Abstract
Background: Breast cancer (BC) and cardiovascular (CV) disease share many risk factors associated with worse outcomes, in terms of cancer relapse, CV events, and quality of life (QoL), that could be counteracted by physical exercise (PE). We aimed to assess the impact of a 12-week differential PE protocol on cardiometabolic profile, QoL, CV- and BC-related long-term outcomes, and physical activity (PA) in a cohort of BC survivors (BCS) not treated with chemotherapy. Methods: 57 BCS participated in a 12-week PE protocol [aerobic exercise training (AET) or resistance exercise training (RET)]. Anthropometric and CV evaluation, health-related (HR)-QoL, daily PA, cortisol, and dehydroepiandrosterone sulfate (DHEA-S) levels were assessed before (T0) and after (T1) PE. We assessed BC and CV outcomes, HR-QoL, CV-QoL, and PA at the follow-up. Results: RET improved waist circumference, DHEA-S, cortisol/DHEA-S, systolic and mean blood pressure, and ventricular/arterial coupling; AET ameliorated sagittal abdomen diameter and pulse wave velocity. Regarding HR-QoL, physical function improved only in AET group. At a mean 34 ± 3.6-month follow-up, we documented no significant differences in CV-QoL, HR-QoL, and PA or CV and BC outcomes. Conclusions: AET and RET determine specific, positive adaptations on many parameters strongly related to CV risk, CV and BC outcomes, and QoL, and should be included in any cardio-oncology rehabilitation program.
Keywords:
breast cancer; breast cancer survivors; cancer therapy-related cardiovascular toxicity; hormone therapy; physical exercise; aerobic exercise training; resistance exercise training; cardiometabolic profile; health-related quality of life; cardiovascular-related quality of life; physical activity 1. Introduction
Breast cancer (BC) is the most common malignancy and the leading cause of cancer-related death among women, although cardiovascular disease (CVD) still represents the most important source of mortality in the female population [1,2].
CVD and BC share several overlapping risk factors, such as age, tobacco habit, obesity, and sedentary behavior (SB), and CV health can influence both cancer outcomes and cancer treatment selection. Moreover, as long-term survival rates after diagnoses of BC are rising, physicians must face up to the long-term sequelae of the various therapeutic options for BC, that is, early or delayed cancer-therapy-related cardiovascular toxicity (CTR-CVT), potentially altering cancer survivorship. There are many clinical and subclinical manifestations of CTR-CVT; in particular, venous thrombosis, thromboembolism, peripheral atherosclerosis, dysrhythmia, valvular dysfunction, pericarditis, and heart failure have been described as complications of hormone therapy (HT) [3,4,5]. Adjuvant HT with aromatase inhibitors (AIs) or selective estrogen receptor modulators (SERMs, usually tamoxifen) exerts oncologic benefits by inhibiting estradiol synthesis or breast estrogen receptor signaling. AIs cause systemic estradiol depletion, whereas tamoxifen has mixed agonistic/antagonistic effects in a tissue-dependent fashion [6]. Since estrogens play a protective role in endothelial function, vascular tone, and cardiac function, as well as in lipid profile and inflammatory status, it looks intuitive that ET may modulate cardiometabolic risk and oxidative stress levels [7]. However, evidence about ET–associated cardiometabolic risks remains incomplete for many reasons. First, no randomized clinical trial has been designed to assess CV risk in this population. Second, clinical trials have described a low number of CV events and predominantly enrolled women with relatively low CV risk and with a short-term follow-up [6].
Apart from ET, cancer is associated with chronic inflammation, which is exacerbated by the effects of CTR-CVT, pre-existing CVD, and negative lifestyle risk factors, such as physical inactivity (PI). This persistent inflammatory status leads to endothelial dysfunction (ED), known as an early risk factor for atherosclerosis and arterial stiffness (AS) [8]. Physical exercise (PE) has positive effects on every aspect of BC progression, including all-cause and breast-cancer-related death. It represents a crucial tool to counteract the proinflammatory cancer-associated burden, to favorably influence the acute and chronic symptoms of BC and to ameliorate or prevent the development of cardiovascular (CV) risk factors and CVD as well [9,10,11,12]. Additionally, aerobic and resistance PE performed at moderate-to-high intensity can significantly improve health-related quality of life (HR-QoL) in breast cancer survivors (BCS) [13,14]. On the contrary, SB and PI have been associated with worsening HR-QoL in this population [15].
