Physical Activity Profiles among Patients Admitted with Acute Exacerbations of Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Procedures
2.2. Data Collection
2.3. Data Management
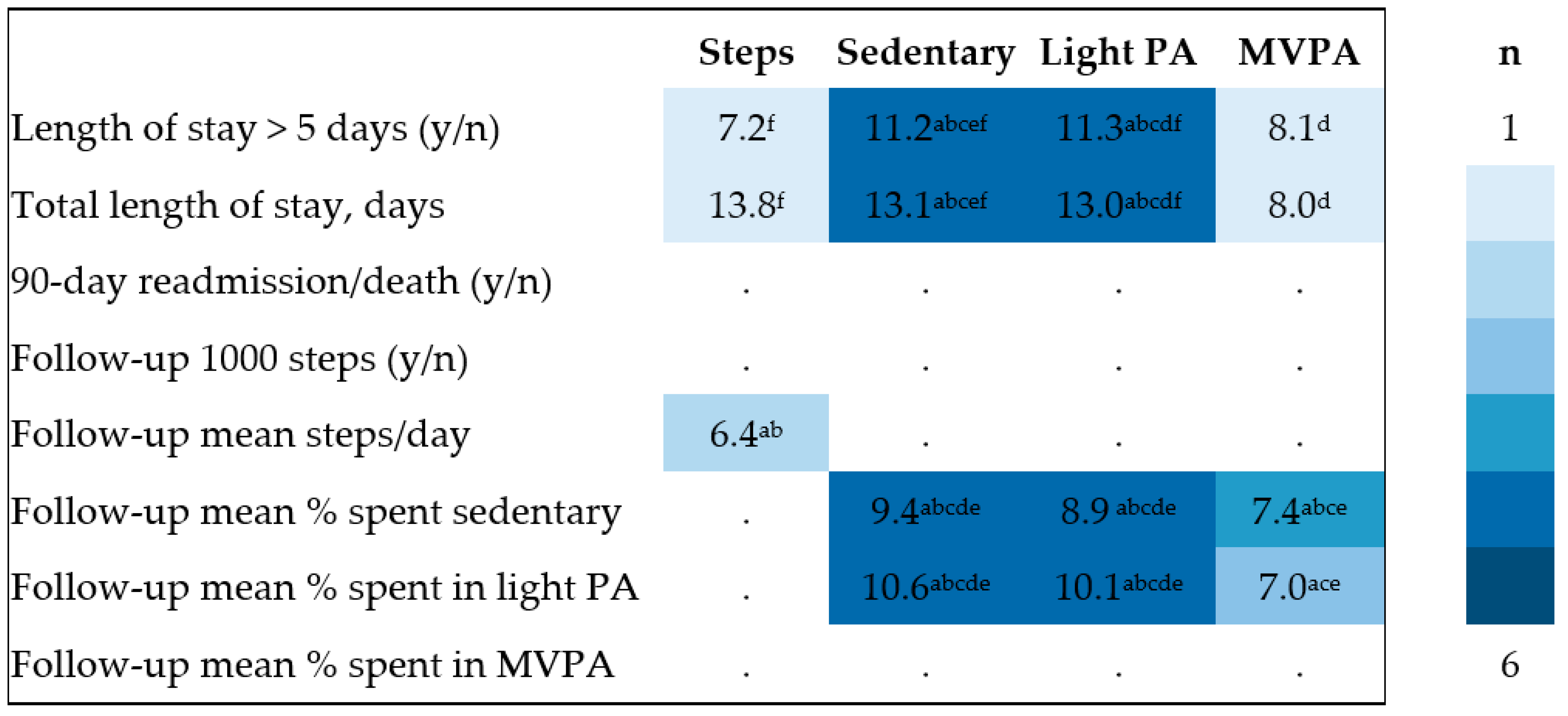
2.4. Analysis Plan
3. Results
3.1. Characterising PA Accumulation Patterns
3.2. Clinical Relevance of Inpatient PA Accumulation
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigare, R.; Dekhuijzen, P.N.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Update on Limb Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. 2014, 189, e15–e62. [Google Scholar] [CrossRef] [PubMed]
- Pitta, F.; Troosters, T.; Probst, V.; Spruit, M.; Decramer, M.; Gosselink, R. Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. Eur. Respir. J. 2006, 27, 1040–1055. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aymerich, J.; Lange, P.; Benet, M.; Schnohr, P.; Antó, J.M. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: A population based cohort study. Thorax 2006, 61, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Waschki, B.; Kirsten, A.; Holz, O.; Müller, K.-C.; Meyer, T.; Watz, H.; Magnussen, H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest 2011, 140, 331–342. [Google Scholar] [CrossRef]
- Hurst John, R.; Jørgen, V.; Antonio, A. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 2010, 363, 1128–1138. [Google Scholar] [CrossRef]
- World Health Organization: Physical Activity. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 14 July 2023).
