Curr. Cardiol. Rep. 2022, 24, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Veronese, G.; Bottiroli, M.; Wang, D.W.; Cipriani, M.; Garascia, A.; Pedrotti, P.; Adler, E.D.; Frigerio, M. Update on acute myocarditis. Trends Cardiovasc. Med. 2021, 31, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Katzmann, J.L.; Schlattmann, P.; Rigopoulos, A.G.; Noutsias, E.; Bigalke, B.; Pauschinger, M.; Tschope, C.; Sedding, D.; Schulze, P.C.; Noutsias, M. Meta-analysis on the immunohistological detection of inflammatory cardiomyopathy in endomyocardial biopsies. Heart Fail. Rev. 2020, 25, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Kažukauskienė, I.; Baltrūnienė, V.; Jakubauskas, A.; Žurauskas, E.; Maneikienė, V.V.; Daunoravičius, D.; Čelutkienė, J.; Ručinskas, K.; Grabauskienė, V. Prevalence and prognostic relevance of myocardial inflammation and cardiotropic viruses in non-ischemic dilated cardiomyopathy. Cardiol. J. 2022, 29, 441–453. [Google Scholar] [CrossRef]
- Ohta-Ogo, K.; Sugano, Y.; Ogata, S.; Nakayama, T.; Komori, T.; Eguchi, K.; Dohi, K.; Yokokawa, T.; Kanamori, H.; Nishimura, S.; et al. Myocardial T-Lymphocytes as a Prognostic Risk-Stratifying Marker of Dilated Cardiomyopathy-Results of the Multicenter Registry to Investigate Inflammatory Cell Infiltration in Dilated Cardiomyopathy in Tissues of Endomyocardial Biopsy (INDICATE Study). Circ. J. 2022, 86, 1092–1101. [Google Scholar] [CrossRef]
- Nakayama, T.; Sugano, Y.; Yokokawa, T.; Nagai, T.; Matsuyama, T.; Ohta-Ogo, K.; Ikeda, Y.; Ishibashi-Ueda, H.; Nakatani, T.; Ohte, N.; et al. Clinical impact of the presence of macrophages in endomyocardial biopsies of patients with dilated cardiomyopathy. Eur. J. Heart Fail. 2017, 19, 490–498. [Google Scholar] [CrossRef]
- Chimenti, C.; Verardo, R.; Scopelliti, F.; Grande, C.; Petrosillo, N.; Piselli, P.; De Paulis, R.; Frustaci, A. Myocardial expression of Toll-like receptor 4 predicts the response to immunosuppressive therapy in patients with virus-negative chronic inflammatory cardiomyopathy. Eur. J. Heart Fail. 2017, 19, 915–925. [Google Scholar] [CrossRef]
- Escher, F.; Kühl, U.; Lassner, D.; Stroux, A.; Westermann, D.; Skurk, C.; Tschöpe, C.; Poller, W.; Schultheiss, H.-P. Presence of perforin in endomyocardial biopsies of patients with inflammatory cardiomyopathy predicts poor outcome. Eur. J. Heart Fail. 2014, 16, 1066–1072. [Google Scholar] [CrossRef]
- Escher, F.; Kühl, U.; Lassner, D.; Stroux, A.; Gross, U.; Westermann, D.; Pieske, B.; Poller, W.; Schultheiss, H. High Perforin-Positive Cardiac Cell Infiltration and Male Sex Predict Adverse Long-Term Mortality in Patients With Inflammatory Cardiomyopathy. J. Am. Heart Assoc. 2017, 6, e005352. [Google Scholar] [CrossRef]
- Woudstra, L.; Biesbroek, P.S.; Emmens, R.W.; Heymans, S.; Juffermans, L.J.; van der Wal, A.C.; van Rossum, A.C.; Niessen, H.W.M.; Krijnen, P.A.J. CD45 is a more sensitive marker than CD3 to diagnose lymphocytic myocarditis in the endomyocardium. Hum. Pathol. 2017, 62, 83–90. [Google Scholar] [CrossRef]
- Noutsias, M.; Hohmann, C.; Pauschinger, M.; Schwimmbeck, P.-L.; Ostermann, K.; Rode, U.; Yacoub, M.H.; Kühl, U.; Schultheiss, H.-P. sICAM-1 correlates with myocardial ICAM-1 expression in dilated cardiomyopathy. Int. J. Cardiol. 2003, 91, 153–161. [Google Scholar] [CrossRef] [PubMed]
- García-Rivas, G.; Castillo, E.C.; Gonzalez-Gil, A.M.; Maravillas-Montero, J.L.; Brunck, M.; Torres-Quintanilla, A.; Elizondo-Montemayor, L.; Torre-Amione, G. The role of B cells in heart failure and implications for future immunomodulatory treatment strategies. ESC Heart Fail. 2020, 7, 1387–1399. [Google Scholar] [CrossRef] [PubMed]
- Vdovenko, D.; Eriksson, U. Regulatory Role of CD4+ T Cells in Myocarditis. J. Immunol. Res. 2018, 2018, 4396351. [Google Scholar] [CrossRef] [PubMed]
- Henke, A.; Huber, S.; Stelzner, A.; Whitton, J.L. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J. Virol. 1995, 69, 6720–6728. [Google Scholar] [CrossRef] [PubMed]
- Santos-Zas, I.; Lemarié, J.; Zlatanova, I.; Cachanado, M.; Seghezzi, J.-C.; Benamer, H.; Goube, P.; Vandestienne, M.; Cohen, R.; Ezzo, M.; et al. Cytotoxic CD8+ T cells promote granzyme B-dependent adverse post-ischemic cardiac remodeling. Nat. Commun. 2021, 12, 1483. [Google Scholar] [CrossRef]
- Bracamonte-Baran, W.; Čiháková, D. Cardiac Autoimmunity: Myocarditis. Adv. Exp. Med. Biol. 2017, 1003, 187–221. [Google Scholar] [CrossRef]
- Eriksson, U.; Ricci, R.; Hunziker, L.; Kurrer, M.O.; Oudit, G.Y.; Watts, T.H.; Sonderegger, I.; Bachmaier, K.; Kopf, M.; Penninger, J.M. Dendritic cell–induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat. Med. 2003, 9, 1484–1490. [Google Scholar] [CrossRef]
- Jurcova, I.; Rocek, J.; Bracamonte-Baran, W.; Zelizko, M.; Netuka, I.; Maluskova, J.; Kautzner, J.; Cihakova, D.; Melenovsky, V.; Maly, J. Complete recovery of fulminant cytotoxic CD8 T-cell-mediated myocarditis after ECMELLA unloading and immunosuppression. ESC Heart Fail. 2020, 7, 1976–1981. [Google Scholar] [CrossRef]
- Streitz, M.; Noutsias, M.; Volkmer, R.; Rohde, M.; Brestrich, G.; Block, A.; Klippert, K.; Kotsch, K.; Ay, B.; Hummel, M.; et al. NS1 Specific CD8+ T-Cells with Effector Function and TRBV11 Dominance in a Patient with Parvovirus B19 Associated Inflammatory Cardiomyopathy. PLoS ONE 2008, 3, e2361. [Google Scholar] [CrossRef]
- Penninger, J.M.; Pummerer, C.; Liu, P.; Neu, N.; Bachmaier, K. Cellular and molecular mechanisms of murine autoimmune myocarditis. Apmis 1997, 105, 1–13. [Google Scholar] [CrossRef]
- Chen, P.; Baldeviano, G.C.; Ligons, D.L.; Talor, M.V.; Barin, J.G.; Rose, N.R.; Cihakova, D. Susceptibility to autoimmune myocarditis is associated with intrinsic differences in CD4+ T cells. Clin. Exp. Immunol. 2012, 169, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Laroumanie, F.; Douin-Echinard, V.; Pozzo, J.; Lairez, O.; Tortosa, F.; Vinel, C.; Delage, C.; Calise, D.; Dutaur, M.; Parini, A.; et al. CD4 + T Cells Promote the Transition From Hypertrophy to Heart Failure During Chronic Pressure Overload. Circulation 2014, 129, 2111–2124. [Google Scholar] [CrossRef] [PubMed]
- Baumeier, C.; Aleshcheva, G.; Harms, D.; Gross, U.; Hamm, C.; Assmus, B.; Westenfeld, R.; Kelm, M.; Rammos, S.; Wenzel, P.; et al. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. Int. J. Mol. Sci. 2022, 23, 6940. [Google Scholar] [CrossRef]
- Hua, X.; Hu, G.; Hu, Q.; Chang, Y.; Hu, Y.; Gao, L.; Chen, X.; Yang, P.-C.; Zhang, Y.; Li, M.; et al. Single-Cell RNA Sequencing to Dissect the Immunological Network of Autoimmune Myocarditis. Circulation 2020, 142, 384–400. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.M.; Cooper, L.T.; Kem, D.C.; Stavrakis, S.; Kosanke, S.D.; Shevach, E.M.; Fairweather, D.; Stoner, J.A.; Cox, C.J.; Cunningham, M.W. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight 2016, 1, e85851. [Google Scholar] [CrossRef] [PubMed]
- Barin, J.G.; Rose, N.R.; Čiháková, D. Macrophage diversity in cardiac inflammation: A review. Immunobiology 2012, 217, 468–475. [Google Scholar] [CrossRef]
- Lafuse, W.P.; Wozniak, D.J.; Rajaram, M.V.S. Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair. Cells 2020, 10, 51. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, H.; Yao, Y.; Tao, Z.; Chen, W.; Huang, F.; Chen, X. Macrophage, a potential targeted therapeutic immune cell for cardiomyopathy. Front. Cell Dev. Biol. 2022, 10, 908790. [Google Scholar] [CrossRef]
- Hou, X.; Chen, G.; Bracamonte-Baran, W.; Choi, H.S.; Diny, N.L.; Sung, J.; Hughes, D.; Won, T.; Wood, M.K.; Talor, M.V.; et al. The Cardiac Microenvironment Instructs Divergent Monocyte Fates and Functions in Myocarditis. Cell Rep. 2019, 28, 172–189.e7. [Google Scholar] [CrossRef]
- Baumeier, C.; Escher, F.; Aleshcheva, G.; Pietsch, H.; Schultheiss, H.-P. Plasminogen activator inhibitor-1 reduces cardiac fibrosis and promotes M2 macrophage polarization in inflammatory cardiomyopathy. Basic Res. Cardiol. 2021, 116, 1. [Google Scholar] [CrossRef]
- Bertani, F.R.; Mozetic, P.; Fioramonti, M.; Iuliani, M.; Ribelli, G.; Pantano, F.; Santini, D.; Tonini, G.; Trombetta, M.; Businaro, L.; et al. Classification of M1/M2-polarized human macrophages by label-free hyperspectral reflectance confocal microscopy and multivariate analysis. Sci. Rep. 2017, 7, 8965. [Google Scholar] [CrossRef] [PubMed]