To date, many different protocols for physical activity (PA) and PE in oncologic patients have been proposed; however, the literature remains insufficient for further detailing prescriptions according to cancer type, types of treatment, and timing of treatment, and no conclusive data about the therapeutic efficacy of each exercise protocol are still available. Finally, there need to be more studies regarding the long-term effects of PE on both BC-related and CV-related outcomes, as well as on QoL and PA, for BCS patients treated with HT [9,16,17,18,19,20,21,22,23,24,25].
Therefore, our study aims to examine the effects of different PE protocols on cardio-metabolic profile, PA and QoL in a population of BCS, not treated with CHT; moreover, in the same population, we evaluated BC-related and CV-related outcomes, CV- and HR-QoL, and PA at a 34-month follow-up.
2. Materials and Methods
This is a single-center, prospective study in which, between April 2016 and April 2017, we screened 76 BCS patients at the Breast Cancer Surgery Unit of the ‘G. Bernabeo’ Hospital (Ortona, Italy). The inclusion criteria were age range between 50 and 65 years; history of BC surgery in the previous 12 months; no history of CHT; no ongoing radiotherapy (RT); eventual ET; lymphedema lower than class 2 of CEAP-L classification [26]; CV and orthopedic eligibility; no dieting or use of nutritional supplements; no participation in any exercise program during the six months prior to the study. The exclusion criteria were previous or ongoing CHT; any history of active CVD (i.e., ischemic heart disease, valvular heart disease, arrhythmias) or recent hospital admission for CVD; any systemic inflammatory disease or any orthopedic condition potentially limiting the physical training; abnormal exercise electrocardiography (ECG) stress test at the screening; dieting or use of nutritional supplements; participation in any exercise program during the six months prior to the study; nonemployed status. A Cardiologist and a Sports Medicine Specialist confirmed CV and orthopedic eligibility through a complete medical examination, 2-D transthoracic echocardiography (TTE) and maximal exercise ECG stress test. According to these criteria, 57 patients were considered eligible. The Ethics Committee of the ‘G. d’Annunzio’ University of Chieti-Pescara approved this study (#312/2015). In acquiescence with the Declaration of Helsinki, all enrolled patients gave written informed consent at the time of their evaluation, stating that data and images may be subsequently used for research purposes (Clinicaltrials.gov registration number: NCT04337736).
Each eligible patient underwent an anthropometric assessment, complete CV evaluation, health-related quality of life (HR-QoL) analysis, a five-day PA recording in a free-living context, and salivary samples collection before (T0) and after (T1) physical training.
After a mean follow-up of 34 months, we evaluated CV and BC outcomes, PA, HR-QoL, and CV quality of life (CV-QoL) with a telephone interview (Figure 1).
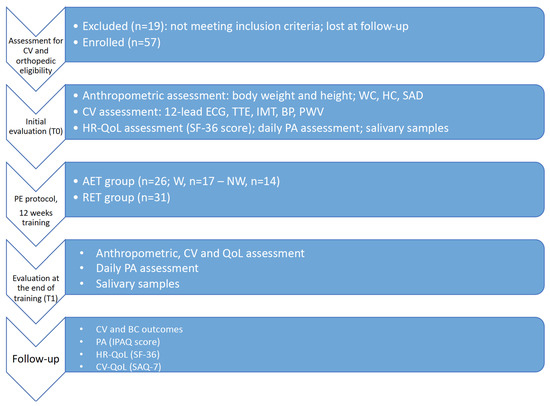
Figure 1.
Flowchart of the study protocol. Abbreviations: CV, cardiovascular; WC, waist circumference; HC, hip circumference; SAD, sagittal abdomen diameter; ECG, electrocardiogram; TTE, transthoracic echocardiogram; IMT, intima-media thickness; BP, blood pressure; PWV, pulse wave velocity; AET, aerobic exercise training; W, walking; NW, Nordic walking; RET, resistance exercise training; QoL, quality of life; PE, physical exercise; PA, physical activity; IPAQ, International Physical Activity Questionnaire; SAQ-7, Seattle Angina Questionnaire-7; SF-36, Short Form Health Survey-36.
