The Effect of Transcutaneous Posterior Tibial Nerve Stimulation on Pain and Quality of Life in Patients with Fibromyalgia: A Single-Blind, Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
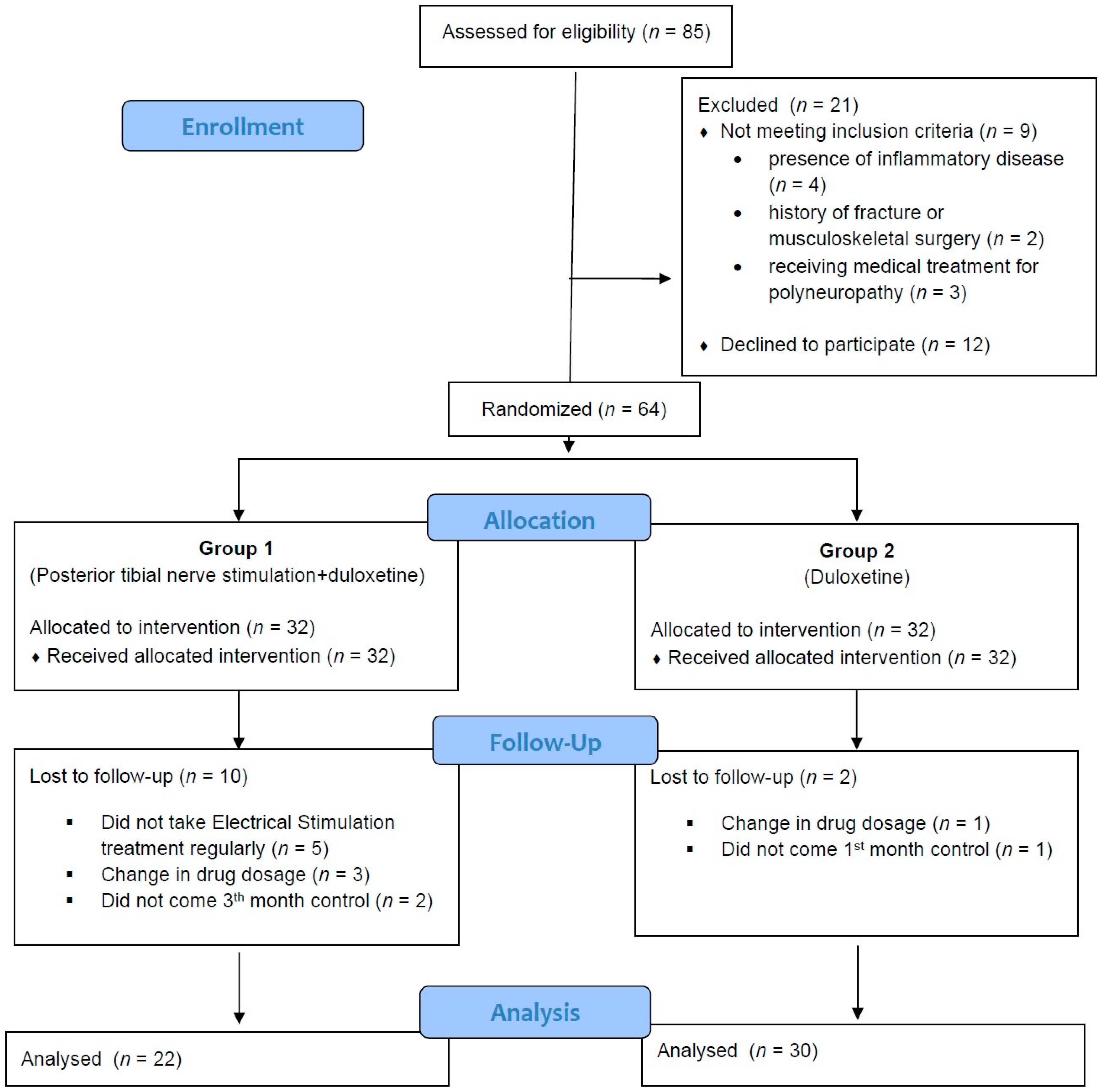
2.1. Study Design and Participants
2.2. Randomization and Blinding
2.3. Baseline Assessment and Outcome Measures
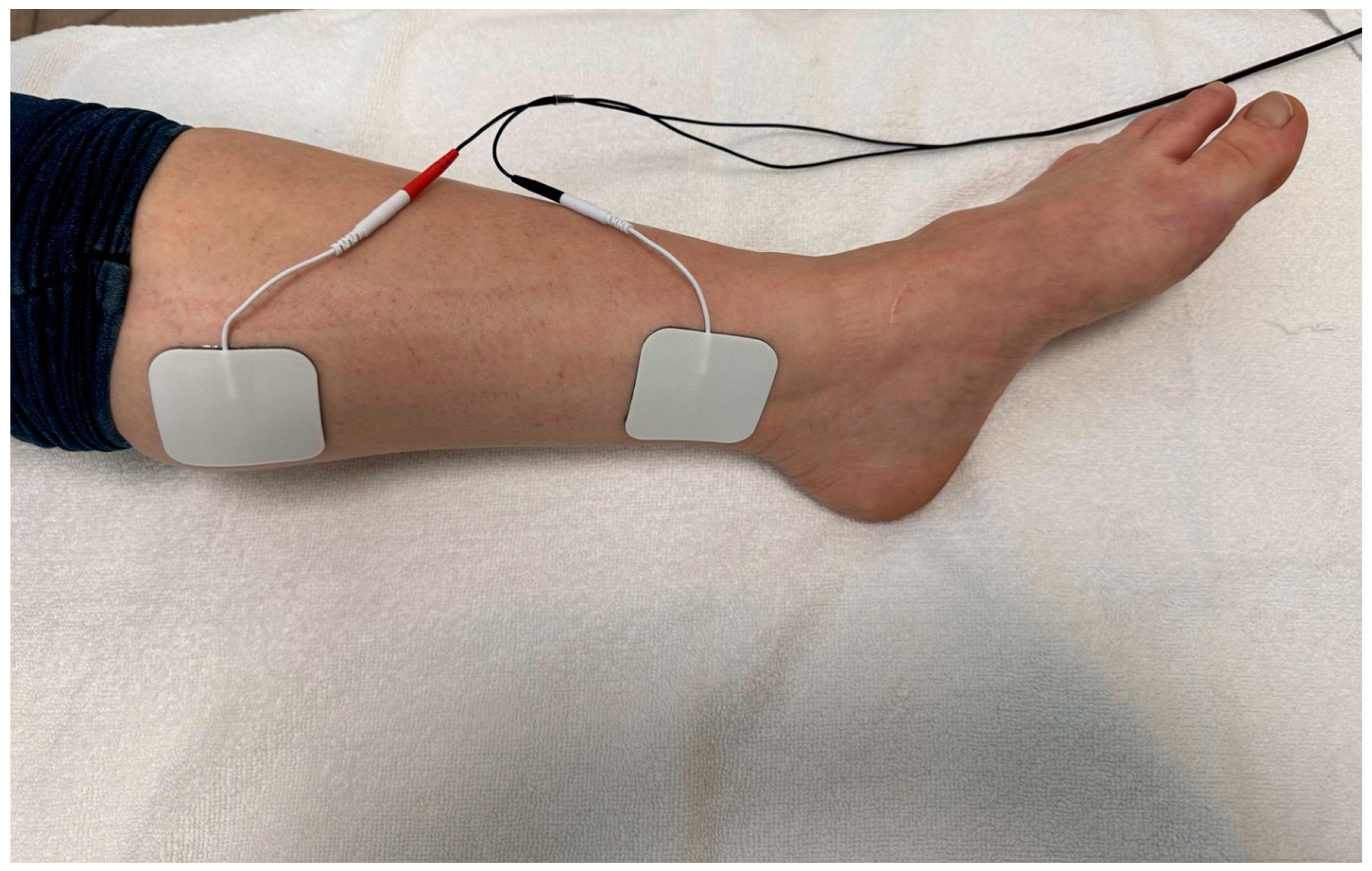
2.4. Intervention
2.5. Sample Size and Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef]
- Sindel, D.; Saral, İ.; Esmaeilzadeh, S. Management approaches in fibromyalgia syndrome. Turk. J. Phys. Med. Rehab. 2012, 58, 136–142. [Google Scholar]
- Cheng, C.W.; Wong, C.S.; Hui, G.K.; Chung, E.K.; Wong, S.H. Fibromyalgia: Is it a neuropathic pain? Pain Manag. 2018, 8, 377–388. [Google Scholar] [CrossRef]
- Chinn, S.; Caldwell, W.; Gritsenko, K. Fibromyalgia Pathogenesis and Treatment Options Update. Curr. Pain Headache Rep. 2016, 20, 25. [Google Scholar] [CrossRef]
- Üçeyler, N.; Zeller, D.; Kahn, A.K.; Kewenig, S.; Kittel-Schneider, S.; Schmid, A.; Casanova-Molla, J.; Reiners, K.; Sommer, C. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013, 136, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Oaklander, A.L.; Herzog, Z.D.; Downs, H.M.; Klein, M.M. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013, 154, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Fluß, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef]
- Lin, T.; Gargya, A.; Singh, H.; Sivanesan, E.; Gulati, A. Mechanism of peripheral nerve stimulation in chronic pain. Pain Med. 2020, 21 (Suppl. 1), S6–S12. [Google Scholar] [CrossRef]
- Slavin, K.V. Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics 2008, 5, 100–106. [Google Scholar] [CrossRef]
- Ong Sio, L.C.; Hom, B.; Garg, S.; Abd-Elsayed, A. Mechanism of Action of Peripheral Nerve Stimulation for Chronic Pain: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 4540. [Google Scholar]