- Kindermann, I.; Kindermann, M.; Kandolf, R.; Klingel, K.; Bültmann, B.; Müller, T.; Lindinger, A.; Böhm, M. Predictors of Outcome in Patients With Suspected Myocarditis. Circulation 2008, 118, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Mahon, N.G.; Madden, B.P.; Caforio, A.L.; Elliott, P.M.; Haven, A.J.; Keogh, B.E.; Davies, M.J.; McKenna, W.J. Immunohistologic evidence of myocardial disease in apparently healthy relatives of patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 2002, 39, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Noutsias, M.; Seeberg, B.; Schultheiss, H.-P.; Kühl, U. Expression of Cell Adhesion Molecules in Dilated Cardiomyopathy. Circulation 1999, 99, 2124–2131. [Google Scholar] [CrossRef]
- Wang, T.; Tian, J.; Jin, Y. VCAM1 expression in the myocardium is associated with the risk of heart failure and immune cell infiltration in myocardium. Sci. Rep. 2021, 11, 19488. [Google Scholar] [CrossRef]
- Troncoso, M.F.; Ortiz-Quintero, J.; Garrido-Moreno, V.; Sanhueza-Olivares, F.; Guerrero-Moncayo, A.; Chiong, M.; Castro, P.F.; García, L.; Gabrielli, L.; Corbalán, R.; et al. VCAM-1 as a predictor biomarker in cardiovascular disease. Biochim. Biophys. Acta-Mol. Basis Dis. 2021, 1867, 166170. [Google Scholar] [CrossRef]
- Toyozaki, T.; Saito, T.; Takano, H.; Yorimitsu, K.; Kobayashi, S.; Ichikawa, H.; Takeda, K.; Inagaki, Y. Expression of intercellular adhesion molecule-1 on cardiac myocytes for myocarditis before and during immunosuppressive therapy. Am. J. Cardiol. 1993, 72, 441–444. [Google Scholar] [CrossRef]
- Salvador, A.M.; Nevers, T.; Velázquez, F.; Aronovitz, M.; Wang, B.; Abadía Molina, A.; Jaffe, I.Z.; Karas, R.H.; Blanton, R.M.; Alcaide, P. Intercellular Adhesion Molecule 1 Regulates Left Ventricular Leukocyte Infiltration, Cardiac Remodeling, and Function in Pressure Overload–Induced Heart Failure. J. Am. Heart Assoc. 2016, 5, e003126. [Google Scholar] [CrossRef]
- Lin, Q.-Y.; Lang, P.-P.; Zhang, Y.-L.; Yang, X.-L.; Xia, Y.-L.; Bai, J.; Li, H.-H. Pharmacological blockage of ICAM-1 improves angiotensin II-induced cardiac remodeling by inhibiting adhesion of LFA-1 + monocytes. Am. J. Physiol. Circ. Physiol. 2019, 317, H1301–H1311. [Google Scholar] [CrossRef]
- Haybar, H.; Shahrabi, S.; Shahjahani, M.; Rezaeeyan, H. Diagnostic Value of HLA Typing in Pathogenesis of Cardiomyopathy. Cardiovasc. Hematol. Disord. Targets 2019, 19, 132–138. [Google Scholar] [CrossRef]
- Meder, B.; Rühle, F.; Weis, T.; Homuth, G.; Keller, A.; Franke, J.; Peil, B.; Lorenzo Bermejo, J.; Frese, K.; Huge, A.; et al. A genome-wide association study identifies 6p21 as novel risk locus for dilated cardiomyopathy. Eur. Heart J. 2014, 35, 1069–1077. [Google Scholar] [CrossRef]
- Bowles, N.E.; Richardson, P.J.; Olsen, E.G.; Archard, L.C. Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet 1986, 1, 1120–1123. [Google Scholar] [CrossRef] [PubMed]
- Jin, O.; Sole, M.J.; Butany, J.W.; Chia, W.K.; McLaughlin, P.R.; Liu, P.; Liew, C.C. Detection of enterovirus RNA in myocardial biopsies from patients with myocarditis and cardiomyopathy using gene amplification by polymerase chain reaction. Circulation 1990, 82, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Kühl, U.; Pauschinger, M.; Noutsias, M.; Seeberg, B.; Bock, T.; Lassner, D.; Poller, W.; Kandolf, R.; Schultheiss, H.-P. High Prevalence of Viral Genomes and Multiple Viral Infections in the Myocardium of Adults With “Idiopathic” Left Ventricular Dysfunction. Circulation 2005, 111, 887–893. [Google Scholar] [CrossRef] [PubMed]
- 76. Mahrholdt, H.; Wagner, A.; Deluigi, C.C.; Kispert, E.; Hager, S.; Meinhardt, G.; Vogelsberg, H.; Fritz, P.; Dippon, J.; Bock, C.T.; et al. Presentation, Patterns of Myocardial Damage, and Clinical Course of Viral Myocarditis. Circulation 2006, 114, 1581–1590. [Google Scholar] [CrossRef]
- Hassan, K.; Kyriakakis, C.; Doubell, A.; Van Zyl, G.; Claassen, M.; Zaharie, D.; Herbst, P. Prevalence of cardiotropic viruses in adults with clinically suspected myocarditis in South Africa. Open Heart 2022, 9, e001942. [Google Scholar] [CrossRef]
- Escher, F.; Aleshcheva, G.; Pietsch, H.; Baumeier, C.; Gross, U.M.; Schrage, B.N.; Westermann, D.; Bock, C.-T.; Schultheiss, H.-P. Transcriptional Active Parvovirus B19 Infection Predicts Adverse Long-Term Outcome in Patients with Non-Ischemic Cardiomyopathy. Biomedicines 2021, 9, 1898. [Google Scholar] [CrossRef]
- Kuhl, U.; Pauschinger, M.; Seeberg, B.; Lassner, D.; Noutsias, M.; Poller, W.; Schultheiss, H.P. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 2005, 112, 1965–1970. [Google Scholar] [CrossRef]
- Kühl, U.; Pauschinger, M.; Schwimmbeck, P.L.; Seeberg, B.; Lober, C.; Noutsias, M.; Poller, W.; Schultheiss, H.-P. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation 2003, 107, 2793–2798. [Google Scholar] [CrossRef]
- Richter, J.; Quintanilla-Martinez, L.; Bienemann, K.; Zeus, T.; Germing, U.; Sander, O.; Kandolf, R.; Häussinger, D.; Klingel, K. An unusual presentation of a common infection. Infection 2013, 41, 565–569. [Google Scholar] [CrossRef]
- Chimenti, C.; Russo, A.; Pieroni, M.; Calabrese, F.; Verardo, R.; Thiene, G.; Russo, M.A.; Maseri, A.; Frustaci, A. Intramyocyte detection of Epstein-Barr virus genome by laser capture microdissection in patients with inflammatory cardiomyopathy. Circulation 2004, 110, 3534–3539. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.; Verardo, R.; Grande, C.; Francone, M.; Frustaci, A. Infarct-like myocarditis with coronary vasculitis and aneurysm formation caused by Epstein–Barr virus infection. ESC Heart Fail. 2020, 7, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Molina, K.; Denfield, S.; Fan, Y.; Moulik, M.; Towbin, J.; Dreyer, W.; Rossano, J. Viral endomyocardial infection in the 1st year post transplant is associated with persistent inflammation in children who have undergone cardiac transplant. Cardiol. Young 2014, 24, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.-E.; Glaser, R.; Kaumaya, P.T.P.; Jones, C.; Williams, M.V. The EBV-Encoded dUTPase Activates NF-κB through the TLR2 and MyD88-Dependent Signaling Pathway. J. Immunol. 2009, 182, 851–859. [Google Scholar] [CrossRef]
- Häusler, M.; Sellhaus, B.; Scheithauer, S.; Gaida, B.; Kuropka, S.; Siepmann, K.; Panek, A.; Berg, W.; Teubner, A.; Ritter, K.; et al. Myocarditis in newborn wild-type BALB/c mice infected with the murine gamma herpesvirus MHV-68. Cardiovasc. Res. 2007, 76, 323–330. [Google Scholar] [CrossRef]
- Rafailidis, P.I.; Mavros, M.N.; Kapaskelis, A.; Falagas, M.E. Antiviral treatment for severe EBV infections in apparently immunocompetent patients. J. Clin. Virol. 2010, 49, 151–157. [Google Scholar] [CrossRef]
- Petrie, B.L.; Melnick, J.L.; Adam, E.; Burek, J.; McCollum, C.H.; Debakey, M.E. Nucleic Acid Sequences of Cytomegalovirus in Cells Cultured from Human Arterial Tissue. J. Infect. Dis. 1987, 155, 158–159. [Google Scholar] [CrossRef]
- Rice, G.P.A.; Schrier, R.D.; Oldstone, M.B.A. Cytomegalovirus infects human lymphocytes and monocytes: Virus expression is restricted to immediate-early gene products. Proc. Natl. Acad. Sci. USA 1984, 81, 6134–6138. [Google Scholar] [CrossRef]
- Melnick, J.L.; Dreesman, G.R.; Mccollum, C.H.; Petrie, B.L.; Burek, J.; Debakey, M.E. Cytomegalovirus Antigen Within Human Arterial Smooth Muscle Cells. Lancet 1983, 322, 644–647. [Google Scholar] [CrossRef]
- Grattan, M.T.; Moreno Cabral, C.E.; Starnes, V.A.; Oyer, P.E.; Stinson, E.B.; Shumway, N.E. Cytomegalovirus Infection Is Associated With Cardiac Allograft Rejection and Atherosclerosis. JAMA J. Am. Med. Assoc. 1989, 261, 3561–3566. [Google Scholar] [CrossRef]
- Saraca, L.M.; Lazzari, L.; di Giuli, C.; Lavagna, A.; Mezzetti, P.; Bovelli, D.; Boschetti, E.; Francisci, D. Cytomegalovirus myocarditis in a patient with systemic lupus erythematosus (SLE) successfully treated with ganciclovir. IDCases 2018, 12, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Barnabei, M.S.; Sjaastad, F.V.; Townsend, D.W.; Bedada, F.B.; Metzger, J.M. Severe dystrophic cardiomyopathy caused by the enteroviral protease 2A-mediated C-terminal dystrophin cleavage fragment. Sci. Transl. Med. 2015, 7, 294ra106. [Google Scholar] [CrossRef] [PubMed]