2.1. Anthropometric Assessment
A second-level anthropometrist assessed bodyweight, stretched height, waist circumference (WC), and hip circumference (HC) according to the International Society for the Advancement of Kinanthropometry’s guidelines [27].
Body weight and stretched stature were measured to the nearest 0.1 kg and 0.1 cm, respectively, with the participants dressed in light clothing and without shoes and in fasting condition, using a stadiometer with a balance-beam scale (Seca 220; Seca, Hamburg, Germany). Body mass index (BMI) was calculated according to the formula by Du Bois et al. [28]. WC and HC were assessed with an anthropometric tape (Seca 200). WC was measured as the smallest circumference between the rib cage and the iliac crest at the end of normal expiration; in contrast, HC was measured at the level of the broadest circumference between the waist and the thighs [27]. Sagittal abdomen diameter (SAD) was measured with the subject lying supine, at the midpoint between the iliac crest and the last rib with a portable, sliding-beam abdominal caliper [29].
2.2. CV Eligibility and Complete CV Evaluation
CV eligibility was assessed through a 12-lead rest electrocardiogram, maximal exercise test, and TTE.
After 10 min of supine rest, a 12-lead electrocardiogram (Stress ECG HD+, Cardioline, Trento, Italy) was achieved.
Then, upon the supervision of a Sports Medicine specialist, participants performed a graded maximal exercise test, i.e., Astrand protocol using 3-min steps, on a cycle ergometer (Cardioline xr50, Cardioline, Trento, Italy), with continuous electrocardiogram monitoring (Stress ECG HD+, Cardioline, Trento, Italy) and blood pressure assessment at the end of each step. According to the Italian Federation of Sports Medicine guidelines, the test lasted until the doctor showed absolute or relative indications for clinically graded exercise test termination [30].
All the TTE were performed using a dedicated MyLab™30Gold CV portable ultrasound (Esaote, Florence, Italy) with a phased array 3.5 MHz cardiac probe. For each participant, general echocardiographic characteristics regarding cardiac chambers volumes and function, including left ventricular (LV) diastolic function and LV global longitudinal strain analysis (GLS), according to the recommendations from the American Society of Echocardiography and the European Association of Cardiovascular Imaging (EACVI) guidelines, were collected [31]. An offline and dedicated software (XStrain™ 2D) was used to perform the two-dimensional speckle-tracking analysis from the apical 4-chambers, 3-chambers, and 2-chambers view. Moreover, we assessed epicardial fat thickness as a marker of visceral adiposity and ventricular/arterial coupling (VAC) as an index of CV efficiency using the single beat method [32,33].
Carotid ultrasound (CUS) was performed using a standard 7.5 MHz linear probe, with each participant lying supine and the neck hyperextended. After manual image acquisition of carotid vessels, we assessed left and right intima-media thickness (IMT) on the common carotid posterior wall, 1 cm from bulb bifurcation on each side. Then, the Quality Intima Media Thickness (QIMTTM) software was used for data analysis, and the mean value between the three measurements for QIMT was obtained for each patient, with a concordance correlation coefficient between the three measurements of 0.99 (95% CI: 0.98–0.99) [34,35,36,37].
Pulse wave velocity (PWV), an index of AS, was evaluated with the photoplethysmographic method (Vascular Explorer, Enverdis, Dusseldorf, Germany), along with systolic, mean, and diastolic blood pressure (SBP, MBP, DBP) with the subject supine for at least 10 min before starting the assessment in a quiet, temperature-controlled room [34,38,39].
All the participants have been examined utilizing TTE, CUS, and photoplethysmographic method before and after the PE protocol (T0-T1).