- Dabby, R.; Sadeh, M.; Goldberg, I.; Finkelshtein, V. Electrical stimulation of the posterior tibial nerve reduces neuropathic pain in patients with polyneuropathy. J. Pain Res. 2017, 10, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Bhide, A.A.; Tailor, V.; Fernando, R.; Khullar, V.; Digesu, G.A. Posterior tibial nerve stimulation for overactive bladder-techniques and efficacy. Int. Urogynecol. J. 2020, 31, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Vera, A.J.; Mondéjar-Ros, R.M.; Franco-Bernal, V.; Molina-Torres, G.; Diaz-Mohedo, E. Efficacy of Posterior Tibial Nerve Stimulation in the Treatment of Fecal Incontinence: A Systematic Review. J. Clin. Med. 2022, 11, 5191. [Google Scholar] [CrossRef] [PubMed]
- Kabay, S.; Kabay, S.C.; Yucel, M.; Ozden, H. Efficiency of posterior tibial nerve stimulation in category IIIB chronic prostatitis/chronic pelvic pain: A Sham-Controlled Comparative Study. Urol. Int. 2009, 83, 33–38. [Google Scholar] [PubMed]
- Thimineur, M.; De Ridder, D. C2 area neurostimulation: A surgical treatment for fibromyalgia. Pain Med. 2007, 8, 639–646. [Google Scholar] [CrossRef]
- Williamson, A.; Hoggart, B. Pain: A review of three commonly used pain rating scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar]
- Melzack, R. The short-form McGill Pain Questionnaire. Pain 1987, 30, 191–197. [Google Scholar] [CrossRef]
- Bennett, R. The Fibromyalgia Impact Questionnaire (FIQ): A review of its development, current version, operating characteristics and uses. Clin. Exp. Rheumatol. 2005, 23 (Suppl. 39), S154–S162. [Google Scholar]
- McPherson, A.; Martin, C.R. A review of the measurement properties of the 36-item short-form health survey (SF-36) to determine its suitability for use in an alcohol-dependent population. J. Psychiatr. Ment. Health Nurs. 2013, 20, 114–123. [Google Scholar] [CrossRef]
- Pierre, M.L.; Friso, B.; Casarotto, R.A.; Haddad, J.M.; Baracat, E.C.; Ferreira, E.A.G. Comparison of transcutaneous electrical tibial nerve stimulation for the treatment of overactive bladder: A multi-arm randomized controlled trial with blinded assessment. Clinics 2021, 76, e3039. [Google Scholar] [CrossRef]
- Bair, M.J.; Krebs, E.E. Fibromyalgia. Ann. Intern. Med. 2020, 172, ITC33–ITC48. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, mechanisms, diagnosis and treatment options update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Nayak, R.; Banik, R.K. Current innovations in peripheral nerve stimulation. Pain Res. Treat. 2018, 2018, 9091216. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.A.; Wall, P.D. Pain mechanisms: A new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, C.S.; Clark, S.R.; Bennett, R.M. Fibromyalgia and quality of life: A comparative analysis. J. Rheumatol. 1993, 20, 475–479. [Google Scholar]
