Neurodynamic Techniques in the Treatment of Mild-to-Moderate Carpal Tunnel Syndrome: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Study Selection and Data Collection
2.4. Risk of Bias Assessment
2.5. Data Synthesis and Statistical Analysis
3. Results
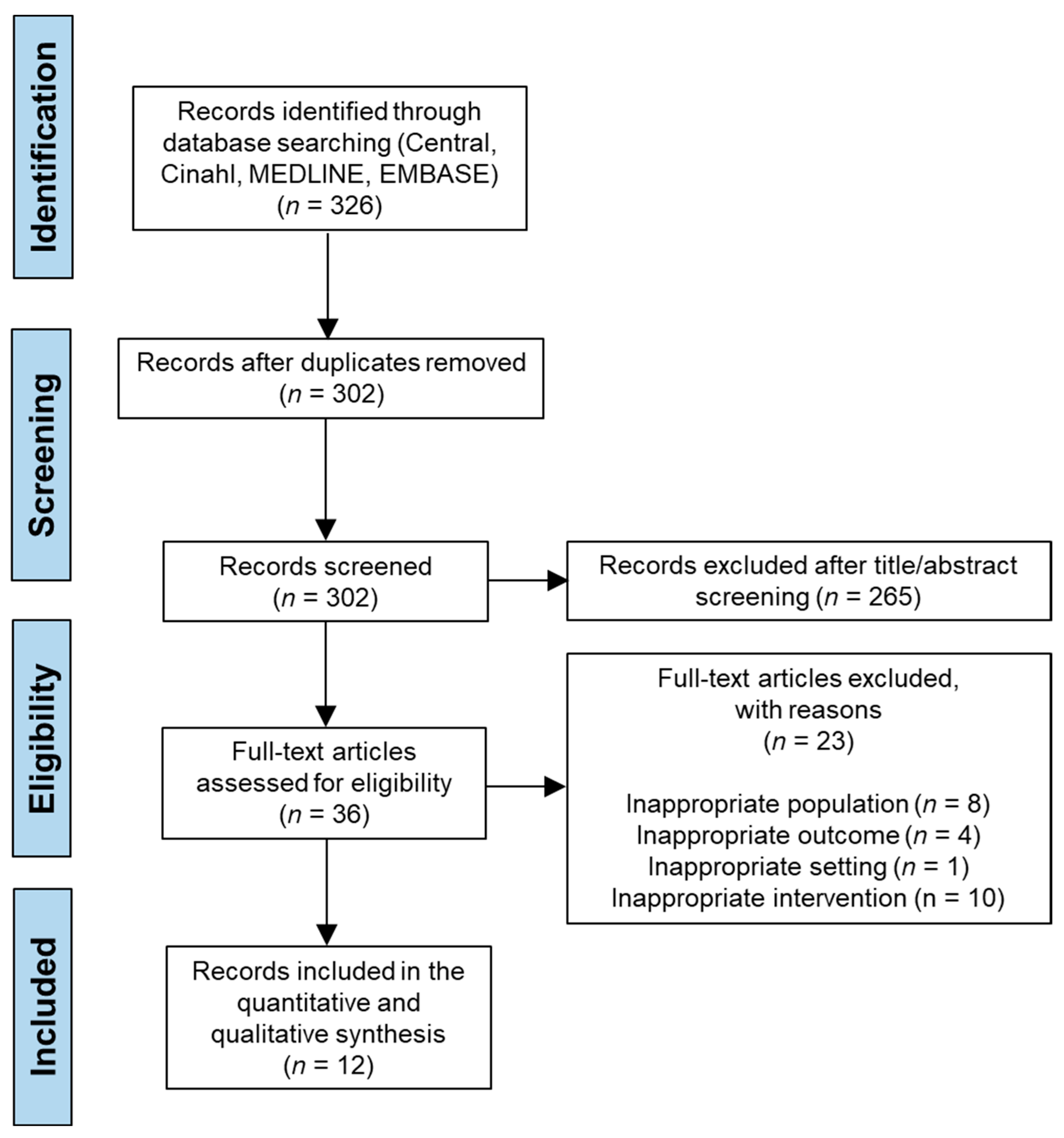
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
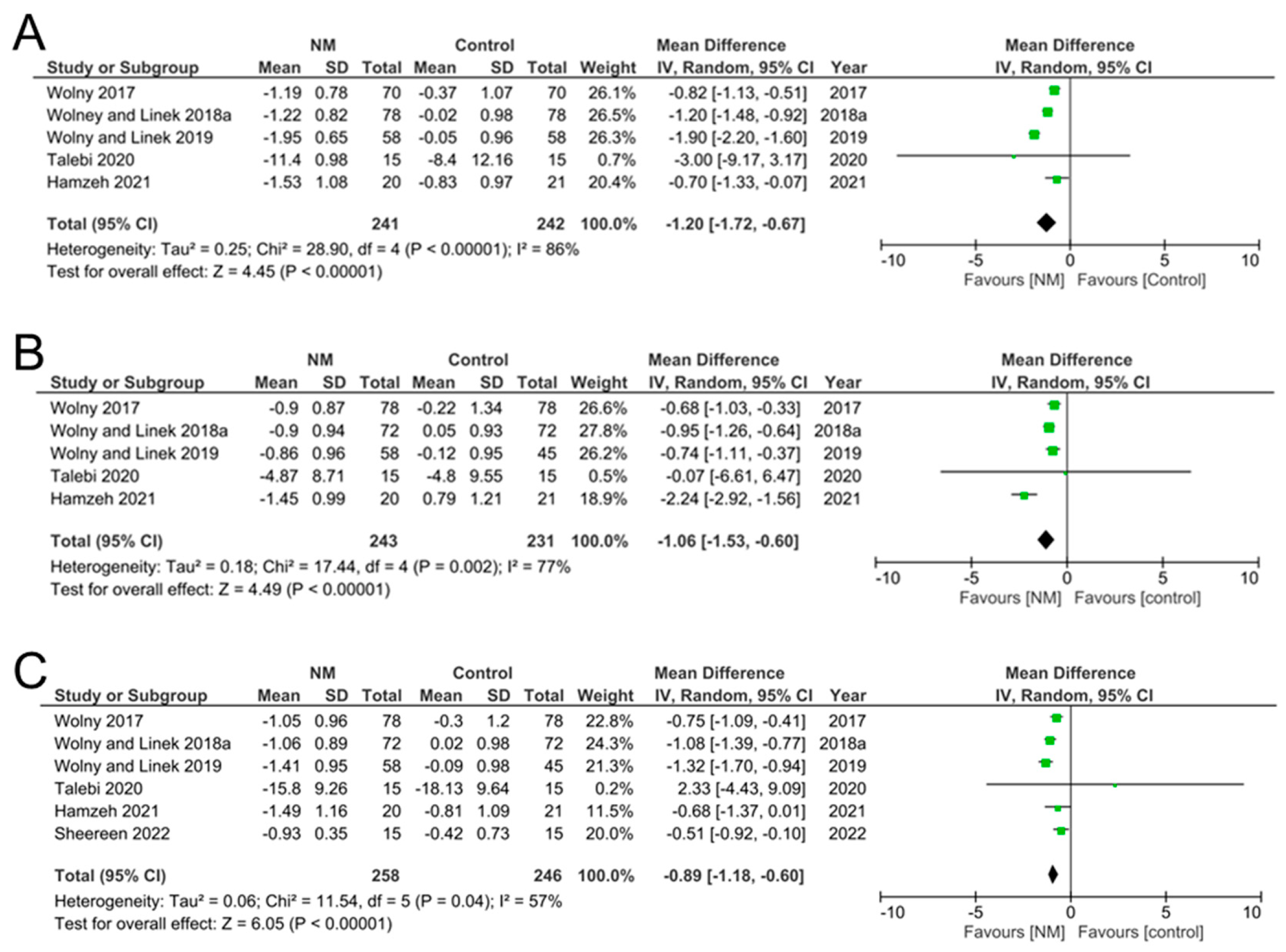
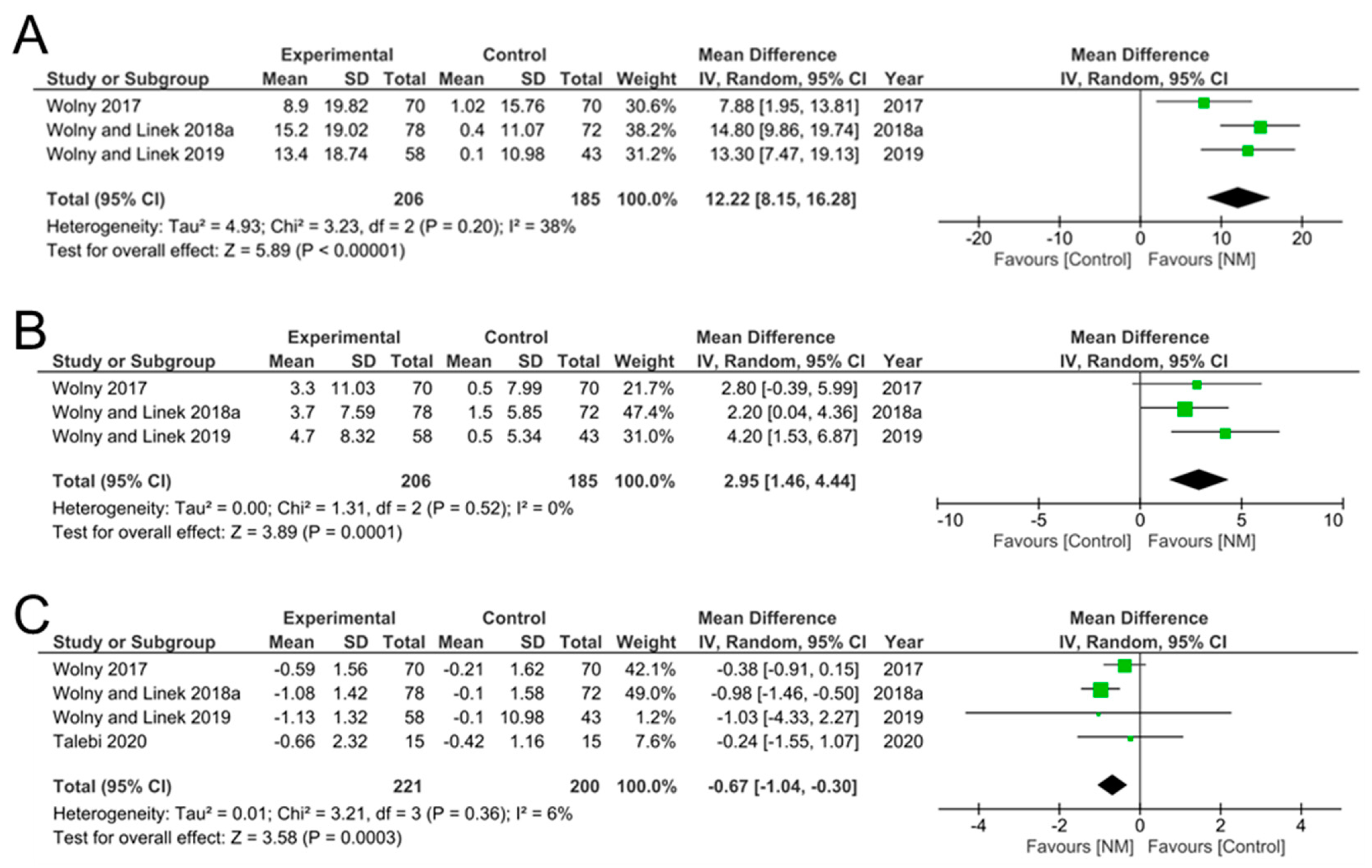
3.4. Data Synthesis
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Damms, N.A.; McCallum, L.M.; Sarrigiannis, P.G.; Zis, P. Pain as a determinant of health-related quality of life in patients with carpal tunnel syndrome; a case-controlled study. Postgrad. Med. 2020, 132, 52–55. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Cleland, J.; Palacios-Ceña, M.; Fuensalida-Novo, S.; Pareja, J.A.; Alonso-Blanco, C. The effectiveness of manual therapy versus surgery on self-reported function, cervical range of motion, and pinch grip force in carpal tunnel syndrome: A randomized clinical trial. J. Orthop. Sports Phys. Ther. 2017, 47, 151–161. [Google Scholar] [CrossRef]
- Hernández-Secorún, M.; Montaña-Cortés, R.; Hidalgo-García, C.; Rodríguez-Sanz, J.; Corral-De-Toro, J.; Monti-Ballano, S.; Hamam-Alcober, S.; Tricás-Moreno, J.M.; Lucha-López, M.O. Effectiveness of conservative treatment according to severity and systemic disease in carpal tunnel syndrome: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 2365. [Google Scholar] [CrossRef]
- Ijaz, M.J.; Karimi, H.; Ahmad, A.; Gillani, S.A.; Anwar, N.; Chaudhary, M.A. Comparative efficacy of routine physical therapy with and without neuromobilization in the treatment of patients with mild to moderate carpal tunnel syndrome. BioMed. Res. Int. 2022, 2022, 2155765. [Google Scholar] [CrossRef] [PubMed]
- Alhusain, F.A.; Almohrij, M.; Althukeir, F.; Alshater, A.; Alghamdi, B.; Masuadi, E.; Basudan, A. Prevalence of carpal tunnel syndrome symptoms among dentists working in Riyadh. Annals Saudi Med. 2019, 39, 104–111. [Google Scholar] [CrossRef]
- Wolny, T.; Linek, P.; Saulicz, E. Overall health status in patients with mild to moderate carpal tunnel syndrome: A case-control study. J. Hand Ther. 2017, 30, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Oguntona, D.A.S.; Jose, D.; Hussein, D. Clinical and neurophysiological characteristics of patients with carpal tunnel syndrome presenting at the tertiary rheumatology out-patient clinic. Saudi Med. J. 2022, 7, 135–140. [Google Scholar] [CrossRef]
- Khosrawi, S.; Maghrouri, R. The prevalence and severity of carpal tunnel syndrome during pregnancy. Adv. Biomed. Res. 2012, 1, 43. [Google Scholar] [CrossRef] [PubMed]
- Pourmemari, M.H.; Shiri, R. Diabetes as a risk factor for carpal tunnel syndrome: A systematic review and meta-analysis. Diabetic Med. 2016, 33, 10–16. [Google Scholar] [CrossRef]
- Sheereen, F.J.; Sarkar, B.; Sahay, P.; Shaphe, M.A.; Alghadir, A.H.; Iqbal, A.; Ali, T.; Ahmad, F. Comparison of two manual therapy programs, including tendon gliding exercises as a common adjunct, while managing the participants with chronic carpal tunnel syndrome. Pain Res. Manag. 2022, 2022, 1975803. [Google Scholar] [CrossRef]
- Erickson, M.I.A.; Lawrence, M.; Stegink Jansen, C.W.; Coker, D.; Amadio, P.; Cleary, C. Hand pain and sensory deficits: Carpal tunnel syndrome. J. Orthop. Sports Phys. Ther. 2019, 49, CPG1–CPG60. [Google Scholar] [CrossRef] [PubMed]