2.3. Quality-of-Life Assessment
The Italian version of the Short Form Health Survey (SF-36) score was used for the assessment of HR-QoL at T0 and T1. This 36-item patient-reported survey offers a consistent and acceptable description of general health status in the general population and in BCS [40,41]. It comprises eight scaled scores (PF, physical function; SC, social function; MH, mental health; P, pain; RLP, role limitation physical; RLM, role limitation mental; EV, vital energy; HP, health perception), which are the weighted sums of the questions in their section. Each scale is directly transformed into a 0–100 score, with a score of 0 equivalent to a maximum disability and a score of 100 equivalent to no disability.
2.4. Daily Physical Activity Measurements
Daily PA was determined under free-living circumstances over five consecutive days, including four weekdays and one weekend day, by using SenseWear Pro3 armbands (BodyMedia, Pittsburgh, PA, USA). This device incorporates the information collected by the two-axis accelerometers and sensors (i.e., skin and near-body temperature, heat flux, and galvanic skin response) with the sex, age, stature, weight, smoking status, and handedness of the user. Thus, it offers details about the intensity and the amount of the daily PA. From the recorded data, we focused on the mean intensity of daily PA (METs), daily steps (Steps), time spent on PA with intensity from 3 to 6 METs (i.e., moderate-intensity PA; MIPAT), and time spent on PA with intensity from 6 METs to 9 METs (i.e., vigorous-intensity PA; VIPAT). In addition, the time spent in sedentary and low-intensity PA (LIPAT) was also considered. The time spent in PA with an intensity < 1.5 METs, excluding nocturnal sleeping, was considered sedentary time (ST), whereas the time spent in physical activities >1.5 METs and <3 METs was considered LIPAT [42]. The participants wore their monitors all day, except while bathing. No rainy days occurred during the recorded periods. The wear time criteria for valid registrations were at least 540 min/day on weekdays and 480 min/day on weekends [43].
2.5. Salivary Samples
Salivary samples were collected three times under a free-living setting using a Salimetrics oral swab (Salimetrics Europe, Suffolk, UK), i.e., a small pad, absorbent and nontoxic, passed within the oral cavity for 2–3 min before being placed inside a labeled tube. The samples were stored at 8 a.m., 5 p.m., and 11 p.m.; refrigerated within 30 min; and frozen at −20 °C within 2 h of collection. On the day of the assay, samples were thawed and centrifuged for 15 min at 3000 rpm to extract saliva and remove the mucin, and the swab was then discarded. Each sample was processed in duplicate. Salivary samplings from 8 a.m. were collected in fasting condition, except for water consumption; in contrast, those from 5 p.m. and 11 p.m. were collected 2 h after any food or beverage consumption, except for water. The sum of the three samplings results was considered representative variable data of daily salivary cortisol excretion [44]. The assays were performed using the High Sensitivity Salivary Cortisol EIA kit and Salivary DHEA-S EIA kit (Salimetrics Eu-rope, Suffolk, UK), with an intra-assay coefficient of variation of 4.6% for cortisol and 7.25% for DHEA-S and an interassay precision of 6% for cortisol and 7.5% for DHEA-S.
2.6. Follow-Up Evaluation
At the follow-up, during a telephone interview, we evaluated HR-QoL, CV and cancer outcomes, CV-QoL, and PA. The Italian version of the Short Form Health Survey (SF-36) score was used to evaluate HR-QoL [40,41]. CV and cancer outcomes were assessed with questions that included hospitalization for CV issues, initiation of novel CV medications, and BC recurrency. CV-QoL was estimated with telephone interview through Seattle Angina Questionnaire-7 (SAQ-7), a shortened version of the full SAQ, previously validated both in the male and female population. This questionnaire includes seven questions to quantify health status in patients with coronary artery disease (CAD). It focuses on three domains that directly evaluate patients’ current health status: Physical Limitation (PL), Angina Frequency (AF), and Quality of Life (QL). Each domain results in a score (from 0 to 100, with 0 denoting the worst and 100 the best possible health status) with an overall final summary score [45,46,47].