- Mas, A.J.; Carmona, L.; Valverde, M.; Ribas, B.; EPISER Study Group. Prevalence and impact of fibromyalgia on function and quality of life in individuals from the general population: Results from a nationwide study in Spain. Clin. Exp. Rheumatol. 2008, 26, 519–526. [Google Scholar]
- Arnold, L.M. Duloxetine and other antidepressants in the treatment of patients with fibromyalgia. Pain Med. 2007, 8, S63–S74. [Google Scholar] [CrossRef]
- Arnold, L.M.; Pritchett, Y.L.; D’Souza, D.N.; Kajdasz, D.K.; Iyengar, S.; Wernicke, J.F. Duloxetine for the treatment of fibromyalgia in women: Pooled results from two randomized, placebo-controlled clinical trials. J. Womens Health 2007, 16, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
| Group 1 (Duloxetine + TPTNS) (n = 22) | Group 2 (Duloxetine) (n = 30) | p Value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 43.18 ±12.22 | 49.43 ± 11.54 | 0.066 i |
| BMI (kg/m2) | 27.71 ± 4.21 | 28.44 ± 3.84 | 0.515 i |
| N (%) | N (%) | ||
| Marital Status | 0.639 f | ||
| Married | 19 (86.4) | 28 (93.3) | |
| Single | 3 (13.6) | 2 (6.7) | |
| Education | (N/A) | ||
| Literate | 3 (13.6) | 0 | |
| Elementary school | 9(40.9) | 16 (53.3) | |
| High school | 5 (22.7) | 11 (36.7) | |
| University | 5 (22.7) | 3 (10.0) | |
| Smoker | 0.468 f | ||
| Yes | 5 (22.7) | 4 (13.3) | |
| No | 17 (77.3) | 26 (86.7) |
| Group 1 (Duloxetine + TPTNS) (n = 22) | Group 2 (Duloxetine) (n = 30) | P2 Value | ||
|---|---|---|---|---|
| NRS activity | Baseline | 7.68 ± 1.94 | 7.57 ± 2.01 | 0.836 m |
| 1st month | 6.05 ± 2.13 * | 6.40 ± 1.89 * | 0.563 m | |
| 3rd month | 5.95 ± 2.01 * | 6.37 ± 1.79 * | 0.271 m | |
| P1 value | <0.001 f | <0.001 f | ||
| NRS rest | Baseline | 6.55 ± 2.18 | 6.40 ± 2.24 | 0.816 i |
| 1st month | 5.14 ± 2.08 * | 5.50 ± 1.85 * | 0.427 m | |
| 3rd month | 5.09 ± 2.14 * | 5.33 ± 1.90 * | 0.718 m | |
| P1 Value | 0.003 r | <0.001 r | ||
| SF MPQ | ||||
| Sensory | Baseline | 17.45 ± 8.50 | 17.63 ± 8.56 | 0.993 m |
