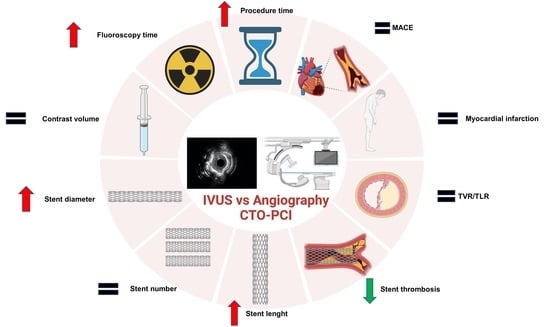
Clinical and Procedural Outcomes of IVUS-Guided vs. Angiography-Guided CTO-PCI: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Research
2.2. Study Selection with Inclusion/Exclusion Criteria
2.3. Outcomes
2.4. Evaluation of Study Quality
2.5. Statistical Analysis
3. Results
3.1. Selected Studies and Baseline Characteristics
3.2. Primary Clinical Outcome
3.3. Secondary Clinical Outcomes
3.4. Procedural Outcomes
3.5. Study Quality
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Fefer, P.; Knudtson, M.L.; Cheema, A.N.; Galbraith, P.D.; Osherov, A.B.; Yalonetsky, S.; Gannot, S.; Samuel, M.; Weisbrod, M.; Bierstone, D.; et al. Current perspectives on coronary chronic total occlusions: The Canadian Multicenter Chronic Total Occlusions Registry. J. Am. Coll. Cardiol. 2012, 59, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Råmunddal, T.; Hoebers, L.P.; Henriques, J.P.; Dworeck, C.; Angerås, O.; Odenstedt, J.; Ioanes, D.; Olivecrona, G.; Harnek, J.; Jensen, U.; et al. Prognostic Impact of Chronic Total Occlusions: A Report From SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc. Interv. 2016, 9, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.S.; Martin-Yuste, V.; Hildick-Smith, D.; Boudou, N.; Sianos, G.; Gelev, V.; Rumoroso, J.R.; Erglis, A.; Christiansen, E.H.; Escaned, J.; et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur. Heart J. 2018, 39, 2484–2493. [Google Scholar] [CrossRef]
- Nikolakopoulos, I.; Choi, J.W.; Khatri, J.J.; Alaswad, K.; Doing, A.H.; Dattilo, P.; Rafeh, N.A.; Maalouf, A.; Jaoudeh, F.A.; Tamez, H.; et al. Follow-up Outcomes After Chronic Total Occlusion Percutaneous Coronary Intervention in Patients With and Without Prior Coronary Artery Bypass Graft Surgery: Insights From the PROGRESS-CTO Registry. J. Invasive Cardiol. 2020, 32, 315–320. [Google Scholar]
- Werner, G.S.; Gastmann, O.; Ferrari, M.; Scholz, K.H.; Schünemann, S.; Figulla, H.R. Determinants of stent restenosis in chronic coronary occlusions assessed by intracoronary ultrasound. Am. J. Cardiol. 1999, 83, 1164–1169. [Google Scholar] [CrossRef]
- Werner, G.S.; Diedrich, J.; Morguet, A.J.; Buchwald, A.B.; Kreuzer, H. Morphology of chronic coronary occlusions and response to interventional therapy—A study by intracoronary ultrasound. Int. J. Card. Imaging 1997, 13, 475–484. [Google Scholar] [CrossRef]
- Truesdell, A.G.; Alasnag, M.A.; Kaul, P.; Rab, S.T.; Riley, R.F.; Young, M.N.; Batchelor, W.B.; Maehara, A.; Welt, F.G.; Kirtane, A.J. Intravascular Imaging During Percutaneous Coronary Intervention: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 81, 590–605. [Google Scholar] [CrossRef]
- Panuccio, G.; Skurk, C.; Landmesser, U.; Abdelwahed, Y.S. Double “full moon” CTO plaque detected by computed tomography could predict high-grade debulking techniques: A case-report. Clin. Case Rep. 2023, 11, e7325. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Bin Song, Y.; Lee, J.-Y.; Lee, S.-J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; Cho, J.Y.; Kim, C.J.; et al. Intravascular Imaging-Guided or Angiography-Guided Complex PCI. N. Engl. J. Med. 2023, 388, 1668–1679. [Google Scholar] [CrossRef]