Last, International Physical Activity Questionnaire—Short Form (IPAQ-SF), whose reliability and validity have been previously recognized, was used to measure physical activity (PA) at the follow-up. It consists of seven questions to estimate the average daily time spent sitting, walking, and engaging in moderate and vigorous PA over the last seven days. Activities that require up to 3 Metabolic Equivalent of Task (METs) have been defined as light-intensity PAs, and activities that range from 3 to 6 METs have been categorized as moderate-intensity PAs; in contrast, those that require more than 6 METs were defined as vigorous-intensity PAs. The IPAQ-SF sum score is then expressed in PA MET/minutes per week [48,49,50,51].
2.7. Physical Exercise Protocols
After basal evaluation and eligibility, BCS were allocated to one of two aerobic exercise protocols (AET), i.e., walking (W) or Nordic walking (NW), or resistance exercise training (RET). We assigned every participant to each PE protocol according to the season, i.e., the women enrolled during spring or summer were assigned to the AET and trained outside, whereas the women enrolled during autumn or winter were assigned to the RET and trained inside in a dedicated gym.
Each group was conducted and supervised by exercise specialists. Total adherence (TA) to PE protocol was expressed as the percentage of total exercise volume (ExV) performed on planned total ExV.
2.7.1. Walking (W) Group
Patients assigned to W group worked out at moderate intensity for 12 weeks, 4-days a week. Exercise intensity was distributed and supervised, as stated in the ratings of the perceived exertion method [52]. From the first to the fourth week, each training session lasted 40 min, with a walking velocity eliciting an effort equal to 10–11 according to the 15-category rating of the perceived exertion scale (RPE). Then, the participants trained for 50 min per each training session at 12–13 RPE from the fifth to the eighth week; from the ninth to the twelfth week, only the training intensity improved from 12–13 to 13–14 RPE. The participants were familiarized with this scale before beginning the training and during the first week of training as well. The Borg scale is particularly helpful to prescribe and monitor exercise intensity in this population because for the same external load, it is possible to have a different internal load, due, for example, to side effects of pharmacological treatments (i.e., fatigue). Exercise trainers checked the exercise intensities of the participants through the talk test and tested their compliance with the training sessions [53].
2.7.2. Nordic Walking (NW) Group
NW participants were introduced to the protocol through 10 lessons on the NW technique, before starting the 12 weeks of supervised workouts. The central part of each lesson, lasting 35 min, shifted its main content from the practice of exercises to the practice of the complete NW technique, maintaining its intensity lower than 10, according to Borg’s rating of perceived exertion scale (RPE) [52]. At the end of this introductory course, the technique appropriateness of each participant was independently verified by two NW instructors of the International Nordic Walking Association (INWA). Sub-groups followed the same training scheme with different contents according to sub-group membership: 12 weeks of supervised training, with a three-times per week cadence; 70 min of training including 15 min of warm-up, 45 min of central phase, and 10 min of cool down. Participants trained from the first to the fourth week at 10–11 RPE, from the fifth to the eighth week at 12–13 RPE, and from the ninth to the twelfth week at 13–14 RPE. The participants were familiarized with Borg’s scale before beginning the training and during the first week of training as well. Compliance with the training sessions was tested through both women’s and exercise trainers’ diaries.
2.7.3. Resistance Exercise Training (RET) Group
RET protocol was structured in 28 sessions, each lasting 50 min. The first six lessons were focused on postural and respiratory training; then, from the 7th to the 16th lessons, each workout was organized in a circuit training manner including eight different exercises, involving the major muscle groups, and executed in a calisthenics way or using the elastic bands. Each exercise lasted 50 s, a rest period of 2 min was observed from one exercise to another. From the 17th to the 28th lesson, just the duration of each exercise (1 min) and that of the rest period (1 min) have been modified. Participants trained from the first to the fourth week at 10–11 RPE, from the fifth to the eighth week at 12–13 RPE, and from the ninth to the twelfth week at 13–14 RPE. The participants were familiarized with Borg’s scale before beginning the training and during the first week of training as well. Compliance with the training sessions was checked through both women’s and exercise trainers’ diaries.