- Bouin, A.; Gretteau, P.-A.; Wehbe, M.; Renois, F.; N’Guyen, Y.; Lévêque, N.; Vu, M.N.; Tracy, S.; Chapman, N.M.; Bruneval, P.; et al. Enterovirus Persistence in Cardiac Cells of Patients With Idiopathic Dilated Cardiomyopathy Is Linked to 5’ Terminal Genomic RNA-Deleted Viral Populations With Viral-Encoded Proteinase Activities. Circulation 2019, 139, 2326–2338. [Google Scholar] [CrossRef]
- Caruso, A.; Rotola, A.; Comar, M.; Favilli, F.; Galvan, M.; Tosetti, M.; Campello, C.; Caselli, E.; Alessandri, G.; Grassi, M.; et al. HHV-6 infects human aortic and heart microvascular endothelial cells, increasing their ability to secrete proinflammatory chemokines. J. Med. Virol. 2002, 67, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Seitz, A.; Martinez Pereyra, V.; Hubert, A.; Klingel, K.; Bekeredjian, R.; Sechtem, U.; Ong, P. Epicardial and microvascular coronary artery spasm in biopsy-proven viral myocarditis. Int. J. Cardiol. 2022, 360, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Vallbracht, K.B.; Schwimmbeck, P.L.; Kühl, U.; Rauch, U.; Seeberg, B.; Schultheiss, H.-P. Differential aspects of endothelial function of the coronary microcirculation considering myocardial virus persistence, endothelial activation, and myocardial leukocyte infiltrates. Circulation 2005, 111, 1784–1791. [Google Scholar] [CrossRef]
- Kühl, U.; Lassner, D.; Wallaschek, N.; Gross, U.M.; Krueger, G.R.F.; Seeberg, B.; Kaufer, B.B.; Escher, F.; Poller, W.; Schultheiss, H.-P. Chromosomally integrated human herpesvirus 6 in heart failure: Prevalence and treatment. Eur. J. Heart Fail. 2015, 17, 9–19. [Google Scholar] [CrossRef]
- Tweedy, J.; Spyrou, M.A.; Pearson, M.; Lassner, D.; Kuhl, U.; Gompels, U.A. Complete Genome Sequence of Germline Chromosomally Integrated Human Herpesvirus 6A and Analyses Integration Sites Define a New Human Endogenous Virus with Potential to Reactivate as an Emerging Infection. Viruses 2016, 8, 19. [Google Scholar] [CrossRef]
- Hakacova, N.; Klingel, K.; Kandolf, R.; Engdahl, E.; Fogdell-Hahn, A.; Higgins, T. First therapeutic use of Artesunate in treatment of human herpesvirus 6B myocarditis in a child. J. Clin. Virol. 2013, 57, 157–160. [Google Scholar] [CrossRef]
- Bültmann, B.D.; Klingel, K.; Sotlar, K.; Bock, C.T.; Baba, H.A.; Sauter, M.; Kandolf, R. Fatal parvovirus B19-associated myocarditis clinically mimicking ischemic heart disease: An endothelial cell-mediated disease. Hum. Pathol. 2003, 34, 92–95. [Google Scholar] [CrossRef]
- Schowengerdt, K.O.; Ni, J.; Denfield, S.W.; Gajarski, R.J.; Bowles, N.E.; Rosenthal, G.; Kearney, D.L.; Price, J.K.; Rogers, B.B.; Schauer, G.M.; et al. Association of parvovirus B19 genome in children with myocarditis and cardiac allograft rejection: Diagnosis using the polymerase chain reaction. Circulation 1997, 96, 3549–3554. [Google Scholar] [CrossRef]
- Das, B.B.; Prusty, B.K.; Niu, J.; Huang, M.-L.; Zhu, H.; Eliassen, E.; Kuypers, J.M.; Jerome, K.R. Detection of parvovirus B19 and human herpesvirus 6 in pediatric dilated cardiomyopathy: Impact after heart transplantation. Ann. Pediatr. Cardiol. 2020, 13, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, U.; Lassner, D.; Dorner, A.; Rohde, M.; Escher, F.; Seeberg, B.; Hertel, E.; Tschope, C.; Skurk, C.; Gross, U.M.; et al. A distinct subgroup of cardiomyopathy patients characterized by transcriptionally active cardiotropic erythrovirus and altered cardiac gene expression. Basic Res. Cardiol. 2013, 108, 372. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.-P.; Bock, C.-T.; Aleshcheva, G.; Baumeier, C.; Poller, W.; Escher, F. Interferon-β Suppresses Transcriptionally Active Parvovirus B19 Infection in Viral Cardiomyopathy: A Subgroup Analysis of the BICC-Trial. Viruses 2022, 14, 444. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.-P.; Piper, C.; Sowade, O.; Waagstein, F.; Kapp, J.-F.; Wegscheider, K.; Groetzbach, G.; Pauschinger, M.; Escher, F.; Arbustini, E.; et al. Betaferon in chronic viral cardiomyopathy (BICC) trial: Effects of interferon-β treatment in patients with chronic viral cardiomyopathy. Clin. Res. Cardiol. 2016, 105, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Elsanhoury, A.; Klein, O.; Sosnowski, M.; Miteva, K.; Lassner, D.; Abou-El-Enein, M.; Pieske, B.; Kühl, U.; Tschöpe, C. Telbivudine in chronic lymphocytic myocarditis and human parvovirus B19 transcriptional activity. ESC Heart Fail. 2018, 5, 818–829. [Google Scholar] [CrossRef]
- Schultheiss, H.-P.; Bock, T.; Pietsch, H.; Aleshcheva, G.; Baumeier, C.; Fruhwald, F.; Escher, F. Nucleoside Analogue Reverse Transcriptase Inhibitors Improve Clinical Outcome in Transcriptional Active Human Parvovirus B19-Positive Patients. J. Clin. Med. 2021, 10, 1928. [Google Scholar] [CrossRef]
- Koenig, T.; Kempf, T.; Schultheiss, H.P.; Cornberg, M.; Bauersachs, J.; Schäfer, A. Fulminant Parvovirus B19 Myocarditis after Chemotherapy: Full Recovery after Antiviral Therapy with Tenofovir. Clin. Res. Cardiol. 2022, 111, 233–236. [Google Scholar] [CrossRef]
- Bojkova, D.; Wagner, J.U.G.; Shumliakivska, M.; Aslan, G.S.; Saleem, U.; Hansen, A.; Luxán, G.; Günther, S.; Pham, M.D.; Krishnan, J.; et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc. Res. 2020, 116, 2207–2215. [Google Scholar] [CrossRef]
- Evans, P.C.; Ed Rainger, G.; Mason, J.C.; Guzik, T.J.; Osto, E.; Stamataki, Z.; Neil, D.; Hoefer, I.E.; Fragiadaki, M.; Waltenberger, J.; et al. Endothelial dysfunction in COVID-19: A position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc. Res. 2020, 116, 2177–2184. [Google Scholar] [CrossRef]
- Hudowenz, O.; Klemm, P.; Lange, U.; Rolf, A.; Schultheiss, H.P.; Hamm, C.; Muller-Ladner, U.; Wegner, F. Case report of severe PCR-confirmed COVID-19 myocarditis in a European patient manifesting in mid January 2020. Eur. Heart J. Case Rep. 2020, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rohun, J.; Dorniak, K.; Faran, A.; Kochańska, A.; Zacharek, D.; Daniłowicz-Szymanowicz, L. Long COVID-19 Myocarditis and Various Heart Failure Presentations: A Case Series. J. Cardiovasc. Dev. Dis. 2022, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Blagova, O.; Lutokhina, Y.; Kogan, E.; Kukleva, A.; Ainetdinova, D.; Novosadov, V.; Rud, R.; Savina, P.; Zaitsev, A.; Fomin, V. Chronic biopsy proven post-COVID myoendocarditis with SARS-CoV-2 persistence and high level of antiheart antibodies. Clin. Cardiol. 2022, 45, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Marchiano, S.; Hsiang, T.Y.; Khanna, A.; Higashi, T.; Whitmore, L.S.; Bargehr, J.; Davaapil, H.; Chang, J.; Smith, E.; Ong, L.P.; et al. SARS-CoV-2 Infects Human Pluripotent Stem Cell-Derived Cardiomyocytes, Impairing Electrical and Mechanical Function. Stem. Cell. Rep. 2021, 16, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Urban, S.; Fułek, M.; Błaziak, M.; Iwanek, G.; Jura, M.; Fułek, K.; Guzik, M.; Garus, M.; Gajewski, P.; Lewandowski, Ł.; et al. COVID-19 Related Myocarditis in Adults: A Systematic Review of Case Reports. J. Clin. Med. 2022, 11, 5519. [Google Scholar] [CrossRef]
- Cambridge, G.; MacArthur, C.G.; Waterson, A.P.; Goodwin, J.F.; Oakley, C.M. Antibodies to Coxsackie B viruses in congestive cardiomyopathy. Br. Heart J. 1979, 41, 692–696. [Google Scholar] [CrossRef]
- Li, Y.; Bourlet, T.; Andreoletti, L.; Mosnier, J.F.; Peng, T.; Yang, Y.; Archard, L.C.; Pozzetto, B.; Zhang, H. Enteroviral capsid protein VP1 is present in myocardial tissues from some patients with myocarditis or dilated cardiomyopathy. Circulation 2000, 101, 231–234. [Google Scholar] [CrossRef]
- Andréoletti, L.; Bourlet, T.; Moukassa, D.; Rey, L.; Hot, D.; Li, Y.; Lambert, V.; Gosselin, B.; Mosnier, J.F.; Stankowiak, C.; et al. Enteroviruses can persist with or without active viral replication in cardiac tissue of patients with end-stage ischemic or dilated cardiomyopathy. J. Infect. Dis. 2000, 182, 1222–1227. [Google Scholar] [CrossRef]
- Pauschinger, M.; Doerner, A.; Kuehl, U.; Schwimmbeck, P.L.; Poller, W.; Kandolf, R.; Schultheiss, H.P. Enteroviral RNA replication in the myocardium of patients with left ventricular dysfunction and clinically suspected myocarditis. Circulation 1999, 99, 889–895. [Google Scholar] [CrossRef]