| 1st month | 15.23 ± 9.11 | 15.03 ± 8.68 * | 1.00 m | |
| 3rd month | 14.05 ± 8.06 * | 14.13 ± 7.74 * | 0.985 m | |
| P1 Value | <0.001 r | <0.001 f | ||
| Affective | Baseline | 7.27 ± 3.83 | 7.27 ± 3.70 | 0.985 m |
| 1st month | 6.41 ± 3.29 | 6.30 ± 3.12 | 0.869 m | |
| 3rd month | 6.05 ± 3.44 * | 6.07 ± 3.28 * | 0.878 m | |
| P1 Value | 0.023 f | 0.005 f | ||
| Total | Baseline | 24.73 ± 11.76 | 24.90 ± 11.71 | 0.978 m |
| 1st month | 21.64 ± 11.87 | 21.33 ± 11.24 * | 0.985 m | |
| 3rd month | 20.09 ± 10.85 * | 20.20 ± 10.33 * | 0.896 m | |
| P1 Value | <0.001 r | <0.001 f | ||
| VAS | Baseline | 7.06 ± 2.21 | 7.12 ± 2.22 | 0.941 m |
| 1st month | 5.28 ± 2.49 * | 5.78 ± 2.22 * | 0.676 m | |
| 3rd month | 5.19 ± 2.25 * | 5.69 ± 2.14 * | 0.553 m | |
| P1 Value | <0.001 r | <0.001 f | ||
| Present Pain Intensity | Baseline | 2.64 ± 0.79 | 2.60 ± 0.72 | 0.934 m |
| 1st month | 2.00 ± 0.87 * | 2.07 ± 0.83 * | 0.646 m | |
| 3rd month | 2.05 ± 0.72 * | 2.13 ± 0.68 * | 0.518 m | |
| P1 Value | <0.001 f | <0.001 f | 0.934 m | |
| FIQ | Baseline | 45.82 ± 7.81 | 50.20 ± 8.77 | 0.068 i |
| 1st month | 38.82 ± 8.57 * | 46.17 ± 10.44 * | 0.084 m | |
| 3rd month | 34.18 ± 10.72 * | 41.47 ± 13.38 * | 0.167 m | |
| P1 Value | <0.001 r | <0.001 r |
| SF-36 | Group 1 (Duloxetine + TPTNS) (n = 22) | Group 2 (Duloxetine) (n = 30) | P2 Value | |
|---|---|---|---|---|
| Physical functioning | Baseline | 43.64 ± 24.11 | 47.00 ± 25.31 | 0.631 i |
| 1st month | 49.09 ± 22.18 | 50.00 ± 23.16 | 0.459 m | |
| 3rd month | 46.36 ± 22.53 | 50.00 ± 22.89 | 0.960 m | |
| P1 Value | 0.065 r | 0.217 r | ||
| Role-Physical | Baseline | 22.73 ± 30.77 | 23.33 ± 31.44 | 0.951 m |
| 1st month | 17.05 ± 24.86 | 18.33 ± 27.02 | 0.973 m | |
| 3rd month | 17.05 ± 28.23 | 19.17 ± 29.86 | 0.782 m | |
| P1 Value | 0.325 f | 0.307 f | ||
| Role-Emotional | Baseline | 12.12 ± 26.32 | 13.33 ± 28.50 | 0.940 m |
| 1st month | 12.12 ± 26.32 | 13.33 ± 28.50 | 0.914 m | |
| 3rd month | 9.09 ± 23.42 | 11.11 ± 26.74 | 0.894 m | |
| P1 Value | 0.549 f | 0.549 f | ||
| Vitality | Baseline | 25.23 ± 18.80 | 25.00 ± 18.98 | 0.926 m |
| 1st month | 28.86 ± 17.92 * | 29.00 ± 18.86 * | 0.915 m | |
| 3rd month | 27.95 ± 17.71 | 28.50 ± 19.26 | 0.862 m | |