2.8. Statistical Analysis
Continuous variables are shown as mean and standard deviation (SD) or median and lower/upper limit (Q1, Q3), while categorical data as absolute numbers and percentages, as appropriate. Adjusted means and standard errors (SE) resulted from linear/logistic regression models. All the variables were tested for normality using the Shapiro–Wilk test. We distributed the participants into two groups according to PE protocol: AET, aerobic exercise training; RET, resistance exercise training. Then, we estimated the absolute change for each variable using the following formula: postexercise program value minus the pre-exercise program value divided by the pre-exercise program, to normalize for T0 values all the variables. According to their distribution, differences between groups and absolute changes were assessed utilizing the Student’s t-test or a nonparametric t-test. A two-tailed p-value of 0.05 was considered statistically significant.
All the statistical analyses were adjusted by age, hormone therapy (HT), radiotherapy (RT), BMI, body surface area (BSA), TA, and ST, to exclude any confounding factors potentially influencing our results. Statistical analysis was completed using the SPSS software package (SPSS 22.0, Chicago, IL, USA) and Prism 6.0 (GraphPad Software, La Jolla, CA, USA).
3. Results
Table 1 shows the baseline characteristics of our population.

Table 1.
General characteristics of the study population, according to PE protocol.
TA to PE protocol was 72.14 ± 24.5%, with a higher TA in RET group as compared to AET group (79.3 ± 13.8% vs. 66 ± 29.9%, p = 0.042).
Table 2 shows the cardiometabolic profile, daily PA levels, and the QoL scores of our population, as for PE protocol (AET or RET), before (T0) and after training (T1).

Table 2.
Pretraining (T0) and post-training (T1) cardiometabolic, QoL profile, and PA levels of the study population, according to PE protocol.
RET group experienced a statistically significant improvement in WC (p < 0.001), DHEA-S (p = 0.002), cortisol (p = 0.002), cortisol/DHEAS (p < 0.001), IMT (p = 0.005), and epicardial fat (p = 0.003), whereas AET group ameliorated DHEAS (p = 0.002) and epicardial fat (p < 0.001).
Among CV parameters, in the RET group, we found an improvement in SBP (p = 0.004), DBP (p = 0.003), MBP (p = 0.001), RWT (p = 0.02), MAPSE (p = 0.003), GLS (p = 0.01), LAEF (p = 0.005), SV (p = 0.002), EA (p = 0.006), EES (p < 0.001), V/A (p < 0.001); AET group showed an improvement in PWV (p < 0.001), GLS (p < 0.001), and SV (p = 0.006).
Regarding daily PA levels, AET group improved METs (p = 0.008) and VIPAT (p = 0.04); in contrast, RET group ameliorates STEPs (p = 0.004). No significant differences where demonstrated in term of ST in both groups.
QoL scores demonstrated an improvement in PF (p < 0.001), P (p = 0.001), HP (p > 0.001) in AET group and in SC (p = 0.03) and MH (p = 0.003) in RET group.
At the analysis of delta absolute change, adjusted for age, BSA, BMI, HT, RT, ST, and TA (Table 3), RET group showed a significant reduction of WC (p = 0.014), DHEAS (p = 0.013), and cortisol/DHEAS (p = 0.001), whereas AET group showed a significant reduction in SAD (p = 0.019). Among CV parameters, RET group showed an improvement in SBP (p = 0.021), MBP (p = 0.038), EES (p = 0.001), EA (p = 0.026), and V/A (p = 0.001), whereas AET group showed an improvement in PWV (p = 0.015). Finally, among QoL score, only AET group showed an improvement in PF (p = 0.03).

Table 3.
Delta absolute change of cardiometabolic and QoL parameters according to PE protocol.
After dividing the population as for PE subgroups (Table 4), at the analysis of delta absolute change, we found that only RET group experienced a significant improvement in WC (p = 0.004), DHEAS (p = 0.026), cortisol/DHEAS (p = 0.001), SBP (p = 0.017), EES (p = 0.001), and V/A (p = 0.001), whereas NW group improved PWV (p = 0.005).

Table 4.
Delta absolute change of cardiometabolic and QoL parameters according to PE subgroups.