- Badorff, C.; Lee, G.H.; Lamphear, B.J.; Martone, M.E.; Campbell, K.P.; Rhoads, R.E.; Knowlton, K.U. Enteroviral protease 2A cleaves dystrophin: Evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat. Med. 1999, 5, 320–326. [Google Scholar] [CrossRef]
- Andréoletti, L.; Ventéo, L.; Douche-Aourik, F.; Canas, F.; Lorin de la Grandmaison, G.; Jacques, J.; Moret, H.; Jovenin, N.; Mosnier, J.F.; Matta, M.; et al. Active Coxsackieviral B infection is associated with disruption of dystrophin in endomyocardial tissue of patients who died suddenly of acute myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 2207–2214. [Google Scholar] [CrossRef]
- Kühl, U.; Lassner, D.; von Schlippenbach, J.; Poller, W.; Schultheiss, H.-P. Interferon-Beta improves survival in enterovirus-associated cardiomyopathy. J. Am. Coll. Cardiol. 2012, 60, 1295–1296. [Google Scholar] [CrossRef] [PubMed]
- Pauschinger, M.; Bowles, N.E.; Fuentes-Garcia, F.J.; Pham, V.; Kühl, U.; Schwimmbeck, P.L.; Schultheiss, H.P.; Towbin, J.A. Detection of adenoviral genome in the myocardium of adult patients with idiopathic left ventricular dysfunction. Circulation 1999, 99, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Bowles, N.E.; Ni, J.; Marcus, F.; Towbin, J.A. The detection of cardiotropic viruses in the myocardium of patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J. Am. Coll. Cardiol. 2002, 39, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Bowles, N.E.; Ni, J.; Kearney, D.L.; Pauschinger, M.; Schultheiss, H.-P.; McCarthy, R.; Hare, J.; Bricker, J.T.; Bowles, K.R.; Towbin, J.A. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J. Am. Coll. Cardiol. 2003, 42, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Donoso Mantke, O.; Meyer, R.; Prösch, S.; Nitsche, A.; Leitmeyer, K.; Kallies, R.; Niedrig, M. High prevalence of cardiotropic viruses in myocardial tissue from explanted hearts of heart transplant recipients and heart donors: A 3-year retrospective study from a German patients’ pool. J. Heart Lung Transpl. 2005, 24, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Breinholt, J.P.; Moulik, M.; Dreyer, W.J.; Denfield, S.W.; Kim, J.J.; Jefferies, J.L.; Rossano, J.W.; Gates, C.M.; Clunie, S.K.; Bowles, K.R.; et al. Viral epidemiologic shift in inflammatory heart disease: The increasing involvement of parvovirus B19 in the myocardium of pediatric cardiac transplant patients. J. Heart Lung Transpl. 2010, 29, 739–746. [Google Scholar] [CrossRef]
- Schmidt-Lucke, C.; Spillmann, F.; Bock, T.; Kühl, U.; Van Linthout, S.; Schultheiss, H.; Tschöpe, C. Interferon Beta Modulates Endothelial Damage in Patients with Cardiac Persistence of Human Parvovirus B19 Infection. J. Infect. Dis. 2010, 201, 936–945. [Google Scholar] [CrossRef]
- Vallbracht, K.B.; Schwimmbeck, P.L.; Kühl, U.; Seeberg, B.; Schultheiss, H.P. Endothelium-dependent flow-mediated vasodilation of systemic arteries is impaired in patients with myocardial virus persistence. Circulation 2004, 110, 2938–2945. [Google Scholar] [CrossRef]
- Schmidt-Lucke, C.; Zobel, T.; Schrepfer, S.; Kuhl, U.; Wang, D.; Klingel, K.; Becher, P.M.; Fechner, H.; Pozzuto, T.; Van Linthout, S.; et al. Impaired Endothelial Regeneration Through Human Parvovirus B19-Infected Circulating Angiogenic Cells in Patients With Cardiomyopathy. J. Infect. Dis. 2015, 212, 1070–1081. [Google Scholar] [CrossRef]
- Schmidt-Lucke, C.; Zobel, T.; Escher, F.; Tschöpe, C.; Lassner, D.; Kühl, U.; Gubbe, K.; Volk, H.-D.; Schultheiss, H.-P. Human Parvovirus B19 (B19V) Up-regulates CXCR4 Surface Expression of Circulating Angiogenic Cells: Implications for Cardiac Ischemia in B19V Cardiomyopathy. J. Infect. Dis. 2018, 217, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.-T.; Klingel, K.; Kandolf, R. Human Parvovirus B19–Associated Myocarditis. N. Engl. J. Med. 2010, 362, 1248–1249. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, H.; Escher, F.; Aleshcheva, G.; Lassner, D.; Bock, C.-T.; Schultheiss, H.-P. Detection of parvovirus mRNAs as markers for viral activity in endomyocardial biopsy-based diagnosis of patients with unexplained heart failure. Sci. Rep. 2020, 10, 22354. [Google Scholar] [CrossRef] [PubMed]
- Escher, F.; Kuhl, U.; Sabi, T.; Suckau, L.; Lassner, D.; Poller, W.; Schultheiss, H.-P.; Noutsias, M. Immunohistological detection of Parvovirus B19 capsid proteins in endomyocardial biopsies from dilated cardiomyopathy patients. Med. Sci. Monit. 2008, 14, CR333–CR338. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Gewartowska, M.; Przybylski, M.; Kuffner, M.; Wiligórska, D.; Gil, R.; Król, Z.; Frontczak-Baniewicz, M. Ultrastructural Changes in Mitochondria in Patients with Dilated Cardiomyopathy and Parvovirus B19 Detected in Heart Tissue without Myocarditis. J. Pers. Med. 2022, 12, 177. [Google Scholar] [CrossRef]
- Bock, C.-T.; Düchting, A.; Utta, F.; Brunner, E.; Sy, B.T.; Klingel, K.; Lang, F.; Gawaz, M.; Felix, S.B.; Kandolf, R. Molecular phenotypes of human parvovirus B19 in patients with myocarditis. World J. Cardiol. 2014, 6, 183–195. [Google Scholar] [CrossRef]
- Rohayem, J.; Dinger, J.; Fischer, R.; Klingel, K.; Kandolf, R.; Rethwilm, A. Fatal myocarditis associated with acute parvovirus B19 and human herpesvirus 6 coinfection. J. Clin. Microbiol. 2001, 39, 4585–4587. [Google Scholar] [CrossRef]
- Tschope, C.; Bock, C.-T.; Kasner, M.; Noutsias, M.; Westermann, D.; Schwimmbeck, P.-L.; Pauschinger, M.; Poller, W.-C.; Kühl, U.; Kandolf, R.; et al. High Prevalence of Cardiac Parvovirus B19 Infection in Patients With Isolated Left Ventricular Diastolic Dysfunction. Circulation 2005, 111, 879–886. [Google Scholar] [CrossRef]
- Bachelier, K.; Biehl, S.; Schwarz, V.; Kindermann, I.; Kandolf, R.; Sauter, M.; Ukena, C.; Yilmaz, A.; Sliwa, K.; Bock, C.-T.; et al. Parvovirus B19-induced vascular damage in the heart is associated with elevated circulating endothelial microparticles. PLoS ONE 2017, 12, e0176311. [Google Scholar] [CrossRef]
- Callon, D.; Berri, F.; Lebreil, A.-L.; Fornès, P.; Andreoletti, L. Coinfection of Parvovirus B19 with Influenza A/H1N1 Causes Fulminant Myocarditis and Pneumonia. An Autopsy Case Report. Pathogens 2021, 10, 958. [Google Scholar] [CrossRef]
- Nikolaou, M.; Lazaros, G.; Karavidas, A.; Hatzianastasiou, S.; Miliopoulos, D.; Adamopoulos, S. Recurrent viral myocarditis: The emerging link toward dilated cardiomyopathy. Hell. J. Cardiol. 2018, 59, 60–63. [Google Scholar] [CrossRef]
- Russcher, A.; Verdonschot, J.; Molenaar-de Backer, M.W.A.; Heymans, S.R.B.; Kroes, A.C.M.; Zaaijer, H.L. Parvovirus B19 DNA detectable in hearts of patients with dilated cardiomyopathy, but absent or inactive in blood. ESC Heart Fail. 2021, 8, 2723–2730. [Google Scholar] [CrossRef]
- Comar, M.; D’Agaro, P.; Campello, C.; Poli, A.; Breinholt 3rd, J.P.; Towbin, J.A.; Vatta, M. Human herpes virus 6 in archival cardiac tissues from children with idiopathic dilated cardiomyopathy or congenital heart disease. J. Clin. Pathol. 2009, 62, 80–83. [Google Scholar] [CrossRef]
- Escher, F.; Kühl, U.; Gross, U.; Westermann, D.; Poller, W.; Tschöpe, C.; Lassner, D.; Schultheiss, H.-P. Aggravation of left ventricular dysfunction in patients with biopsy-proven cardiac human herpesvirus A and B infection. J. Clin. Virol. 2015, 63, 1–5. [Google Scholar] [CrossRef]
- Ace, O.; Domb, S. Myocarditis as the initial presentation of Epstein-Barr virus infection in a 17-year-old male patient. Can. Fam. Physician 2019, 65, 897–899. [Google Scholar]
- Walenta, K.; Kindermann, I.; Gärtner, B.; Kandolph, R.; Link, A.; Böhm, M. Dangerous Kisses: Epstein-Barr Virus Myocarditis Mimicking Myocardial Infarction. Am. J. Med. 2006, 119, e3–e6. [Google Scholar] [CrossRef]
- Watanabe, M.; Panetta, G.L.; Piccirillo, F.; Spoto, S.; Myers, J.; Serino, F.M.; Costantino, S.; Di Sciascio, G. Acute Epstein-Barr related myocarditis: An unusual but life-threatening disease in an immunocompetent patient. J. Cardiol. Cases 2020, 21, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Zhu, B.-L.; Li, D.-R.; Zhao, D.; Maeda, H. Epstein–Barr virus myocarditis as a cause of sudden death: Two autopsy cases. Int. J. Legal Med. 2005, 119, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Antonakaki, D.; Mirza, S.A.; Sundar, S.; Grapsa, J. Epstein-Barr futile myocarditis requiring urgent orthotopic heart transplantation. Perfusion 2016, 31, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Roubille, C.; Brunel, A.S.; Gahide, G.; Vernhet Kovacsik, H.; Le Quellec, A. Cytomegalovirus (CMV) and acute myocarditis in an immunocompetent patient. Intern. Med. 2010, 49, 131–133. [Google Scholar] [CrossRef]