- Carlson, H.; Colbert, A.; Frydl, J.; Arnall, E.; Elliott, M.; Carlson, N. Current options for nonsurgical management of carpal tunnel syndrome. Int. J. Clin. Rheumatol. 2010, 5, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Macdermid, J.C. Is surgical intervention more effective than non-surgical treatment for carpal tunnel syndrome? a systematic review. J. Orthop. Surg. Res. 2011, 6, 17. [Google Scholar] [CrossRef]
- Wolny, T.; Linek, P. Is manual therapy based on neurodynamic techniques effective in the treatment of carpal tunnel syndrome? A randomized controlled trial. Clin. Rehab. 2019, 33, 408–417. [Google Scholar] [CrossRef]
- Lewis, K.J.; Coppieters, M.W.; Ross, L.; Hughes, I.; Vicenzino, B.; Schmid, A.B. Group education, night splinting and home exercises reduce conversion to surgery for carpal tunnel syndrome: A multicentre randomised trial. J. Physiother. 2020, 66, 97–104. [Google Scholar] [CrossRef] [PubMed]
- FernÁNdez-De-Las-PeÑAs, C.; Ortega-Santiago, R.; Salom-Moreno, J.; Arias-BurÍA, J.L.; Fahandezh-Saddi, D.H.; Cleland, J.A.; Pareja, J.A. Cost-effectiveness evaluation of manual physical therapy versus surgery for carpal tunnel syndrome: Evidence from a randomized clinical trial. J. Orthop. Sports Phys. Ther. 2019, 49, 55–63. [Google Scholar] [CrossRef]
- Basson, A.; Olivier, B.; Ellis, R.; Coppieters, M.; Stewart, A.; Mudzi, W. The effectiveness of neural mobilization for neuromusculoskeletal conditions: A systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2017, 47, 593–615. [Google Scholar] [CrossRef]
- Oskouei, A.E.; Talebi, G.A.; Shakouri, S.K.; Ghabili, K. Effects of neuromobilization manoeuvre on clinical and electrophysiological measures of patients with carpal tunnel syndrome. J. Phys. Ther. Sci. 2014, 26, 1017–1022. [Google Scholar] [CrossRef][Green Version]
- McKeon, J.M.M.; Yancosek, K.E. Neural gliding techniques for the treatment of carpal tunnel syndrome: A systematic review. J. Sport Rehab. 2008, 17, 324–341. [Google Scholar] [CrossRef][Green Version]
- Duman, I.; Davul, S.; Hallaçeli, H.; Dogramaci, Y.; Vedat, U. Excursion of the median, ulnar and radial nerves during the nerve gliding exercises used in The Orthopedic Physiotherapy: A cadaveric study. Mustafa Kemal Üniversitesi Tıp. Dergisi. 2021, 12, 144–148. [Google Scholar] [CrossRef]
- Hamzeh, H.; Madi, M.; Alghwiri, A.A.; Hawamdeh, Z. The long-term effect of neurodynamics vs exercise therapy on pain and function in people with carpal tunnel syndrome: A randomized parallel-group clinical trial. J. Hand Ther. 2021, 34, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Nunez De Arenas-Arroyo, S.; Cavero-Redondo, I.; Torres-Costoso, A.N.A.; Reina-GutiÉRrez, S.; ÁLvarez-Bueno, C.; MartÍNez-VizcaÍNo, V. Short-term effects of neurodynamic techniques for treating carpal tunnel syndrome: A systematic review with meta-analysis. J. Orthop. Sports Phys. Ther. 2021, 51, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Kurniawti, I.R. Comparison on effectiveness of nerve mobilization and Kinesio Taping toward changes in Carpal Tunnel syndrome. J. Phys. Conf. Ser. 2019, 1529, 032034. [Google Scholar] [CrossRef]
- Alam, M.; Khan, M.; Ahmed, S.I.; Ali, S.S. Effectiveness of neural mobilization and ultrasound therapy on pain severity in carpal tunnel syndrome. Biomed. Res. Ther. 2018, 5, 2187–2193. [Google Scholar] [CrossRef]