- Panuccio, G.; Neri, G.; Macrì, L.M.; Salerno, N.; De Rosa, S.; Torella, D. Use of Impella device in cardiogenic shock and its clinical outcomes: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2022, 40, 101007. [Google Scholar] [CrossRef] [PubMed]
- Karatasakis, A.; Danek, B.A.; Karmpaliotis, D.; Alaswad, K.; Jaffer, F.A.; Yeh, R.W.; Patel, M.P.; Bahadorani, J.N.; Wyman, R.M.; Lombardi, W.L.; et al. Impact of Proximal Cap Ambiguity on Outcomes of Chronic Total Occlusion Percutaneous Coronary Intervention: Insights From a Multicenter US Registry. J. Invasive Cardiol. 2016, 28, 391–396. [Google Scholar] [PubMed]
- Rathore, S.; Terashima, M.; Suzuki, T. Value of intravascular ultrasound in the management of coronary chronic total occlusions. Catheter. Cardiovasc. Interv. 2009, 74, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Di Mario, C.; Mashayekhi, K.A.; Garbo, R.; Pyxaras, S.A.; Ciardetti, N.; Werner, G.S. Recanalisation of coronary chronic total occlusions. EuroIntervention 2022, 18, 535–561. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Moher, D.; Cook, D.J.; Eastwood, S.; Olkin, I.; Rennie, D.; Stroup, D.F. Improving the quality of reports of meta-analyses of randomised controlled trials: The QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999, 354, 1896–1900. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Hong, S.-J.; Kim, B.-K.; Shin, D.-H.; Kim, J.-S.; Hong, M.-K.; Gwon, H.-C.; Kim, H.-S.; Yu, C.W.; Park, H.S.; Chae, I.-H.; et al. Usefulness of intravascular ultrasound guidance in percutaneous coronary intervention with second-generation drug-eluting stents for chronic total occlusions (from the Multicenter Korean-Chronic Total Occlusion Registry). Am. J. Cardiol. 2014, 114, 534–540. [Google Scholar] [CrossRef]
- Kim, B.-K.; Shin, D.-H.; Hong, M.-K.; Park, H.S.; Rha, S.-W.; Mintz, G.S.; Kim, J.-S.; Kim, J.S.; Lee, S.-J.; Kim, H.-Y.; et al. Clinical Impact of Intravascular Ultrasound-Guided Chronic Total Occlusion Intervention With Zotarolimus-Eluting Versus Biolimus-Eluting Stent Implantation: Randomized Study. Circ. Cardiovasc. Interv. 2015, 8, e002592. [Google Scholar] [CrossRef]
- Tian, N.-L.; Gami, S.-K.; Ye, F.; Zhang, J.-J.; Liu, Z.-Z.; Lin, S.; Ge, Z.; Shan, S.-J.; You, W.; Chen, L.; et al. Angiographic and clinical comparisons of intravascular ultrasound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: Two-year results from a randomised AIR-CTO study. EuroIntervention 2015, 10, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Vemmou, E.; Khatri, J.; Doing, A.H.; Dattilo, P.; Toma, C.; Sheikh, A.; Alaswad, K.; Jefferson, B.K.; Patel, T.N.; Chandwaney, R.H.; et al. Impact of Intravascular Ultrasound Utilization for Stent Optimization on 1-Year Outcomes After Chronic Total Occlusion Percutaneous Coronary Intervention. J. Invasive Cardiol. 2020, 32, 392–399. [Google Scholar] [PubMed]