- Padala, S.K.; Kumar, A.; Padala, S. Fulminant cytomegalovirus myocarditis in an immunocompetent host: Resolution with oral valganciclovir. Tex. Heart Inst. J. 2014, 41, 523–529. [Google Scholar] [CrossRef]
- Magno Palmeira, M.; Umemura Ribeiro, H.Y.; Garcia Lira, Y.; Machado Jucá Neto, F.O.; da Silva Rodrigues, I.A.; Fernandes da Paz, L.N.; Nascimento Pinheiro Mda, C. Heart failure due to cytomegalovirus myocarditis in immunocompetent young adults: A case report. BMC Res. Notes 2016, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Oualim, S.; Elouarradi, A.; Hafid, S.; Naitelhou, A.; Sabry, M. A misleading CMV myocarditis during the COVID-19 pandemic: Case report. Pan Afr. Med. J. 2020, 36, 167. [Google Scholar] [CrossRef] [PubMed]
- Kytö, V.; Vuorinen, T.; Saukko, P.; Lautenschlager, I.; Lignitz, E.; Saraste, A.; Voipio-Pulkki, L.-M. Cytomegalovirus Infection of the Heart Is Common in Patients with Fatal Myocarditis. Clin. Infect. Dis. 2005, 40, 683–688. [Google Scholar] [CrossRef]
- Schonian, U.; Crombach, M.; Maisch, B. Assessment of cytomegalovirus DNA and protein expression in patients with myocarditis. Clin. Immunol. Immunopathol. 1993, 68, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, G.; Pellegrini, C.; Maurelli, M.; Belliato, M.; Sciutti, F.; Bottazzi, A.; Sepe, P.A.; Resasco, T.; Camporotondo, R.; Bruno, R.; et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020, 22, 911–915. [Google Scholar] [CrossRef]
- Basso, C.; Leone, O.; Rizzo, S.; De Gaspari, M.; van der Wal, A.C.; Aubry, M.-C.; Bois, M.C.; Lin, P.T.; Maleszewski, J.J.; Stone, J.R. Pathological features of COVID-19-associated myocardial injury: A multicentre cardiovascular pathology study. Eur. Heart J. 2020, 41, 3827–3835. [Google Scholar] [CrossRef]
- Pietsch, H.; Escher, F.; Aleshcheva, G.; Baumeier, C.; Morawietz, L.; Elsaesser, A.; Schultheiss, H.-P. Proof of SARS-CoV-2 genomes in endomyocardial biopsy with latency after acute infection. Int. J. Infect. Dis. 2021, 102, 70–72. [Google Scholar] [CrossRef]
- Escher, F.; Pietsch, H.; Aleshcheva, G.; Bock, T.; Baumeier, C.; Elsaesser, A.; Wenzel, P.; Hamm, C.; Westenfeld, R.; Schultheiss, M.; et al. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020, 7, 2440–2447. [Google Scholar] [CrossRef]
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.-P.; et al. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281. [Google Scholar] [CrossRef]
- Wenzel, P.; Kopp, S.; Göbel, S.; Jansen, T.; Geyer, M.; Hahn, F.; Kreitner, K.-F.; Escher, F.; Schultheiss, H.-P.; Münzel, T. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc. Res. 2020, 116, 1661–1663. [Google Scholar] [CrossRef]
- Bräuninger, H.; Stoffers, B.; Fitzek, A.D.E.; Meißner, K.; Aleshcheva, G.; Schweizer, M.; Weimann, J.; Rotter, B.; Warnke, S.; Edler, C.; et al. Cardiac SARS-CoV-2 infection is associated with pro-inflammatory transcriptomic alterations within the heart. Cardiovasc. Res. 2022, 118, 542–555. [Google Scholar] [CrossRef]
- Liu, H.; Gai, S.; Wang, X.; Zeng, J.; Sun, C.; Zhao, Y.; Zheng, Z. Single-cell analysis of SARS-CoV-2 receptor ACE2 and spike protein priming expression of proteases in the human heart. Cardiovasc Res 2020, 116, 1733–1741. [Google Scholar] [CrossRef]
- Tucker, N.R.; Chaffin, M.; Bedi, K.C., Jr.; Papangeli, I.; Akkad, A.D.; Arduini, A.; Hayat, S.; Eraslan, G.; Muus, C.; Bhattacharyya, R.P..; et al. Myocyte-Specific Upregulation of ACE2 in Cardiovascular Disease: Implications for SARS-CoV-2-Mediated Myocarditis. Circulation 2020, 142, 708–710. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, Z.; Xiong, C.; Qian, H. Direct mechanisms of SARS-CoV-2-induced cardiomyocyte damage: An update. Virol. J. 2022, 19, 108. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, K.H.; Lee, N.; Cho, S.H.; Kim, S.Y.; Kim, E.K.; Park, J.-H.; Choi, E.-Y.; Choi, J.-O.; Park, H.; et al. COVID-19 vaccination-related myocarditis: A Korean nationwide study. Eur. Heart J. 2023, 44, 2234–2243. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kenzaka, T.; Matsumoto, M.; Nishio, R.; Kawasaki, S.; Akita, H. A case report of myocarditis combined with hepatitis caused by herpes simplex virus. BMC Cardiovasc. Disord. 2018, 18, 134. [Google Scholar] [CrossRef] [PubMed]
- Kuchynka, P.; Palecek, T.; Hrbackova, H.; Vitkova, I.; Simek, S.; Nemecek, E.; Aster, V.; Louch, W.E.; Aschermann, M.; Linhart, A. Herpes simplex virus-induced cardiomyopathy successfully treated with acyclovir. Wien. Klin. Wochenschr. 2010, 122, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Colombo, D.; Albore, M.; Del Nonno, F.; Bolino, G.; D’Ambrosio, M.; Salvi, A.; Cecannecchia, C.; Falasca, L. Fatal fulminant HSV-2 myocarditis: A complicated presentation. Int. J. Infect. Dis. 2022, 114, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Teravanichpong, S.; Chuangsuwanich, T. Fatal varicella in a healthy girl. J. Med. Assoc. Thai. 1990, 73, 648–651. [Google Scholar]
- Tsintsof, A.; Delprado, W.J.; Keogh, A.M. Varicella zoster myocarditis progressing to cardiomyopathy and cardiac transplantation. Br. Heart J. 1993, 70, 93–95. [Google Scholar] [CrossRef]
- Donoiu, I.; Istratoaie, O. Varicella-zoster myocarditis mimicking acute myocardial infarction. Curr. Health Sci. J. 2014, 40, 78–80. [Google Scholar] [CrossRef]
- Chaudhari, M.; Sharma, S.; Jha, R.K.; Ahuja, R.S.; Bansal, S. Varicella myopericarditis mimicking acute myocardial infarction with ARDS—A rare association in an immunocompetent young adult. Indian Heart J. 2016, 68 (Suppl. S2), S274–S275. [Google Scholar] [CrossRef]
- Ioannou, A.; Tsappa, I.; Metaxa, S.; Missouris, C.G. Ventricular Fibrillation following Varicella Zoster Myocarditis. Case Rep. Cardiol. 2017, 2017, 1017686. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, R.; Kucuk, M.; Dibeklioglu, S.E. Report of a Myocarditis Outbreak among Pediatric Patients: Human Herpesvirus 7 as a Causative Agent? J. Trop. Pediatr. 2018, 64, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Ichida, F.; Saji, T.; Ogawa, S.; Waki, K.; Kaneko, M.; Tahara, M.; Soga, T.; Ono, Y.; Yasukochi, S. Clinical Features of Acute and Fulminant Myocarditis in Children-2nd Nationwide Survey by Japanese Society of Pediatric Cardiology and Cardiac Surgery. Circ. J. 2016, 80, 2362–2368. [Google Scholar] [CrossRef] [PubMed]
- Belkin, M.N.; Uriel, N. Heart health in the age of highly active antiretroviral therapy: A review of HIV cardiomyopathy. Curr. Opin. Cardiol. 2018, 33, 317–324. [Google Scholar] [CrossRef]
- Hekimian, G.; Jovanovic, T.; Brechot, N.; Lebreton, G.; Leprince, P.; Trouillet, J.L.; Schmidt, M.; Nieszkowska, A.; Besset, S.; Chastre, J.; et al. When the heart gets the flu: Fulminant influenza B myocarditis: A case-series report and review of the literature. J. Crit. Care 2018, 47, 61–64. [Google Scholar] [CrossRef]
- Frustaci, A.; Abdulla, A.K.; Caldarulo, M.; Buffon, A. Fatal measles myocarditis. Cardiologia 1990, 35, 347–349. [Google Scholar]
- Harada, T.; Ohtaki, E.; Tobaru, T.; Kitahara, K.; Sumiyoshi, T.; Hosoda, S. Rubella-associated perimyocarditis—A case report. Angiology 2002, 53, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, O.A.; Mackie, D.; Bratincsak, A.; Bradley, J.S.; Perry, J.C. Spectrum of Viral Pathogens Identified in Children with Clinical Myocarditis (Pre-Coronavirus Disease-2019, 2000–2018): Etiologic Agent Versus Innocent Bystander. J. Pediatr. 2022, 242, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, A.; Matoba, Y.; Sasayama, S. Dilated cardiomyopathy associated with hepatitis C virus infection. Circulation 1995, 92, 2519–2525. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, A.; Shimada, T.; Chapman, N.M.; Tracy, S.M.; Mason, J.W. Myocarditis and heart failure associated with hepatitis C virus infection. J. Card. Fail. 2006, 12, 293–298. [Google Scholar] [CrossRef]
- Premkumar, M.; Rangegowda, D.; Vashishtha, C.; Bhatia, V.; Khumuckham, J.S.; Kumar, B. Acute viral hepatitis e is associated with the development of myocarditis. Case Rep. Hepatol. 2015, 2015, 458056. [Google Scholar] [CrossRef]