- Tal-Akabi, A.; Rushton, A. An investigation to compare the effectiveness of carpal bone mobilisation and neurodynamic mobilisation as methods of treatment for carpal tunnel syndrome. Man. Ther. 2000, 5, 214–222. [Google Scholar] [CrossRef][Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Bialosky, J.E.; Bishop, M.D.; Price, D.D.; Robinson, M.E.; Vincent, K.R.; George, S.Z. A randomized sham-controlled trial of a neurodynamic technique in the treatment of carpal tunnel syndrome. J. Orthop. Sports Ther. 2009, 39, 709–723. [Google Scholar] [CrossRef]
- Wolny, T.; Saulice, E.; Linek, P.; Mysliwiec, A.; Saulicz, M. Effect of manual therapy and neurodynamic techniques vs ultrasound and laser on 2PD in patients with CTS: A randomized controlled trial. J. Hand Ther. 2016, 29, 235–245. [Google Scholar] [CrossRef]
- Wolny, T.P.; Saulicz, E.P.; Linek, P.P.; Shacklock, M.M.P.T.; Myśliwiec, A.P. Efficacy of manual therapy including neurodynamic techniques for the treatment of carpal tunnel syndrome: A randomized controlled trial. J. Manip. Physiol. Ther. 2017, 40, 263–272. [Google Scholar] [CrossRef]
- Wolny, T.; Linek, P. Neurodynamic techniques versus “sham” therapy in the treatment of carpal tunnel syndrome: A randomized placebo-controlled trial. Arch. Phys. Med. Rehab. 2018, 99, 843–854. [Google Scholar] [CrossRef]
- Wolny, T.; Linek, P. The effect of manual therapy including neurodynamic techniques on the overall health status of people with carpal tunnel syndrome: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2018, 41, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Marryam, M.; Yasmeen, R.; Malik, T.M.; Malik, A.N.; Amjad, I. A comparison of the effectiveness of neurodynamics versus nerve and tendon gliding exercises alone for carpal tunnel syndrome. Pak. Armed. Forces Med. J. 2018, 68, 924–929. [Google Scholar]
- Talebi, G.A.; Saadat, P.; Javadian, Y.; Taghipour, M. Comparison of two manual therapy techniques in patients with carpal tunnel syndrome: A randomized clinical trial. Caspian J. Intern. Med. 2020, 11, 163–170. [Google Scholar]
- Paquette, P.; Higgins, J.; Danino, M.A.; Harris, P.; Lamontagne, M.; Gagnon, D.H. Effects of a preoperative neuromobilization program offered to individuals with carpal tunnel syndrome awaiting carpal tunnel decompression surgery: A pilot randomized controlled study. J. Hand Surg. 2021, 34, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulos, E.; Karanasios, S.; Gioftsos, G.; Tatsios, P.; Koumantakis, G.; Papandreou, M. The effectiveness of neuromobilization exercises in carpal tunnel syndrome: Systematic review and meta-analysis. Physother. Theory Pract. 2022, 28, 1–40. [Google Scholar] [CrossRef]
- Ballestero-Perez, R.; Plaza-Manzano, G.; Urraca-Gesto, A.; Romo-Romo, F.; Atin-Arratibel, M.d.L.; Pecos-Martin, D.; Gallego-Izquierdo, T.; Romero-Franco, N. Effectiveness of nerve gliding exercises on carpal tunnel syndrome: A systematic review. J. Manip. Physiol. Ther. 2017, 40, 50–59. [Google Scholar] [CrossRef]
- Graham, B.; Peljovich, A.E.; Afra, R.; Cho, M.S.; Gray, R.; Stephenson, J.; Gurman, A.; MacDermid, J.; Mlady, G.; Patel, A.T.; et al. The American Academy of Orthopaedic Surgeons evidence-based clinical practice guideline on management of carpal tunnel syndrome. J. Bone Joint Surg. Am. 2016, 98, 1750–1754. [Google Scholar] [CrossRef]