- Baldwin, C.; Van Kessel, G.; Phillips, A.; Johnston, K. Accelerometry shows inpatients with acute medical or surgical conditions spend little time upright and are highly sedentary: Systematic review. Phys. Ther. 2017, 97, 1044–1065. [Google Scholar] [CrossRef]
- Kirk, A.G.; Behm, K.J.; Kimmel, L.A.; Ekegren, C.L. Levels of physical activity and sedentary behavior during and after hospitalization: A systematic review. Arch. Phys. Med. Rehabil. 2021, 102, 1368–1378. [Google Scholar] [CrossRef]
- Borges, R.C.; Carvalho, C.R.F. Physical activity in daily life in Brazilian COPD patients during and after exacerbation. COPD J. Chronic Obstr. Pulm. Dis. 2012, 9, 596–602. [Google Scholar] [CrossRef]
- Orme, M.W.; Harvey-Dunstan, T.C.; Boral, I.; Chaplin, E.J.; Hussain, S.F.; Morgan, M.D.; Steiner, M.C.; Singh, S.J.; Greening, N.J. Changes in physical activity during hospital admission for chronic respiratory disease. Respirology 2019, 24, 652–657. [Google Scholar] [CrossRef]
- Pitta, F.; Troosters, T.; Probst, V.S.; Spruit, M.A.; Decramer, M.; Gosselink, R. Physical activity and hospitalization for exacerbation of COPD. Chest 2006, 129, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Geelen, S.J.G.; van Dijk-Huisman, H.C.; de Bie, R.A.; Veenhof, C.; Engelbert, R.; van der Schaaf, M.; Lenssen, A.F. Barriers and enablers to physical activity in patients during hospital stay: A scoping review. Syst. Rev. 2021, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Osadnik, C.R. Hospitalizations for patients with acute respiratory exacerbations: In pursuit of rest or recovery? Respirology 2019, 24, 618–619. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.L.Y.; Alison, J.A.; McKenzie, D.K.; McKeough, Z.J. Physical activity levels improve following discharge in people admitted to hospital with an acute exacerbation of chronic obstructive pulmonary disease. Chron. Respir. Dis. 2016, 13, 23–32. [Google Scholar] [CrossRef]