- Sengupta, P.; Biswas, S.; Roy, T. Hepatitis E-Induced Acute Myocarditis in an Elderly Woman. Case Rep. Gastroenterol. 2019, 13, 342–349. [Google Scholar] [CrossRef]
- Simon, F.; Paule, P.; Oliver, M. Chikungunya virus-induced myopericarditis: Toward an increase of dilated cardiomyopathy in countries with epidemics? Am. J. Trop. Med. Hyg. 2008, 78, 212–213. [Google Scholar] [CrossRef]
- Cotella, J.I.; Sauce, A.L.; Saldarriaga, C.I.; Perez, G.E.; Farina, J.M.; Wyss, F.; Sosa Liprandi, A.; Mendoza, I.; Munera, A.G.; Alexander, B.; et al. Chikungunya and the Heart. Cardiology 2021, 146, 324–334. [Google Scholar] [CrossRef]
- Miranda, C.H.; Borges Mde, C.; Schmidt, A.; Pazin-Filho, A.; Rossi, M.A.; Ramos, S.G.; Lopes da Fonseca, B.A. A case presentation of a fatal dengue myocarditis showing evidence for dengue virus-induced lesion. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 127–130. [Google Scholar] [CrossRef]
- Tahir, H.; Daruwalla, V.; Hayat, S. Myocarditis leading to severe dilated cardiomyopathy in a patient with dengue Fever. Case Rep. Cardiol. 2015, 2015, 319312. [Google Scholar] [CrossRef] [PubMed]
- Farias, L.A.B.G.; Beserra, F.L.C.N.; Fernandes, L.; Teixeira, A.A.R.; Ferragut, J.M.; Girão, E.S.; Pires Neto, R.d.J. Myocarditis Following Recent Chikungunya and Dengue Virus Coinfection: A Case Report. Arq. Bras. Cardiol. 2019, 113, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Aletti, M.; Lecoules, S.; Kanczuga, V.; Soler, C.; Maquart, M.; Simon, F.; Leparc-Goffart, I. Transient myocarditis associated with acute Zika virus infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 64, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Pergam, S.A.; DeLong, C.E.; Echevarria, L.; Scully, G.; Goade, D.E. Myocarditis in West Nile Virus infection. Am. J. Trop. Med. Hyg. 2006, 75, 1232–1233. [Google Scholar] [CrossRef]
- Kushawaha, A.; Jadonath, S.; Mobarakai, N. West nile virus myocarditis causing a fatal arrhythmia: A case report. Cases J. 2009, 2, 7147. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Ji, W.; Bhaya, B.; Ahsan, C. A Rare Case of Cardiac Recovery after Acute Myocarditis from West Nile Virus Infection: A Review of the Current Literature. Case Rep. Cardiol. 2022, 2022, 8517728. [Google Scholar] [CrossRef]
- Xu, J.; Brooks, E.G. Giant Cell Myocarditis: A Brief Review. Arch. Pathol. Lab. Med. 2016, 140, 1429–1434. [Google Scholar] [CrossRef]
- Cooper, L.T.; ElAmm, C. Giant cell myocarditis. Herz 2012, 37, 632–636. [Google Scholar] [CrossRef]
- Fluschnik, N.; Escher, F.; Blankenberg, S.; Westermann, D. Fatal recurrence of fulminant giant cell myocarditis and recovery after initialisation of an alternative immunosuppressive regime. Case Rep. 2014, 2014, bcr2014206386. [Google Scholar] [CrossRef]
- Cooper, L.T.; Berry, G.J.; Shabetai, R. Idiopathic Giant-Cell Myocarditis—Natural History and Treatment. N. Engl. J. Med. 1997, 336, 1860–1866. [Google Scholar] [CrossRef]
- Frustaci, A.; Chimenti, C.; Pieroni, M.; Salvatori, L.; Morgante, E.; Sale, P.; Ferretti, E.; Petrangeli, E.; Gulino, A.; Russo, M.A. Cell death, proliferation and repair in human myocarditis responding to immunosuppressive therapy. Mod. Pathol. 2006, 19, 755–765. [Google Scholar] [CrossRef]
- Kandolin, R.; Lehtonen, J.; Salmenkivi, K.; Räisänen-Sokolowski, A.; Lommi, J.; Kupari, M. Diagnosis, Treatment, and Outcome of Giant-Cell Myocarditis in the Era of Combined Immunosuppression. Circ. Heart Fail. 2013, 6, 15–22. [Google Scholar] [CrossRef]
- Fruhwald, F.; Lassner, D.; Fruhwald, S.; Gross, U.M.; Dapunt, O.; Schultheiss, H.-P. Immunosuppressive treatment in fulminant myocarditis and gene expression pattern associated with, but no histological confirmation of giant cell myocarditis. ESC Heart Fail. 2017, 4, 190–192. [Google Scholar] [CrossRef][Green Version]
- Blauwet, L.A.; Cooper, L.T. Idiopathic giant cell myocarditis and cardiac sarcoidosis. Heart Fail. Rev. 2013, 18, 733–746. [Google Scholar] [CrossRef]
- Lassner, D.; Kuhl, U.; Siegismund, C.S.; Rohde, M.; Elezkurtaj, S.; Escher, F.; Tschope, C.; Gross, U.M.; Poller, W.; Schultheiss, H.-P. Improved diagnosis of idiopathic giant cell myocarditis and cardiac sarcoidosis by myocardial gene expression profiling. Eur. Heart J. 2014, 35, 2186–2195. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Minhas, K.M.; Irizarry, R.A.; Ye, S.Q.; Edness, G.; Breton, E.; Conte, J.V.; Tomaselli, G.; Garcia, J.G.N.; Hare, J.M. Gene expression in giant cell myocarditis: Altered expression of immune response genes. Int. J. Cardiol. 2005, 102, 333–340. [Google Scholar] [CrossRef]
- Escher, F.; Pietsch, H.; Aleshcheva, G.; Wenzel, P.; Fruhwald, F.; Stumpf, C.; Westermann, D.; Bauersachs, J.; Enseleit, F.; Ruschitzka, F.; et al. Evaluation of Myocardial Gene Expression Profiling for Superior Diagnosis of Idiopathic Giant-Cell Myocarditis and Clinical Feasibility in a Large Cohort of Patients with Acute Cardiac Decompensation. J. Clin. Med. 2020, 9, 2689. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Clark, A. Impact of microRNA regulation on variation in human gene expression. Genome Res. 2012, 22, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Hoefig, K.; Heissmeyer, V. MicroRNAs grow up in the immune system. Curr. Opin. Immunol. 2008, 20, 281–287. [Google Scholar] [CrossRef]
- Romaine, S.P.R.; Tomaszewski, M.; Condorelli, G.; Samani, N.J. MicroRNAs in cardiovascular disease: An introduction for clinicians. Heart 2015, 101, 921–928. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Colpaert, R.; Calore, M. Epigenetics and microRNAs in cardiovascular diseases. Genomics 2021, 113, 540–551. [Google Scholar] [CrossRef]
- Halushka, P.; Goodwin, A.; Halushka, M. Opportunities for microRNAs in the Crowded Field of Cardiovascular Biomarkers. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 211–238. [Google Scholar] [CrossRef]
- Forero, D.A.; González-Giraldo, Y.; Castro-Vega, L.J.; Barreto, G.E. qPCR-based methods for expression analysis of miRNAs. Biotechniques 2019, 67, 192–199. [Google Scholar] [CrossRef]
- Besler, C.; Urban, D.; Watzka, S.; Lang, D.; Rommel, K.-P.; Kandolf, R.; Klingel, K.; Thiele, H.; Linke, A.; Schuler, G.; et al. Endomyocardial miR-133a Levels Correlate with Myocardial Inflammation, Improved Left Ventricular Function, and Clinical Outcome in Patients with Inflammatory Cardiomyopathy. Eur. J. Heart Fail. 2016, 18, 1442–1451. [Google Scholar] [CrossRef]
- Ferreira, L.; Frade, A.; Santos, R.; Teixeira, P.; Baron, M.; Navarro, I.; Benvenuti, L.; Fiorelli, A.; Bocchi, E.; Stolf, N.; et al. MicroRNAs miR-1, miR-133a, miR-133b, miR-208a and miR-208b are dysregulated in Chronic Chagas disease Cardiomyopathy. Int. J. Cardiol. 2014, 175, 409–417. [Google Scholar] [CrossRef]
- Bao, J.-L.; Lin, L. MiR-155 and miR-148a reduce cardiac injury by inhibiting NF-κB pathway during acute viral myocarditis. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2349–2356. [Google Scholar] [PubMed]
- Chen, Z.; Liu, H.; Zhang, J.; Zhang, S.; Zhao, L.; Liang, W. Upregulated microRNA-214 enhances cardiac injury by targeting ITCH during coxsackievirus infection. Mol. Med. Rep. 2015, 12, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Kühl, U.; Rohde, M.; Lassner, D.; Gross, U.M.; Escher, F.; Schultheiss, H.-P. miRNA as activity markers in Parvo B19 associated heart disease. Herz 2012, 37, 637–643. [Google Scholar] [CrossRef]
- Corsten, M.F.; Papageorgiou, A.; Verhesen, W.; Carai, P.; Lindow, M.; Obad, S.; Summer, G.; Coort, S.L.M.; Hazebroek, M.; van Leeuwen, R.; et al. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ. Res. 2012, 111, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, U.; Lassner, D.; Gast, M.; Stroux, A.; Rohde, M.; Siegismund, C.; Wang, X.; Escher, F.; Gross, M.; Skurk, C.; et al. Differential Cardiac MicroRNA Expression Predicts the Clinical Course in Human Enterovirus Cardiomyopathy. Circ. Heart Fail. 2015, 8, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; He, M.; Wang, J.; Chen, P.; Wang, F.; Lai, J.; Li, C.; Yu, T.; Zuo, H.; Cui, G.; et al. Circulating miR-4763-3p Is a Novel Potential Biomarker Candidate for Human Adult Fulminant Myocarditis. Mol. Ther. Methods Clin. Dev. 2020, 17, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, J.; Zhou, R.; Zheng, N. Expression and Significance of Circulating microRNA-29b in Adult Fulminant Myocarditis. Acta Acad. Med. Sin. 2022, 44, 102–109. [Google Scholar] [CrossRef]