- Karjalanen, T.; Raatikainen, S.; Jaatinen, K.; Lusa, V. Update on efficacy of conservative treatments for carpal tunnel syndrome. J. Clin. Med. 2022, 11, 950. [Google Scholar] [CrossRef]
- Matesanz-García, L.; Cáceres-Pajuelo, J.E.; Cuenca-Martínez, F.; La Touche, R.; Goicoechea-García, C.; Fernández-Carnero, J. Effects of neural mobilizations through movement representation techniques for the improvement of neural mechanosensitivity of the median nerve region: A randomized controlled trial. Somat. Mot. Res. 2021, 38, 267–276. [Google Scholar] [CrossRef]
- Boudier-Revéret, M.; Gilbert, K.; Allégue, D.; Moussadyk, M.; Brismée, J.M.; Sizer, P., Jr.; Feipel, V.; Dugailly, P.-M.; Sobczak, S. Effect of neurodynamic mobilization on fluid dispersion in median nerve at the level of the carpal tunnel: A cadaveric study. Musculoskelet. Sci. Pract. 2017, 31, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Shacklock, M. Clinical Neurodynamics: A New System of Neuromusculoskeletal Treatment, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Del Barrio, S.J.; Gracia, E.B.; García, C.H.; de Miguel, E.E.; Moreno, J.T.; Marco, S.R.; Laita, L.C. Conservative treatment in patients with mild to moderate carpal tunnel syndrome: A systematic review. Neurología 2018, 33, 590–601. [Google Scholar]
- Fernandez-de-Las-Penas, C.; Arias-Buria, J.L.; Cleland, J.A.; Pareja, J.A.; Plaza-Manzano, G.; Ortega-Santiago, R. Manual therapy versus surgery for carpal tunnel syndrome: 4-year follow-up from a randomized controlled trial. Phys. Ther. 2020, 100, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]



| Study | Study Year | Country | Age (Years); Gender | No. of Participants | Study Inclusion Criteria | Outcome Measure | Intervention | Frequency of Interventions |
|---|---|---|---|---|---|---|---|---|
| Tal-Akabi and Rushton. [25] | 2000 | UK | 29–85; male and female | 21 (7 NM; 7 CBM; 7 Control) | CTS diagnosis by physician: positive Phalen’s test, positive Tinel’s sign, and positive electrodiagnostic test. | NPRS FBS ULTT2a | Neurodynamic modulation Carpal bone mobilization (CBM) No intervention | Not stated |
| Bialosky et al. [28] | 2009 | USA | 18–70; male and female | 40 (19 NM; 20 Controls) | Presence of CTS as defined by pain or paresthesia in the median nerve distribution and/or clinical examination findings consistent with CTS. CTS was present for greater than 12 weeks with pain rating of 4/10. | MVAS DASH Grip strength NCS | Neurodynamic technique “directly stresses the median nerve through shoulder, elbow, and wrist movements” or “indirectly stresses the median nerve through shoulder, elbow, and wrist movements” Sham—no stress across median nerve | 2 session per week over 3 weeks |
| Wolny et al. [29] | 2016 | Poland | 26–72; male and female | 210: (140 NM; 70 Control) | CTS diagnosis by physician: numbness and tingling of the median nerve, paresthesia, positive Phalen’s test, positive Tinel’s sign, and pain in wrist radiating to shoulder | 2PD | Neuromodulation: manual therapy Electrophysical modalities (laser and ultrasound) | 20 sessions over 10 weeks |
| Wolny et al. [30] | 2017 | Poland | >18; male and female | 140 (70 NM; 70 Control) | Numbness and tingling; nighttime paresthesia; positive Phalen test and Tinel sign Pain radiating to the shoulder NCS diminished nerve conduction values, increases motor latency | NCS BCTQ NPRS | Manual therapy group: neurodynamic techniques and carpal bone mobilizations Electrotherapy group: Laser and Ultrasound | 20 sessions over 10 weeks |