| P1 Value | 0.004 f | 0.001 f | ||
| Mental health | Baseline | 43.64 ± 23.03 | 46.00 ± 23.62 | 0.720 i |
| 1st month | 49.09 ± 19.37 | 50.53 ± 20.49 | 0.858 m | |
| 3rd month | 46.18 ± 21.93 | 48.40 ± 22.47 | 0.985 m | |
| P1 Value | 0.128 r | 0.216 r | ||
| Social functioning | Baseline | 41.48 ± 26.56 | 46.58 ± 26.40 | 0.495 i |
| 1st month | 49.43 ± 26.30 | 48.67 ± 26.09 | 0.482 m | |
| 3rd month | 46.59 ± 28.13 | 48.25 ± 27.57 | 0.509 m | |
| P1 Value | 0.294 r | 0.108 f | ||
| Bodily pain | Baseline | 38.52 ± 18.12 | 39.58 ± 18.08 | 0.835 i |
| 1st month | 43.52 ± 24.54 | 44.67 ± 22.72 | 0.953 m | |
| 3rd month | 41.59 ± 22.80 | 43.25 ± 21.47 | 0.902 m | |
| P1 Value | 0.295 r | 0.150 r | ||
| General health perception | Baseline | 32.95 ± 17.91 | 34.00 ± 18.63 | 0.925 m |
| 1st month | 31.36 ± 19.10 | 32.67 ± 19.77 | 0.906 i | |
| 3rd month | 30.45 ± 18.96 | 32.00 ± 19.72 | 0.696 m | |
| P1 Value | 0.254 r | 0.368 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarı, İ.F.; İlhanlı, İ.; Mızrak, T.; Kulaklı, F.; Kasap, Z. The Effect of Transcutaneous Posterior Tibial Nerve Stimulation on Pain and Quality of Life in Patients with Fibromyalgia: A Single-Blind, Randomized Controlled Trial. J. Clin. Med. 2023, 12, 4989. https://doi.org/10.3390/jcm12154989
Sarı İF, İlhanlı İ, Mızrak T, Kulaklı F, Kasap Z. The Effect of Transcutaneous Posterior Tibial Nerve Stimulation on Pain and Quality of Life in Patients with Fibromyalgia: A Single-Blind, Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(15):4989. https://doi.org/10.3390/jcm12154989
Chicago/Turabian StyleSarı, İlker Fatih, İlker İlhanlı, Tuba Mızrak, Fazıl Kulaklı, and Zerrin Kasap. 2023. "The Effect of Transcutaneous Posterior Tibial Nerve Stimulation on Pain and Quality of Life in Patients with Fibromyalgia: A Single-Blind, Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 15: 4989. https://doi.org/10.3390/jcm12154989
APA StyleSarı, İ. F., İlhanlı, İ., Mızrak, T., Kulaklı, F., & Kasap, Z. (2023). The Effect of Transcutaneous Posterior Tibial Nerve Stimulation on Pain and Quality of Life in Patients with Fibromyalgia: A Single-Blind, Randomized Controlled Trial. Journal of Clinical Medicine, 12(15), 4989. https://doi.org/10.3390/jcm12154989