- Obradovic, D.; Rommel, K.-P.; Blazek, S.; Klingel, K.; Gutberlet, M.; Lücke, C.; Büttner, P.; Thiele, H.; Adams, V.; Lurz, P.; et al. The potential role of plasma miR-155 and miR-206 as circulatory biomarkers in inflammatory cardiomyopathy. ESC Heart Fail. 2021, 8, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Marketou, M.; Kontaraki, J.; Patrianakos, A.; Kochiadakis, G.; Anastasiou, I.; Fragkiadakis, K.; Plevritaki, A.; Papadaki, S.; Chlouverakis, G.; Parthenakis, F. Peripheral Blood MicroRNAs as Potential Biomarkers of Myocardial Damage in Acute Viral Myocarditis. Genes 2021, 12, 420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Wang, D.-G.; Jiang, X.; Zhang, M.; Lv, K. Serum exosome microRNA panel as a noninvasive biomarker for molecular diagnosis of fulminant myocarditis. Mol. Ther.-Methods Clin. Dev. 2020, 20, 142–151. [Google Scholar] [CrossRef]
- Fan, K.-L.; Li, M.-F.; Cui, F.; Feng, F.; Kong, L.; Zhang, F.-H.; Hao, H.; Yin, M.-X.; Liu, Y. Altered exosomal miR-181d and miR-30a related to the pathogenesis of CVB3 induced myocarditis by targeting SOCS3. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2208–2215. [Google Scholar] [CrossRef]
- Aleshcheva, G.; Pietsch, H.; Escher, F.; Schultheiss, H.-P. MicroRNA profiling as a novel diagnostic tool for identification of patients with inflammatory and/or virally induced cardiomyopathies. ESC Heart Fail. 2021, 8, 408–422. [Google Scholar] [CrossRef]
- Li, Y.; Shi, X.; Zuo, Y.; Li, T.; Liu, L.; Shen, Z.; Shen, J.; Zhang, R.; Wang, S. Multiplexed Target Enrichment Enables Efficient and In-Depth Analysis of Antimicrobial Resistome in Metagenomes. Microbiol. Spectr. 2022, 10, e0229722. [Google Scholar] [CrossRef]
- Deng, X.; Achari, A.; Federman, S.; Yu, G.; Somasekar, S.; Bartolo, I.; Yagi, S.; Mbala-Kingebeni, P.; Kapetshi, J.; Ahuka-Mundeke, S.; et al. Metagenomic sequencing with spiked primer enrichment for viral diagnostics and genomic surveillance. Nat. Microbiol. 2020, 5, 443–454. [Google Scholar] [CrossRef]
- Greninger, A.L.; Roychoudhury, P.; Xie, H.; Casto, A.; Cent, A.; Pepper, G.; Koelle, D.M.; Huang, M.L.; Wald, A.; Johnston, C.; et al. Ultrasensitive Capture of Human Herpes Simplex Virus Genomes Directly from Clinical Samples Reveals Extraordinarily Limited Evolution in Cell Culture. mSphere 2018, 3, e00283-18. [Google Scholar] [CrossRef]
- Wylie, T.N.; Wylie, K.M.; Herter, B.N.; Storch, G.A. Enhanced virome sequencing using targeted sequence capture. Genome Res. 2015, 25, 1910–1920. [Google Scholar] [CrossRef]
- Metsky, H.C.; Siddle, K.J.; Gladden-Young, A.; Qu, J.; Yang, D.K.; Brehio, P.; Goldfarb, A.; Piantadosi, A.; Wohl, S.; Carter, A.; et al. Capturing sequence diversity in metagenomes with comprehensive and scalable probe design. Nat. Biotechnol. 2019, 37, 160–168. [Google Scholar] [CrossRef]
- Metsky, H.C.; Matranga, C.B.; Wohl, S.; Schaffner, S.F.; Freije, C.A.; Winnicki, S.M.; West, K.; Qu, J.; Baniecki, M.L.; Gladden-Young, A.; et al. Zika virus evolution and spread in the Americas. Nature 2017, 546, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Sam, S.S.; Rogers, R.; Gillani, F.S.; Tsongalis, G.J.; Kraft, C.S.; Caliendo, A.M. Evaluation of a Next-Generation Sequencing Metagenomics Assay to Detect and Quantify DNA Viruses in Plasma from Transplant Recipients. J. Mol. Diagn. 2021, 23, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Carbo, E.C.; Russcher, A.; Kraakman, M.E.M.; de Brouwer, C.S.; Sidorov, I.A.; Feltkamp, M.C.W.; Kroes, A.C.M.; Claas, E.C.J.; de Vries, J.J.C. Longitudinal Monitoring of DNA Viral Loads in Transplant Patients Using Quantitative Metagenomic Next-Generation Sequencing. Pathogens 2022, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Matranga, C.B.; Andersen, K.G.; Winnicki, S.; Busby, M.; Gladden, A.D.; Tewhey, R.; Stremlau, M.; Berlin, A.; Gire, S.K.; England, E.; et al. Enhanced methods for unbiased deep sequencing of Lassa and Ebola RNA viruses from clinical and biological samples. Genome Biol. 2014, 15, 519. [Google Scholar] [CrossRef]
- Kwok, H.; Wu, C.W.; Palser, A.L.; Kellam, P.; Sham, P.C.; Kwong, D.L.; Chiang, A.K. Genomic diversity of Epstein-Barr virus genomes isolated from primary nasopharyngeal carcinoma biopsy samples. J. Virol. 2014, 88, 10662–10672. [Google Scholar] [CrossRef]
- Chalkias, S.; Gorham, J.M.; Mazaika, E.; Parfenov, M.; Dang, X.; DePalma, S.; McKean, D.; Seidman, C.E.; Seidman, J.G.; Koralnik, I.J. ViroFind: A novel target-enrichment deep-sequencing platform reveals a complex JC virus population in the brain of PML patients. PLoS ONE 2018, 13, e0186945. [Google Scholar] [CrossRef]
- Heidecker, B.; Williams, S.H.; Jain, K.; Oleynik, A.; Patriki, D.; Kottwitz, J.; Berg, J.; Garcia, J.A.; Baltensperger, N.; Lovrinovic, M.; et al. Virome Sequencing in Patients With Myocarditis. Circ. Heart Fail. 2020, 13, e007103. [Google Scholar] [CrossRef]
- Aziz, N.; Zhao, Q.; Bry, L.; Driscoll, D.K.; Funke, B.; Gibson, J.S.; Grody, W.W.; Hegde, M.R.; Hoeltge, G.A.; Leonard, D.G.; et al. College of American Pathologists’ laboratory standards for next-generation sequencing clinical tests. Arch. Pathol. Lab. Med. 2015, 139, 481–493. [Google Scholar] [CrossRef]
- Lopez-Labrador, F.X.; Brown, J.R.; Fischer, N.; Harvala, H.; Van Boheemen, S.; Cinek, O.; Sayiner, A.; Madsen, T.V.; Auvinen, E.; Kufner, V.; et al. Recommendations for the introduction of metagenomic high-throughput sequencing in clinical virology, part I: Wet lab procedure. J. Clin. Virol. 2021, 134, 104691. [Google Scholar] [CrossRef]
- de Vries, J.J.C.; Brown, J.R.; Couto, N.; Beer, M.; Le Mercier, P.; Sidorov, I.; Papa, A.; Fischer, N.; Oude Munnink, B.B.; Rodriquez, C.; et al. Recommendations for the introduction of metagenomic next-generation sequencing in clinical virology, part II: Bioinformatic analysis and reporting. J. Clin. Virol. 2021, 138, 104812. [Google Scholar] [CrossRef]
- Lassner, D.; Siegismund, C.S.; Kühl, U.; Rohde, M.; Stroux, A.; Escher, F.; Schultheiss, H.-P. CCR5del32 genotype in human enteroviral cardiomyopathy leads to spontaneous virus clearance and improved outcome compared to wildtype CCR5. J. Transl. Med. 2018, 16, 249. [Google Scholar] [CrossRef]
- Bissel, S.J.; Winkler, C.C.; Deltondo, J.; Wang, G.; Williams, K.; Wiley, C.A. Coxsackievirus B4 myocarditis and meningoencephalitis in newborn twins. Neuropathology 2014, 34, 429–437. [Google Scholar] [CrossRef]
- Cognet, T.; Lairez, O.; Marchal, P.; Roncalli, J.; Galinier, M. A Family History of Dilated Cardiomyopathy Induced by Viral Myocarditis. Case Rep. Cardiol. 2012, 2012, 204371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amdani, S.M.; Kim, H.S.; Orvedahl, A.; John, A.O.; Said, A.; Simpson, K. Successful treatment of fulminant neonatal enteroviral myocarditis in monochorionic diamniotic twins with cardiopulmonary support, intravenous immunoglobulin and pocapavir. BMJ. Case Rep. 2018, 2018, bcr-2017. [Google Scholar] [CrossRef] [PubMed]
- Lota, A.S.; Hazebroek, M.R.; Theotokis, P.; Wassall, R.; Salmi, S.; Halliday, B.P.; Tayal, U.; Verdonschot, J.; Meena, D.; Owen, R.; et al. Genetic Architecture of Acute Myocarditis and the Overlap with Inherited Cardiomyopathy. Circulation 2022, 146, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Narula, N.; Giuliani, L.; Di Toro, A. Genetic Basis of Myocarditis: Myth or Reality? Myocarditis 2020, 45–89. [Google Scholar] [CrossRef]
- Baggio, C.; Gagno, G.; Porcari, A.; Paldino, A.; Artico, J.; Castrichini, M.; Dal Ferro, M.; Bussani, R.; Merlo, M. Myocarditis: Which Role for Genetics? Curr. Cardiol. Rep. 2021, 23, 58. [Google Scholar] [CrossRef]