| Wolny and Linek [31] | 2018a | Poland | 26–72; male and female | 150 (78 NM; 72 Control) | CTS diagnosed based on history, clinical examination: numbness and tingling of the median nerve, paresthesia, positive Phalen’s test, positive Tinel’s sign, and pain in wrist radiating to shoulder and NCS | BCTQ NCS Grip strength Pinch strength | Neuromodulation: neurodynamic techniques Sham glide proximal mobilization | 20 sessions over 10 weeks |
| Wolny and Linek [32] | 2018b | Poland | 26–72; male and female | 189 (102 NM; 87 Control) | CTS diagnosed based on history, clinical examination: numbness and tingling of the median nerve, paresthesia, positive Phalen’s test, positive Tinel’s sign, and pain in wrist radiating to shoulder and NCS | SF-36 PF RF BP GH | Manual therapy including neurodynamic techniques Control group: no therapy | 20 sessions over 10 weeks |
| Maryam et al. [33] | 2018 | Pakistan | 25–55; male and female | 27 (13 NM; 14 Control) | Clinically diagnosed mild-to-moderate CTS | BCTQ QuickDASH | Control group: nerve tendon gliding exercises with electrotherapy (TENS, Ultrasound) Experimental group: Neurodynamic techniques with electrotherapy (TENS and Ultrasound) | 3 sessions per week over 4 weeks |
| Wolny and Linek [14] | 2019 | Poland | 53.85 ± 9.60 | 103 (58 NM; 43 Control) | CTS diagnosed based on history, clinical examination: numbness and tingling of the median nerve, paresthesia, positive Phalen’s test, positive Tinel’s sign, and pain in wrist radiating to shoulder and NCS | BCTQ NPRS NCS Grip strength | Neurodynamic techniques No treatment | 20 sessions over 10 weeks |
| Talebi et al. [34] | 2020 | Iran | 30–50; male and female | 30 (15 NM; 15 Control) | Positive findings in the clinical examination (complains of pain, numbness or tingling in the first three digits for 6 months, positive Phalen’s sign) and electro-diagnostic findings. | BCTQ VAS Distal latency of median nerve | Mechanical interface group Nerve mobilization group | 3 sessions per week over 4 weeks |
| Hamzeh et al. [21] | 2021 | Jordan | >18; male and female | 41 (20 NM; 21 Control) | CTS diagnosed based on history, clinical examination: numbness and tingling of the median nerve, paresthesia, positive Phalen’s test, presence of flick sign, nerve conduction <50 m/s, and/or increased latency >4 m/s. | BCTQ QuickDASH NPRS Wrist ROM Grip strength | Neurodynamic techniques Control group (exercise therapy) | 60 min weekly session for 4 weeks |
| Paquette et al. [35] | 2021 | Canada | 18–70; male and female | 30 (12 NM; 13 Control) | CTS diagnosis, confirmed by electrodiagnostic test | Ultrasound WHYMPI DASH Grip strength | Neurodynamic techniques Control group (no interventions) | 45 repetitions/day for 4 weeks |
| Sheereen et al. [10] | 2022 | India | 30–59; male and female | 30 (15 NM; 15 Control) | Pain, tingling, or paresthesia in the hand including thumb, index finger, middle finger and radial half of the ring finger, VAS of 4–7, positive Tinel’s sign and Phalen’s test, sleep disturbance caused by hand pain, positive nerve conduction study for distal motor latency of >4.4 m/s. | BCTQ VAS NCS Grip strength | Neurodynamic techniques Control group (carpal bone mobilization) | 3 alternate days for 3 weeks |