- Boyd, C.M.; Landefeld, C.S.; Counsell, S.R.; Palmer, R.M.; Fortinsky, R.H.; Kresevic, D.; Burant, C.; Covinsky, K.E. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J. Am. Geriatr. Soc. 2008, 56, 2171–2179. [Google Scholar] [CrossRef]
- Demeyer, H.; Mohan, D.; Burtin, C.; Vaes, A.W.; Heasley, M.; Bowler, R.P.; Casaburi, R.; Cooper, C.B.; Corriol-Rohou, S.; Frei, A. Objectively measured physical activity in patients with COPD: Recommendations from an international task force on physical activity. Chronic Obstr. Pulm. Dis. J COPD Found. 2021, 8, 528. [Google Scholar] [CrossRef]
- MacDonald, M.I.; Osadnik, C.R.; Bulfin, L.; Leahy, E.; Leong, P.; Shafuddin, E.; Hamza, K.; King, P.T.; Bardin, P.G. MULTI-PHACET: Multidimensional clinical phenotyping of hospitalised acute COPD exacerbations. ERJ Open Res. 2021, 7, 00198-2021. [Google Scholar] [CrossRef]
- Leidy, N.K. Functional Status and the Forward Progress of Merry-Go-Rounds: Toward a Coherent Analytical Framework. Nurs. Res. 1994, 43, 196–202. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Van Remoortel, H.; Raste, Y.; Louvaris, Z.; Giavedoni, S.; Burtin, C.; Langer, D.; Wilson, F.; Rabinovich, R.; Vogiatzis, I.; Hopkinson, N.S. Validity of six activity monitors in chronic obstructive pulmonary disease: A comparison with indirect calorimetry. PLoS ONE 2012, 7, e39198. [Google Scholar] [CrossRef]
- Demeyer, H.; Burtin, C.; Van Remoortel, H.; Hornikx, M.; Langer, D.; Decramer, M.; Gosselink, R.; Janssens, W.; Troosters, T. Standardizing the analysis of physical activity in patients with COPD following a pulmonary rehabilitation program. Chest 2014, 146, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.E.; John, D.; Freedson, P.S. Validation and comparison of ActiGraph activity monitors. J. Sci. Med. Sport 2011, 14, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Norman, E.; Mylotte, A.; Watson, P. Factors associated with length of stay and re-admissions in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care. Med. 2001, 163, A508. [Google Scholar]