- Kalogeropoulos, A.S.; Alsanjari, O.; Davies, J.R.; Keeble, T.R.; Tang, K.H.; Konstantinou, K.; Vardas, P.; Werner, G.S.; Kelly, P.A.; Karamasis, G.V. Impact of Intravascular Ultrasound on Chronic Total Occlusion Percutaneous Revascularization. Cardiovasc. Revascularization Med. 2021, 33, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Räber, L.; Mintz, G.S.; Koskinas, K.C.; Johnson, T.W.; Holm, N.R.; Onuma, Y.; Radu, M.D.; Joner, M.; Yu, B.; Jia, H.; et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An. expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur. Heart J. 2018, 39, 3281–3300. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, H.; Münzel, T.; Gori, T. Coronary Stent Thrombosis-Predictors and Prevention. Dtsch. Arztebl. Int. 2020, 117, 320–326. [Google Scholar] [CrossRef]
- Park, J.J.; Chae, I.-H.; Cho, Y.-S.; Kim, S.-W.; Yang, H.-M.; Seo, J.-B.; Kim, S.-Y.; Oh, I.-Y.; Yoon, C.-H.; Suh, J.-W.; et al. The recanalization of chronic total occlusion leads to lumen area increase in distal reference segments in selected patients: An intravascular ultrasound study. JACC Cardiovasc. Interv. 2012, 5, 827–836. [Google Scholar] [CrossRef]
- Barbato, E.; Gallinoro, E.; Abdel-Wahab, M.; Andreini, D.; Carrié, D.; Di Mario, C.; Dudek, D.; Escaned, J.; Fajadet, J.; Guagliumi, G.; et al. Management strategies for heavily calcified coronary stenoses: An EAPCI clinical consensus statement in collaboration with the EURO4C-PCR group. Eur. Heart J. 2023, ehad342. [Google Scholar] [CrossRef]
- Blessing, R.; Buono, A.; Ahoopai, M.; Geyer, M.; Knorr, M.; Brandt, M.; Steven, S.; Drosos, I.; Muenzel, T.; Wenzel, P.; et al. Use of intravascular ultrasound for optimal vessel sizing in chronic total occlusion percutaneous coronary intervention. Front. Cardiovasc. Med. 2022, 9, 922366. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhao, L.; Chen, K.; Xia, S. The Clinical Effects of Intravascular Ultrasound-Guided Percutaneous Coronary Intervention in Patients with Chronic Total Occlusion: A Meta-Analysis. Cardiol. Res. Pract. 2022, 2022, 4170060. [Google Scholar] [CrossRef]
- Chugh, Y.; Buttar, R.; Kwan, T.; Vemmou, E.; Karacsonyi, J.; Nikolakopoulos, I.; Garcia, S.; Goessl, M.; Wang, Y.; Chavez, I.; et al. Outcomes of Intravascular Ultrasound-Guided Versus Angiography-Guided Percutaneous Coronary Interventions in Chronic Total Occlusions: A Systematic Review and Meta-Analysis. J. Invasive Cardiol. 2022, 34, E310–E318. [Google Scholar] [PubMed]




| K-CTO | CTO-IVUS | Air-CTO | Progress-CTO | Kalogeropoulos et al. | |
|---|---|---|---|---|---|
| Year | 2014 | 2015 | 2015 | 2020 | 2021 |
| Study type | Observational | Randomized Controlled Trial | Randomized controlled trial | Observational | Observational |
| Sample Size | 402 IG: 201 AG: 201 | 402 IG: 201 AG: 201 | 230 IG: 115 AG: 115 | 922 IG: 344 AG: 578 | 364 IG: 182 AG: 182 |
| Follow-Up (Years) | 2 | 1 | 1 | 1 | 4 |
| Primary Endpoint | Definite or probable stent thrombosis | Cardiac Death | In-stent late lumen loss (LLL) | CD, MI, TVR | All cause death, CD, MI, TVR |
| Procedural Success | NR | IG 99 AG 98 | IG 91 AG 68 | NR | NR |
| Retrograde Approach (%) | NR | IG: 7 AG 9.5 | IG: 10.4 AG: 19.1 | IG: 28.8 AG: 21.4 | 25.5 IG: 30.2 AG: 20.9 |
| Anterograde Approach (%) | NR | IG: 93 AG: 90.5 | IG: 89.6 AG: 80.9 | IG: AWE 53.5 ADR:17.4 AG: AWE 57.1 ADR: 19.8 | IG: AWE 60.4 ADR: 9.3 AG: AWE 69.2 ADR: 9.9 |
| Second-Generation DES (%) | 100 | 100 | IG 27.8 AG 20.0 | NR | 100 |