| Study | Number of Patients Excluded from Final Analysis | Reason for Exclusion |
|---|---|---|
| Tal-Akabi and Rushton, 2000 [25] | NM group, n = 0 Control group, n = 0 | N/A |
| Bialosky et al., 2009 [28] | NM group, n = 1 Control group, n = 0 | Did not respond or keep up follow up appointments. |
| Wolny et al., 2016 [29] | NM group, n = 10 Control group, n = 10 | 6 lacked final results of nerve conduction, 2 did not complete final examination form, 2 had comorbidities that resulted in exclusion. 5 lacked final nerve conduction results, 3 resigned from the experiment, 2 had other reasons for withdrawal. |
| Wolny et al., 2017 [30] | NM group, n = 10 Control group, n = 10 | 6 lacked final results of nerve conduction, 2 did not complete final examination form, 2 had comorbidities that resulted in exclusion. 5 lacked final nerve conduction results, 3 resigned from the experiment, 2 had other reasons for withdrawal. |
| Wolny and Linek, 2018a [31] | NM group, n = 12 Control group, n = 18 | 2 resigned, 7 lacked final results of nerve conduction, 3 had other diseases as comorbidities. 4 resigned, 10 lacked final results of nerve conduction, 4 had other diseases as comorbidities. |
| Wolny and Linek, 2018b [32] | NM group, n = 10 Control group, n = 25 | 3 resigned, 4 lacked final nerve conduction results, 3 had other diseases as comorbidities. 12 resigned, 8 lacked final nerve conduction results, 5 had other diseases as comorbidities. |
| Marryam et al., 2018 [33] | NM group, n = 2 Control group, n = 3 | Did not complete follow up examinations. |
| Wolny and Linek, 2019 [14] | NM group, n = 2 Control group, n = 10 | Lacked final nerve conduction results. 6 lacked final nerve conduction results, 4 had other diseases as comorbidities. |
| Talebi et al., 2020 [34] | NM group, n = 5 Control group, n = 6 | 5 lost to follow-up. 4 lost to follow-up, 2 resigned for personal reasons. |
| Hamzeh et al., 2021 [21] | NM group, n = 6 Control group, n = 4 | 2 lost contact, 1 resigned, 1 had stroke, 1 left the country and 1 had an additional hip fracture. 4 lost contact. |
| Paquette et al., 2021 [35] | NM group, n = 0 Control group, n = 0 | N/A |
| Sheereen et al., 2022 [10] | NM group, n = 0 Control group, n = 0 | N/A |
| Study | Groups | Pre-Intervention (Mean ± SD) | Post-Intervention (Mean ± SD) | Difference (± SD) |
|---|---|---|---|---|
| Wolny et al., 2017 [30] | NM group, n = 70 Control group, n = 70 | 2.89 ± 0.79 2.86 ± 0.84 | 1.84 ± 0.55 2.56 ± 0.86 | −1.05 ± 0.96 −0.30 ± 1.20 |
| Wolny and Linek, 2018a [31] | NM group, n = 78 Control group, n = 72 | 2.92 ± 0.70 2.96 ± 0.68 | 1.86 ± 0.55 2.98 ± 0.70 | −1.06 ± 0.89 0.02 ± 0.98 |
| Wolny and Linek, 2019 [14] | NM group, n = 58 Control group, n = 45 | 2.93 ± 0.68 2.96 ± 0.69 | 1.52 ± 0.66 2.87 ± 0.70 | −1.41 ± 0.95 −0.09 ± 0.98 |
| Talebi et al., 2020 [34] | NM group, n = 15 Control group, n = 15 | 23.93 ± 7.30 24.73 ± 8.50 | 8.13 ± 5.70 6.6 ± 4.54 | −15.80 ± 9.26 −18.13 ± 9.64 |
| Hamzeh et al., 2021 [21] | NM group, n = 20 Control group, n = 21 | 2.99 ± 0.87 2.67 ± 0.80 | 1.50 ± 0.77 1.86 ± 0.74 | −1.49 ± 1.16 −0.81 ± 1.09 |
| Shereen et al., 2022 [10] | NM group, n = 15 Control group, n = 15 | 2.28 ± 0.32 2.30 ± 0.47 | 1.35 ± 0.15 1.88 ± 0.56 | −0.93 ± 0.35 −0.42 ± 0.73 |
| Study | Primary Outcome | Groups | Pre-Intervention (Mean ± SD) | Post-Intervention (Mean ± SD) | Difference (± SD) |