- Anthonisen, N.R.; Manfreda, J.; Warren, C.P.; Hershfield, E.S.; Harding, G.K.; Nelson, N.A. Antibiotic Therapy in Exacerbations of Chronic Obstructive Pulmonary Disease. Ann. Intern. Med. 1987, 106, 196–204. [Google Scholar] [CrossRef]
- Benzo, R.; Wetzstein, M.; Neuenfeldt, P.; McEvoy, C. Implementation of physical activity programs after COPD hospitalizations: Lessons from a randomized study. Chron. Respir. Dis. 2015, 12, 5–10. [Google Scholar] [CrossRef]
- Orme, M.W.; Weedon, A.E.; Saukko, P.M.; Esliger, D.W.; Morgan, M.D.; Steiner, M.C.; Downey, J.W.; Sherar, L.B.; Singh, S.J. Findings of the chronic obstructive pulmonary disease-sitting and exacerbations trial (COPD-SEAT) in reducing sedentary time using wearable and mobile technologies with educational support: Randomized controlled feasibility trial. JMIR mHealth uHealth 2018, 6, e9398. [Google Scholar] [CrossRef]
- Torres-Sánchez, I.; Valenza, M.C.; Cabrera-Martos, I.; López-Torres, I.; Benítez-Feliponi, Á.; Conde-Valero, A. Effects of an Exercise Intervention in Frail Older Patients with Chronic Obstructive Pulmonary Disease Hospitalized due to an Exacerbation: A Randomized Controlled Trial. COPD J. Chronic Obst. Pulm. Dis. 2017, 14, 37–42. [Google Scholar] [CrossRef]
- Greening, N.J.; Williams, J.E.A.; Hussain, S.F.; Harvey-Dunstan, T.C.; Bankart, M.J.; Chaplin, E.J.; Vincent, E.E.; Chimera, R.; Morgan, M.D.; Singh, S.J.; et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: Randomised controlled trial. BMJ Brit. Med. J. 2014, 349, g4315. [Google Scholar] [CrossRef]