- Arbustini, E.; Behr, E.R.; Carrier, L.; Van Duijn, C.; Evans, P.; Favalli, V.; Van Der Harst, P.; Haugaa, K.H.; Jondeau, G.; Kääb, S.; et al. Interpretation and actionability of genetic variants in cardiomyopathies: A position statement from the European Society of Cardiology Council on cardiovascular genomics. Eur. Heart J. 2022, 43, 1901–1916. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Chimenti, C.; Calabrese, F.; Pieroni, M.; Thiene, G.; Maseri, A. Immunosuppressive therapy for active lymphocytic myocarditis: Virological and immunologic profile of responders versus nonresponders. Circulation 2003, 107, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Russo, M.A.; Chimenti, C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: The TIMIC study. Eur. Heart J. 2009, 30, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Escher, F.; Kühl, U.; Lassner, D.; Poller, W.; Westermann, D.; Pieske, B.; Tschöpe, C.; Schultheiss, H.-P. Long-term outcome of patients with virus-negative chronic myocarditis or inflammatory cardiomyopathy after immunosuppressive therapy. Clin. Res. Cardiol. 2016, 105, 1011–1020. [Google Scholar] [CrossRef]
- De Luca, G.; Campochiaro, C.; Sartorelli, S.; Peretto, G.; Dagna, L. Therapeutic strategies for virus-negative myocarditis: A comprehensive review. Eur. J. Intern. Med. 2020, 77, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Pappalardo, F.; Mangieri, A.; Dinarello, C.A.; Dagna, L.; Tresoldi, M. Treating life-threatening myocarditis by blocking interleukin-1. Crit. Care Med. 2016, 44, e751–e754. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Savage, E.; Wazir, T.; Drake, M.; Cuthbert, R.; Wright, G. Fulminant myocarditis and macrophage activation syndrome secondary to adult-onset still’s disease successfully treated with tocilizumab. Rheumatology 2014, 53, 1352–1353. [Google Scholar] [CrossRef]
- Jibbe, A.; Brett, C.N.; Rajpara , A.; Whitsitt , J.; Hamblin , M. A Case of Autoimmune Myocarditis Treated with IL-17 Inhibition. Kansas J. Med. 2021, 14, 201–202. [Google Scholar] [CrossRef]
- Frustaci, A.; Galea, N.; Dominici, L.; Verardo, R.; Alfarano, M.; Scialla, R.; Richetta, A.G. Interleukin-17A-Correlated Myocarditis in Patients with Psoriasis: Cardiac Recovery following Secukinumab Administration. J. Clin. Med. 2023, 12, 4010. [Google Scholar] [CrossRef]
- Klein, A.L.; Imazio, M.; Cremer, P.; Brucato, A.; Abbate, A.; Fang, F.; Insalaco, A.; LeWinter, M.; Lewis, B.S.; Lin, D.; et al. Phase 3 Trial of Interleukin-1 Trap Rilonacept in Recurrent Pericarditis. N. Engl. J. Med. 2021, 384, 31–41. [Google Scholar] [CrossRef]
- Klein, A.L.; Imazio, M.; Brucato, A.; Cremer, P.; LeWinter, M.; Abbate, A.; Lin, D.; Martini, A.; Beutler, A.; Chang, S.; et al. RHAPSODY: Rationale for and design of a pivotal Phase 3 trial to assess efficacy and safety of rilonacept, an interleukin-1α and interleukin-1β trap, in patients with recurrent pericarditis. Am. Heart J. 2020, 228, 81–90. [Google Scholar] [CrossRef]
- Herrmann, J. Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 2020, 17, 474–502. [Google Scholar] [CrossRef]
- Van Der Ree, M.H.; Van Der Meer, A.J.; De Bruijne, J.; Maan, R.; Van Vliet, A.; Welzel, T.M.; Zeuzem, S.; Lawitz, E.J.; Rodriguez-Torres, M.; Kupcova, V.; et al. Long-term safety and efficacy of microRNA-targeted therapy in chronic hepatitis C patients. Antiviral. Res. 2014, 111, 53–59. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Piper, J.; Lynch, J.M.; Seto, A.G.; Hong, S.J.; Mustoe, T.A.; Maari, C.; Pestano, L.A.; Dalby, C.M.; Jackson, A.L.; et al. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. J. Investig. Dermatol. 2019, 139, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Täubel, J.; Hauke, W.; Rump, S.; Viereck, J.; Batkai, S.; Poetzsch, J.; Rode, L.; Weigt, H.; Genschel, C.; Lorch, U.; et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: Results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur. Heart J. 2021, 42, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Foinquinos, A.; Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Gyöngyösi, M.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D.; et al. Preclinical development of a miR-132 inhibitor for heart failure treatment. Nat. Commun. 2020, 11, 633. [Google Scholar] [CrossRef]
- Hazebroek, M.R.; Henkens, M.T.H.M.; Raafs, A.G.; Verdonschot, J.A.J.; Merken, J.J.; Dennert, R.M.; Eurlings, C.; Abdul Hamid, M.A.; Wolffs, P.F.G.; Winkens, B.; et al. Intravenous immunoglobulin therapy in adult patients with idiopathic chronic cardiomyopathy and cardiac parvovirus B19 persistence: A prospective, double-blind, randomized, placebo-controlled clinical trial. Eur. J. Heart Fail. 2021, 23, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, Y.; Su, G.; Li, Y.; Shuai, X. Intravenous immunoglobulin therapy for acute myocarditis in children and adults a meta-analysis. Int. Heart J. 2019, 60, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, D. A comparative study of valaciclovir, valganciclovir, and artesunate efficacy in reactivated HHV-6 and HHV-7 infections associated with chronic fatigue syndrome/myalgic encephalomyelitis. Microbiol. Immunol. 2022, 66, 193–199. [Google Scholar] [CrossRef]
| Virus | Classification | Cardiac Tropism | Clinical Manifestation * | Pathophysiological Aspects | Treatment |
|---|---|---|---|---|---|
| Adenoviruses | dsDNA | cardiomyocytes | acute MC | direct cardiomyocyte damage | interferon β [80] |
| Epstein–Barr virus | dsDNA | B cells; CD8+ T cells [81]; cardiomyocytes [82,83]; endothelial cells [83] | acute MC; DCM [75]; reduced allograft survival [84] | indirect vascular damage linked to viral replication [85]; indirect (immune-mediated) damage [86]; possible direct cardiomyocyte infection [82,83] | ganciclovir [87] |
| Cytomegalovirus | dsDNA | endothelial cells [88]; leukocytes [89]; smooth muscle cells [90] | acute MC; DCM [75]; reduced allograft survival [91] | indirect (immune-mediated) damage; development of vasculopathy in donor hearts [91] | ganciclovir [92] |
| Enteroviruses | ssRNA | cardiomyocytes | acute MC; chronic MC; autoimmune DCMi; DCM [75] | direct cardiomyocyte damage [93]; non-cytopathic viral persistence [94] | interferon β [80] |
| Human herpes virus 6 | dsDNA | T cells; endothelial cells [95] | acute MC; coronary artery spasm [96]; DCM [75]; | viral persistence [79]; cardiomyocyte damage secondary to endothelial dysfunction [97]; iciHHV-6 reactivation and HHV-6 superinfection [98,99] | artesunate [100]; valganciclovir [98] |
| Parvovirus B19 | ssDNA | endothelial cells [101] | coronary artery spasm [96]; viral DCMi; autoimmune DCMi; DCM [75]; reduced allograft survival [84,102,103] | viral persistence [79]; cardiomyocyte damage secondary to endothelial dysfunction [97,101]; viral transcriptional reactivation-induced pathogenic cellular gene expression [104] | interferon β [105,106]; telbivudine [107,108]; tenofovir [109]; IVIg |
| SARS-CoV-2 | ssRNA | ACE2- and TRMPSS2-expressing cardiomyocytes [110], endothelial cells and macrophages [12,111] | acute MC [112]; chronic MC [113,114] | direct cardiomyocyte damage [115]; endothelial dysfunction; “long COVID” autoimmune myocarditis [113,114] | remdesivir; lopinavir; ritonavir [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumeier, C.; Harms, D.; Aleshcheva, G.; Gross, U.; Escher, F.; Schultheiss, H.-P. Advancing Precision Medicine in Myocarditis: Current Status and Future Perspectives in Endomyocardial Biopsy-Based Diagnostics and Therapeutic Approaches. J. Clin. Med. 2023, 12, 5050. https://doi.org/10.3390/jcm12155050
Baumeier C, Harms D, Aleshcheva G, Gross U, Escher F, Schultheiss H-P. Advancing Precision Medicine in Myocarditis: Current Status and Future Perspectives in Endomyocardial Biopsy-Based Diagnostics and Therapeutic Approaches. Journal of Clinical Medicine. 2023; 12(15):5050. https://doi.org/10.3390/jcm12155050
Chicago/Turabian StyleBaumeier, Christian, Dominik Harms, Ganna Aleshcheva, Ulrich Gross, Felicitas Escher, and Heinz-Peter Schultheiss. 2023. "Advancing Precision Medicine in Myocarditis: Current Status and Future Perspectives in Endomyocardial Biopsy-Based Diagnostics and Therapeutic Approaches" Journal of Clinical Medicine 12, no. 15: 5050. https://doi.org/10.3390/jcm12155050
APA StyleBaumeier, C., Harms, D., Aleshcheva, G., Gross, U., Escher, F., & Schultheiss, H.-P. (2023). Advancing Precision Medicine in Myocarditis: Current Status and Future Perspectives in Endomyocardial Biopsy-Based Diagnostics and Therapeutic Approaches. Journal of Clinical Medicine, 12(15), 5050. https://doi.org/10.3390/jcm12155050