|---|---|---|---|---|---|
| Wolny et al., 2017 [30] | BCTQ-SSS | NM group, n = 70 | 2.97 ± 0.63 | 1.78 ± 0.47 | −1.19 ± 0.78 |
| Control group, n = 70 | 2.94 ± 0.74 | 2.57 ± 0.77 | −0.37 ± 1.07 | ||
| BCTQ-FSS | NM group, n = 70 | 2.80 ± 0.94 | 1.90 ± 0.62 | −0.9 ± 0.87 | |
| Control group, n = 70 | 2.77 ± 0.94 | 2.55 ± 0.95 | −0.22 ± 1.34 | ||
| Wolny and Linek, 2018a [31] | BCTQ-SSS | NM group, n = 78 | 2.99 ± 0.67 | 1.77 ± 0.48 | −1.22 ± 0.82 |
| Control group, n = 72 | 2.88 ± 0.72 | 2.86 ± 0.72 | −0.02 ± 0.98 | ||
| BCTQ-FSS | NM group, n = 78 | 2.84 ± 0.72 | 1.94 ± 0.61 | −0.9 ± 0.94 | |
| Control group, n = 72 | 3.04 ± 0.64 | 3.09 ± 0.68 | 0.05 ± 0.93 | ||
| Wolny and Linek, 2019 [14] | BCTQ-SSS | NM group, n = 58 | 3.03 ± 0.65 | 1.08 ± 0.68 | −1.95 ± 0.65 |
| Control group, n = 45 | 2.92 ± 0.71 | 2.87 ± 0.68 | −0.05 ± 0.96 | ||
| BCTQ-FSS | NM group, n = 58 | 2.82 ± 0.71 | 1.96 ± 0.64 | −0.86 ± 0.96 | |
| Control group, n = 45 | 2.99 ± 0.67 | 2.87 ± 0.71 | −0.12 ± 0.95 | ||
| Talebi et al., 2020 [34] | BCTQ-SSS | NM group, n = 15 | 30.66 ± 7.82 | 19.26 ± 5.48 | −11.40 ± 0.98 |
| Control group, n = 15 | 30.13 ± 8.95 | 21.73 ± 8.22 | −8.40 ± 12.16 | ||
| BCTQ-FSS | NM group, n = 15 | 17.20 ± 6.77 | 12.33 ± 5.48 | −4.87 ± 8.71 | |
| Control group, n = 15 | 19.33 ± 8.05 | 14.53 ± 5.13 | −4.8 ± 9.55 | ||
| Hamzeh et al., 2021 [21] | BCTQ-SSS | NM group, n = 20 | 3.17 ± 0.86 | 1.64 ± 0.66 | −1.53 ± 1.08 |
| Control group, n = 21 | 2.71 ± 0.76 | 1.88 ± 0.60 | −0.83 ± 0.97 | ||
| BCTQ-FSS | NM group, n = 20 | 2.80 ± 0.87 | 1.35 ± 0.48 | −1.45 ± 0.99 | |
| Control group, n = 21 | 2.63 ± 0.84 | 1.84 ± 0.87 | −0.79 ± 1.21 | ||
| Shereen et al., 2022 [10] | BCTQ | NM group, n = 15 | 2.28 ± 0.32 | 1.35 ± 0.15 | −0.93 ± 0.35 |
| Control group, n = 15 | 2.3 ± 0.47 | 1.88 ± 0.56 | −0.42 ± 0.73 |
| Study | Groups | Pre-Intervention (Mean ± SD) | Post-Intervention (Mean ± SD) | Difference (± SD) |
|---|---|---|---|---|
| Wolny et al., 2017 [30] | NM group, n = 70 Control group, n = 70 | 26.20 ± 15.70 38.20 ± 11.10 | 35.10 ± 12.10 39.22 ± 11.19 | 8.90 ± 19.82 1.02 ± 15.76 |
| Wolny and Linek, 2018a [31] | NM group, n = 78 Control group, n = 72 | 24.60 ± 15.30 24.70 ± 7.89 | 39.80 ± 11.30 25.10 ± 7.77 | 15.20 ± 19.02 0.40 ± 11.07 |
| Wolny and Linek, 2019 [14] | NM group, n = 58 Control group, n = 43 | 24.9 ± 15.1 25.8 ± 7.81 | 38.3 ± 11.1 25.90 ± 7.72 | 13.40 ± 18.74 0.10 ± 10.98 |
| Study | Groups | Pre-Intervention (Mean ± SD) | Post-Intervention (Mean ± SD) | Difference (± SD) |
|---|---|---|---|---|
| Wolny et al., 2017 [30] | NM group, n = 70 Control group, n = 70 | 53.2 ± 7.80 54.8 ± 5.6 | 56.5 ± 7.8 55.3 ± 5.7 | 3.30 ± 11.03 0.50 ± 7.99 |
| Wolny and Linek, 2018a [31] | NM group, n = 78 Control group, n = 72 | 52.4 ± 3.53 52.6 ± 3.94 | 56.1 ± 6.72 54.1 ± 4.32 | 3.70 ± 7.59 1.50 ± 5.85 |
| Wolny and Linek, 2019 [14] | NM group, n = 58 Control group, n = 43 | 51.10 ± 5.15 53.1 ± 3.44 | 55.8 ± 6.92 53.6 ± 4.08 | 4.70 ± 8.32 0.50 ± 5.34 |
| Study | Groups | Pre-Intervention (Mean ± SD) | Post-Intervention (Mean ± SD) | Difference (± SD) |
|---|---|---|---|---|
| Wolny et al., 2017 [30] | NM group, n = 70 Control group, n = 70 | 5.61 ± 1.08 5.45 ± 1.12 | 5.02 ± 1.13 5.24 ± 1.17 | −0.59 ± 1.56 −0.21 ± 1.62 |
| Wolny and Linek, 2018a [31] | NM group, n = 78 Control group, n = 72 | 5.51 ± 1.08 5.43 ± 1.11 | 4.43 ± 0.81 5.33 ± 1.13 | −1.08 ± 1.42 −0.10 ± 1.58 |