- Valeiro, B.; Rodríguez, E.; Pérez, P.; Gómez, A.; Mayer, A.I.; Pasarín, A.; Ibañez, J.; Ferrer, J.; Ramon, M.A. Promotion of physical activity after hospitalization for COPD exacerbation: A randomized control trial. Respirology 2023, 28, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.-Y.; Benzo, R.; Ries, A.L.; Soler, X. Physical activity monitoring in patients with chronic obstructive pulmonary disease. Chronic Obstr. Pulm. Dis. 2014, 1, 155. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.W.; Alison, J.A.; Stamatakis, E.; Dennis, S.M.; McKeough, Z.J. Patterns and correlates of sedentary behaviour accumulation and physical activity in people with chronic obstructive pulmonary disease: A cross-sectional study. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 156–164. [Google Scholar] [CrossRef] [PubMed]
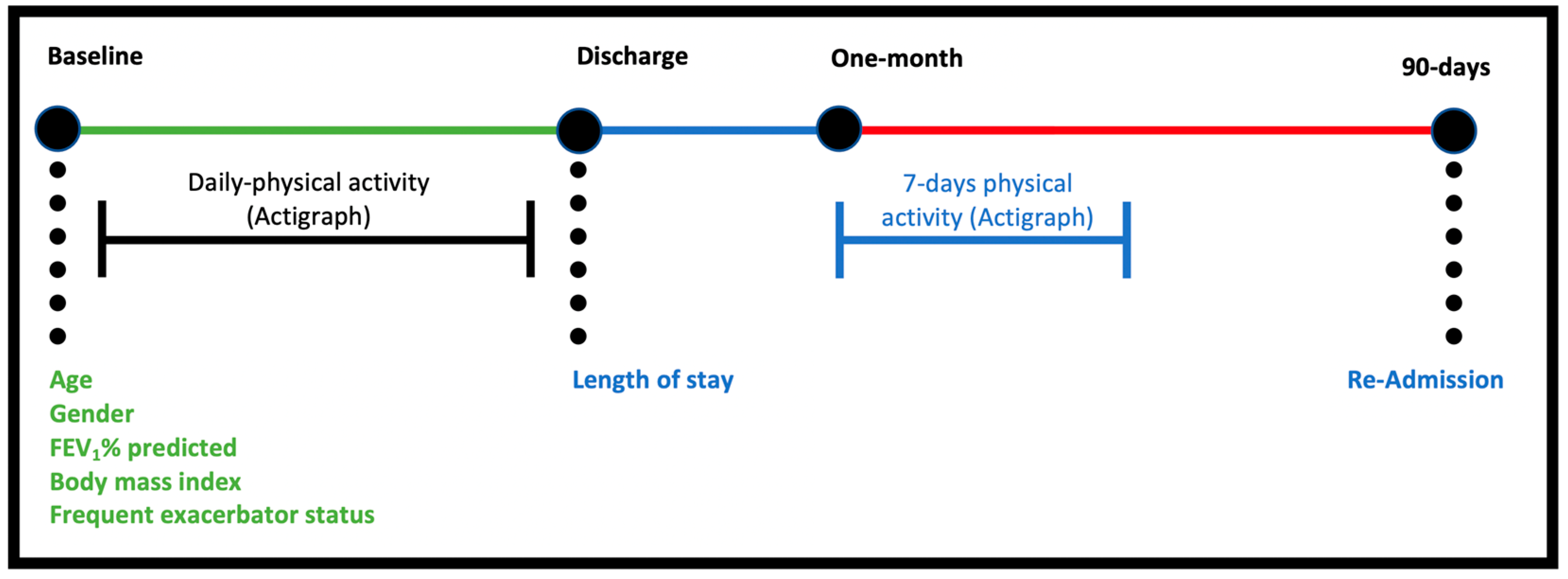

| Type of Data | Units of Measurement/Analysis Characteristics |
|---|---|
| Exposure variable: Physical activity | |
| Physical activity amount/duration | Steps/time spent active per day |
| Sedentary behaviour | Time spent sedentary per day |
| Physical activity intensity | MET-derived cut-offs (light, moderate, vigorous) |
| Outcome variables: Clinical outcomes | |
| Length of stay | Days |
| 90-day readmissions | Yes or no |
| PA at one-month follow-up | Steps/day |
| Covariates | |
| Age | Years, days |
| Body mass index | Kg/m2 |
| Gender | Male, female, non-binary |
| Frequent exacerbator status | 0 or ≥1 prior event in the last 12 months |
| FEV1% predicted | FEV1% predicted |
| Descriptive Variable | N = 68 (at Recruitment) |
|---|---|
| Age (years) | 69.2 (9.0) |
| Gender: | |
| Male | 38 (56%) |
| Female | 30 (44%) |
| Body mass index (kg/m2) | 27.2 (7.5) |
| FEV1% predicted | 45.0 (17.7) |
| TLCO % predicted | 44.2 (20.3) |
| Smoking status: | |
| Current | 17 (25%) |
| Former | 49 (72%) |
| Never | 1 (1.5%) |