| Study | Year | Age | Diabetes (%) | HTN (%) | 3 Vessel Disease (%) | DES (%) | Male Sex (%) | CKD (%) | Femoral Access (%) | Radial Access (%) | LVEF (%) | LAD CTO(%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K-CTO | 2014 | 62 | 30.5 | 59 | 31 | 100 | 77 | NR | NR | NR | NR | 39.0 |
| CTO-IVUS | 2015 | 61.2 | 34.3 | 63.2 | 34.8 | 100 | 80.6 | NR | 73.1 | NR | 56.8 | 44.3 |
| AIR-CTO | 2015 | 66.5 | 28.3 | 72.6 | NR | 100 | 84.35 | NR | 11.7 | 42.1 | NR | 40.4 |
| PROGRESS-CTO | 2020 | 64.8 | 51 | 90.9 | NR | 99.4 | 82.5 | NR | NR | 58.7 | 50.8 | 28.1 |
| KALOGEROPOULOS ET AL. | 2021 | 66.2 | 22.2 | 70.0 | NR | 100 | 82.1 | 18.4 | NR | NR | Good (>55%) IG 71.4 AG 69.8 Mildy reduced (30-49%) IG 24.2 AG 27.5 Reduced (<30%) IG 2.7 AG 2.7 | 26.6 |
| K-CTO | CTO-IVUS | Air-CTO | Progress-CTO | Kalogeropoulos et al. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IG | AG | IG | AG | IG | AG | IG | AG | IG | AG | |
| Stent length | 44.9 ± 21.2 | 37.3 ± 20.6 | 43.6 ± 18.7 | 41.5 ± 17.6 | 55.0 ± 23.0 | 52.0 ± 25.0 | 74.5 ± 47.0 | 68.9 ± 45.3 | 60.0 ± 39.4 | 38.0 ± 28.5 |
| Stent diameter | 2.9 ± 0.3 | 2.8 ± 0.3 | 2.9 ± 0.5 | 2.8 ± 0.4 | 3.0 ± 0.4 | 2.8 ± 0.3 | NR | NR | 3.5 ± 0.7 | 3.2 ± 0.3 |
| Stent number | 1.7 ± 0.7 | 1.4 ± 0.6 | 1.7 ± 0.8 | 1.6 ± 0.7 | 1.6 ± 0.9 | 1.5 ± 0.8 | 2.3 ± 0.74 | 2.0 ± 1.4 | 2.4 ± 0.7 | 3.0 ± 0.7 |
| Procedure time (min) | NR | NR | 95.0 ± 50.0 | 88.0 ± 47.0 | 87.0 ± 48.0 | 90.0 ± 57.0 | 136.0 ± 80.0 | 126.0 ± 71.0 | 138.0 ± 98.2 | 108.0 ± 60.7 |
| Fluoroscopy time (min) | NR | NR | 41.0 ± 26.0 | 37.0 ± 24.0 | 77.0 ± 69.0 | 70.0 ± 61.0 | 49.4 ± 32.0 | 44.7 ± 28.9 | 36.7 ± 26.3 | 33.9 ± 24.2 |
| Contrast volume (mL) | NR | NR | 299.0 ± 128.0 | 295 ± 123.0 | 293 ± 141.0 | 293.0 ± 136.0 | 216.0 ± 107.0 | 240 ± 124.0 | 250.0 ± 88.0 | 250.0 ± 97.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panuccio, G.; Abdelwahed, Y.S.; Carabetta, N.; Salerno, N.; Leistner, D.M.; Landmesser, U.; De Rosa, S.; Torella, D.; Werner, G.S. Clinical and Procedural Outcomes of IVUS-Guided vs. Angiography-Guided CTO-PCI: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 4947. https://doi.org/10.3390/jcm12154947
Panuccio G, Abdelwahed YS, Carabetta N, Salerno N, Leistner DM, Landmesser U, De Rosa S, Torella D, Werner GS. Clinical and Procedural Outcomes of IVUS-Guided vs. Angiography-Guided CTO-PCI: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(15):4947. https://doi.org/10.3390/jcm12154947
Chicago/Turabian StylePanuccio, Giuseppe, Youssef S. Abdelwahed, Nicole Carabetta, Nadia Salerno, David Manuel Leistner, Ulf Landmesser, Salvatore De Rosa, Daniele Torella, and Gerald S. Werner. 2023. "Clinical and Procedural Outcomes of IVUS-Guided vs. Angiography-Guided CTO-PCI: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 15: 4947. https://doi.org/10.3390/jcm12154947
APA StylePanuccio, G., Abdelwahed, Y. S., Carabetta, N., Salerno, N., Leistner, D. M., Landmesser, U., De Rosa, S., Torella, D., & Werner, G. S. (2023). Clinical and Procedural Outcomes of IVUS-Guided vs. Angiography-Guided CTO-PCI: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(15), 4947. https://doi.org/10.3390/jcm12154947