| Wolny and Linek, 2019 [14] | NM group, n = 58 Control group, n = 43 | 5.62 ± 1.11 5.51 ± 1.17 | 4.49 ± 0.72 5.41 ± 1.18 | −1.13 ± 1.32 −0.10 ± 1.67 |
| Talebi et al., 2020 [34] | NM group, n = 15 Control group, n = 15 | 6.26 ± 1.85 6.18 ± 1.65 | 5.60 ± 1.40 5.76 ± 1.15 | −0.66 ± 2.32 −0.42 ± 1.16 |
| Study | Groups | Pre-Intervention (Mean ± SD) | Post-Intervention (Mean ± SD) | Difference (± SD) |
|---|---|---|---|---|
| Tal-Akabi and Rushton, 2000 [25] | NM group, n = 7 Control group, n = 7 | 2.42 ± 1.51 2.00 ± 1.29 | 1.57 ± 1.40 2.14 ± 0.69 | −0.85 ± 2.06 0.14 ± 1.46 |
| Bialosky et al., 2009 [28] | NM group, n = 19 Control group, n = 20 | 22.70 ± 16.30 14.90 ± 15.80 | 16.0 ± 15.0 7.90 ±12.10 | −6.70 ± 22.15 −7.00 ± 19.90 |
| Wolny et al., 2017 [30] | NM group, n = 70 Control group, n = 70 | 5.72 ± 1.49 5.25 ± 1.75 | 1.47 ± 1.20 3.58 ± 1.93 | −4.25 ± 1.91 −1.67 ± 2.60 |
| Wolny and Linek, 2019 [14] | NM group, n = 58 Control group, n = 43 | 5.86 ± 1.46 5.71 ± 1.34 | 1.38 ± 1.01 5.46 ± 1.05 | −4.48 ± 1.42 −0.25 ± 1.70 |
| Talebi et al., 2020 [34] | NM group, n = 15 Control group, n = 15 | 6.40 ± 1.45 6.80 ± 1.65 | 3.53 ± 2.23 3.93 ± 1.90 | −2.87 ± 2.66 −2.87 ± 2.52 |
| Hamzeh et al., 2021 [21] | NM group, n = 20 Control group, n = 21 | 4.17 ± 2.23 3.17 ± 2.49 | 1.06 ± 1.75 2.09 ± 2.43 | −3.11 ± 2.83 −1.08 ± 3.48 |
| Sheereen et al., 2022 [10] | NM group, n = 15 Control group, n = 15 | 6.30 ± 0.65 6.20 ± 0.47 | 2.02 ± 0.49 2.30 ± 0.58 | −4.28 ± 0.81 −3.90 ± 0.75 |
| Study | Groups | Pre-Intervention (Mean ± SD) | Post-Intervention (Mean ± SD) | Difference (± SD) |
|---|---|---|---|---|
| Wolny and Linek, 2018a [31] | NM group, n = 78 Control group, n = 72 | 27.7 ± 6.66 29.6 ± 5.67 | 28.4 ± 6.11 30.3 ± 5.38 | 0.70 ± 9.04 0.70 ± 7.82 |
| Wolny and Linek, 2019 [14] | NM group, n = 58 Control group, n = 43 | 28.10 ± 6.11 29.4 ± 6.02 | 28.80 ± 5.62 30.10 ± 5.74 | 0.70 ± 8.30 0.70 ± 8.32 |
| Hamzeh et al., 2021 [21] | NM group, n = 20 Control group, n = 21 | 24.88 ± 16.59 23.43 ± 17.21 | 35.41 ± 13.30 29.64 ± 18.67 | 20.53 ± 21.26 6.21 ± 25.39 |
| Paquette et al., 2021 [35] | NM group, n = 12 Control group, n = 13 | 4.24 ± 1.98 3.89 ± 1.71 | 3.00 ± 1.38 3.35 ± 1.14 | −1.24 ± 2.41 −0.54 ± 2.06 |
| Sheereen et al., 2022 [10] | NM group, n = 15 Control group, n = 15 | 17.08 ± 2.02 17.42 ± 1.20 | 21.26 ± 3.34 21.04 ± 2.06 | 4.18 ± 3.90 3.62 ± 2.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaheer, S.A.; Ahmed, Z. Neurodynamic Techniques in the Treatment of Mild-to-Moderate Carpal Tunnel Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 4888. https://doi.org/10.3390/jcm12154888
Zaheer SA, Ahmed Z. Neurodynamic Techniques in the Treatment of Mild-to-Moderate Carpal Tunnel Syndrome: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(15):4888. https://doi.org/10.3390/jcm12154888
Chicago/Turabian StyleZaheer, Sheikh Azka, and Zubair Ahmed. 2023. "Neurodynamic Techniques in the Treatment of Mild-to-Moderate Carpal Tunnel Syndrome: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 15: 4888. https://doi.org/10.3390/jcm12154888
APA StyleZaheer, S. A., & Ahmed, Z. (2023). Neurodynamic Techniques in the Treatment of Mild-to-Moderate Carpal Tunnel Syndrome: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(15), 4888. https://doi.org/10.3390/jcm12154888