| Pack/years | 41.8 [25.1, 55.5] |
| GOLD Stage: | |
| I | 3 (5%) |
| II | 22 (35%) |
| III | 24 (39%) |
| IV | 13 (21%) |
| Hypertension | 40 (59%) |
| Ischaemic heart disease | 21 (31%) |
| Diabetes | 8 (12%) |
| Anxiety | 25 (37%) |
| Depression | 22 (32%) |
| Usual respiratory care: | |
| LABA | 63 (93%) |
| LAMA | 63 (93%) |
| ICS | 57 (84%) |
| Long-term oxygen therapy | 15 (22%) |
| Dyspnoea (eMRC Dyspnoea Scale): | |
| 2 | 17 (25%) |
| 3 | 18 (26%) |
| 4 | 17 (25%) |
| 5a | 11 (16%) |
| 5b | 4 (6%) |
| No. exacerbations past year | 1.0 [1.0, 3.0] |
| Frequent exacerbator status (2+ in last year) (n, %) | 19 (32%) |
| Descriptive Variable | N = 68 (at Recruitment) |
|---|---|
| Exacerbation type *: | |
| 1 | 20 (29%) |
| 2 | 16 (24%) |
| 3 | 26 (38%) |
| Inpatient corticosteroid use (IV/oral) | 59 (87%) |
| Inpatient antibiotic use (IV/oral) | 60 (88%) |
| Evidence of chest x-ray consolidation | 18 (26%) |
| Use of BiPAP in emergency department | 19 (28%) |
| Use of BiPAP on ward | 16 (24%) |
| Length of stay (days) | 4.0 [3.0, 7.0] |
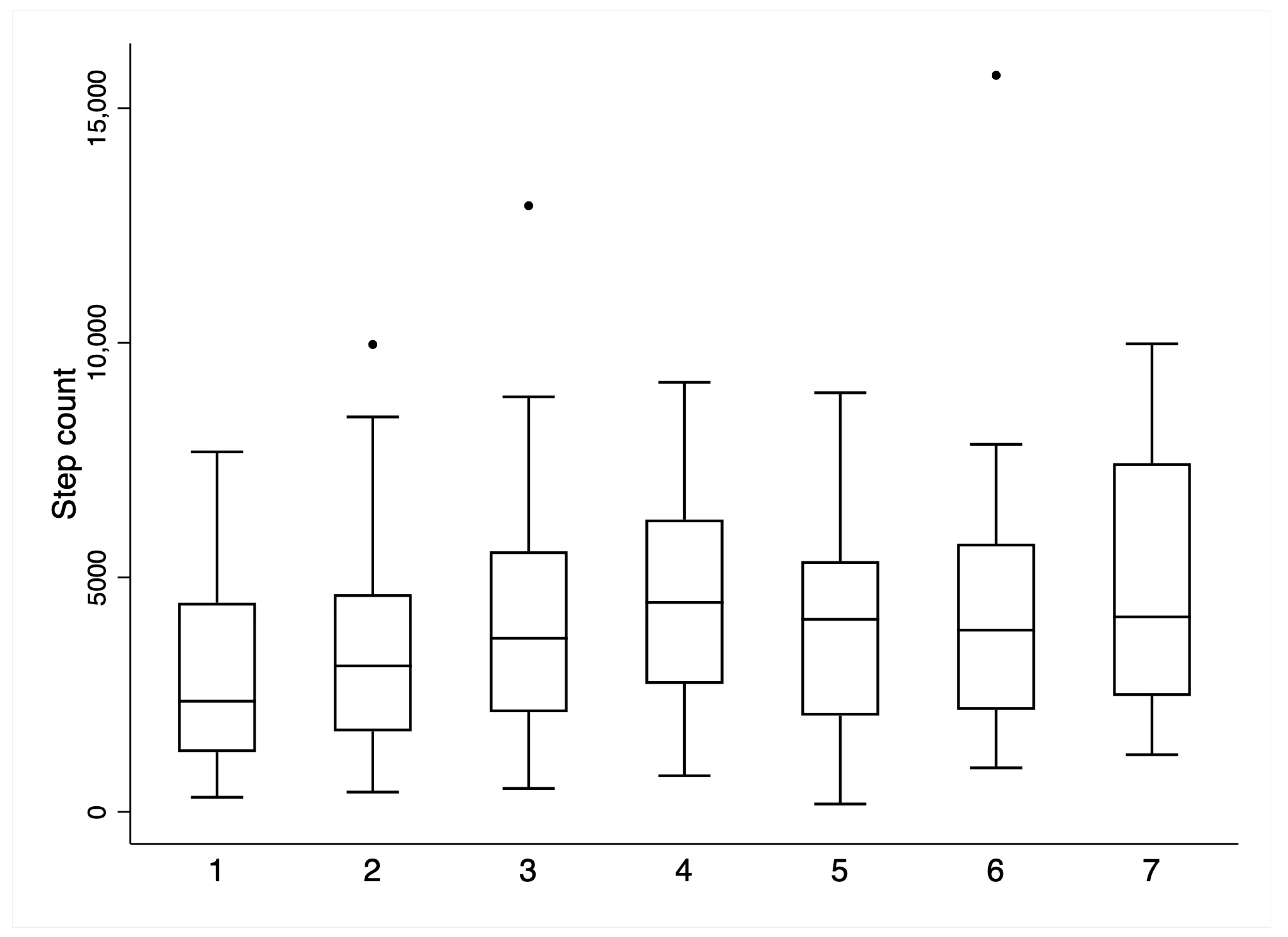
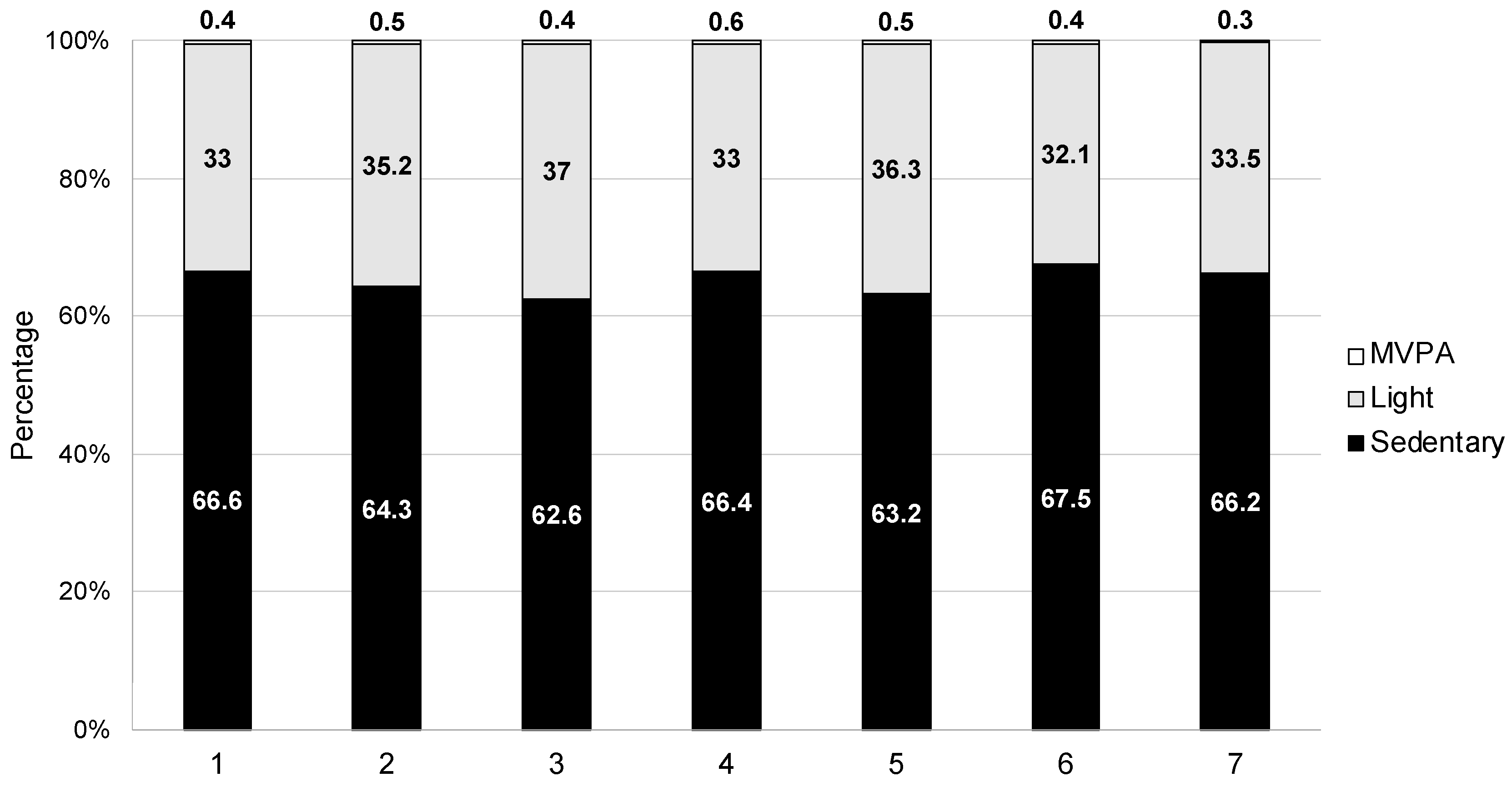
| Day 1 (N = 31) | Day 2 (N = 47) | Day 3 (N = 40) | Day 4 (N = 26) | Day 5 (N = 21) | Day 6 (N = 16) | Day 7 (N = 8) | Inpatient Total (N = 68) | Follow-Up Total (N = 51) | |
|---|---|---|---|---|---|---|---|---|---|
| Discharge day, n | - | 7 | 19 | 11 | 5 | 7 | 7 | - | - |
| Step count | 2995.5 (2040.2) | 3476.0 (2240.0) | 4119.1 (2568.9) | 4542.7 (2121.8) | 4012.0 (2387.1) | 4587.6 (3623.0) | 4915.9 (3154.1) | 3817.0 (2144.2) | 6173.7 (3564.6) |
| Mean % sedentary time | 66.6 (12.6) | 64.3 (14.0) | 62.6 (15.6) | 66.4 (11.8) | 63.2 (16.5) | 67.5 (17.7) | 66.2 (22.6) | 63.4 (12.9) | 49.4 (14.7) |
| Mean % light time | 33.0 (12.3) | 35.2 (14.0) | 37.0 (15.4) | 33.0 (11.7) | 36.3 (16.2) | 32.1 (17.5) | 33.5 (22.5) | 36.2 (12.7) | 49.3 (14.0) |
| Mean % MVPA time | 0.4 (0.6) | 0.5 (0.7) | 0.4 (0.6) | 0.6 (0.8) | 0.5 (0.5) | 0.4 (0.3) | 0.3 (0.3) | 0.5 (0.6) | 1.3 (1.8) |
| Factor | N | Mean Steps/Day | p-Value | Mean % Time in Sedentary | p-Value | Mean % Time in Light | p-Value | Mean % Time in MVPA | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Censor * no | 48 | 4175.7 (1996.0) | 0.15 | 62.6 (13.7) | 0.75 | 36.8 (13.5) | 0.80 | 0.3 [0.2, 0.8] | 0.403 |
| Censor * yes | 17 | 3322.4 (2289.3) | 63.8 (10.8) | 35.9 (10.9) | 0.3 [0.2, 0.5] | ||||
| LOS < 5 | 42 | 3892.5 (2210.0) | 0.71 | 59.9 (12.4) | 0.004 | 39.6 (12.3) | 0.004 | 0.3 [0.2, 0.7] | 0.387 |
| LOS ≥ 5 | 26 | 3695.0 (2070.4) | 69.0 (11.8) | 30.6 (11.6) | 0.3 [0.2, 0.6] | ||||
| GOLD 1 and 2 | 25 | 3712.0 (2040.2) | 0.47 | 62.2 (13.2) | 0.78 | 37.1 (12.9) | 0.84 | 0.6 [0.2, 0.9] | 0.016 |
| GOLD 3 and 4 | 36 | 4121.2 (2254.6) | 63.2 (13.5) | 36.5 (13.5) | 0.2 [0.2, 0.4] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byron, C.; Osadnik, C.R. Physical Activity Profiles among Patients Admitted with Acute Exacerbations of Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2023, 12, 4914. https://doi.org/10.3390/jcm12154914
Byron C, Osadnik CR. Physical Activity Profiles among Patients Admitted with Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine. 2023; 12(15):4914. https://doi.org/10.3390/jcm12154914
Chicago/Turabian StyleByron, Christopher, and Christian R. Osadnik. 2023. "Physical Activity Profiles among Patients Admitted with Acute Exacerbations of Chronic Obstructive Pulmonary Disease" Journal of Clinical Medicine 12, no. 15: 4914. https://doi.org/10.3390/jcm12154914
APA StyleByron, C., & Osadnik, C. R. (2023). Physical Activity Profiles among Patients Admitted with Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine, 12(15), 4914. https://doi.org/10.3390/jcm12154914