Table 5 shows the follow-up data in general population and in PE subgroups (AET and RET); 21 patients were lost at the follow-up. The mean follow-up was 34 ± 3.6 months in the whole population. Regarding BC outcomes, two BC recurrences were described, one in the AET group and one in the RET group; in contrast, two patients required the introduction of novel CV medications, and one patient was hospitalized for CV issues, both in the AET group. No significant differences in SAQ-7 score, HR-QoL, and PA measured in MET-minutes per week were found between the two groups at the follow-up. The mean IPAQ-score was 2160 in the whole population, indicative of a sufficiently active lifestyle. Participants in the RET group experienced a higher percentage of low-level PA (p = 0.012).

Table 5.
Cardiovascular and cancer outcomes, physical activity, CV, and HR-QoL at the follow-up, according to PE protocol.
4. Discussion
To the best of our knowledge, our study is the first explorative analysis focused on the effects of a 12-week supervised PE protocol on cardiometabolic status, QoL, and PA in a population of BCS, not treated with anthracyclines or HER-2 inhibitors, providing data on BC and CV outcomes, PA, and QoL at a 34-month follow-up.
According to our results, a 12-week supervised PE protocol was associated with an improvement in cardiometabolic profile, with peculiar differences according to the type of PE. In particular, RET determined with an improvement in WC, DHEA-S, cortisol/DHEA-S, SBP, MBP, and V/A coupling; in contrast, AET ameliorated SAD and PWV. Among HR-QoL parameters, we only observed an improvement of PF in the AET group. At the follow-up analysis, no differences were documented in CV-QoL, HR-QoL, and PA, according to PE subgroups.
Central or abdominal obesity, measured as WC or SAD, represents one of the risk factors for diagnosing metabolic syndrome and is strongly related to mortality and cardiorespiratory fitness [54,55,56,57]. Pischon et al. demonstrated, in a group of 359,387 participants from the European Prospective Investigation into Cancer and Nutrition (EPIC) population, that a 5-cm increase of WC leads to an increased higher risk of death of 17% for men and 13% for women [58]. Moreover, Dyrstad et al. highlighted that even a small increase in WC is associated with a significant reduction in cardiorespiratory fitness, the latter being a meaningful marker of CV health in BCS [59,60]. SAD has been identified as a robust anthropometric measure of visceral adipose fat, being considerably associated with insulin resistance and cardiometabolic risk [61,62]. Additionally, in the premenopausal women population, SAD correlates with epicardial adipose tissue [63]. About 65% of BCS are overweight or obese, with a significant risk for cancer relapse and all-cause mortality. Furthermore, both medical anticancer therapy and the inactivity related to cancer could increase the distribution of fat mass, with a reduction in lean mass and bone density. On the other hand, PE could counteract any increase in WC and SAD, as demonstrated in our study, in line with the available data in the literature [64,65,66].
The effects of PE on the hormonal milieu in healthy women have been widely demonstrated, with a decrease in circulating sex hormones potentially protective from the risk of breast cancer [67,68]. In cancer patients, PE may correlate to endogenous stress reduction, which is related to many cardiometabolic outcomes and psychophysical health [69,70]. Many studies demonstrated that AET reduces cortisol level in BCS, whereas there are still insufficient data about the effects of different PE protocol on DHEA-S level in this population [71,72,73]. Our results demonstrated that a RET protocol could significantly reduce DHEA-S and cortisol/DHEA-S levels, regardless of HT, with a possible protective role on cancer relapse, as androgen receptors modulate BC cell growth both with a genomic and nongenomic pathway [74]. However, these results should be confirmed and analyzed at a long-term follow-up, as a potential anti-metastatic role for DHEA-S in BC has been recently advocated [75].
Among CV parameters, much evidence documented the role of RET in the reduction of SBP, both in hypertensive patients, postmenopausal women with and without arterial hypertension, and BCS, with a strong recommendation from the European Society of Cardiology (ESC) to encourage the incorporation of RET both in primary and secondary CV prevention PE protocol [34,76,77,78,79]. This effect could be mediated by peripheral vascular resistance and arterial stiffness reduction, renal and muscular sympathetic activity decline, and modulation of cardiac and renal baroreceptors [80]. Our data align with the available evidence in the literature [81,82]. VAC, as the ratio between end-systolic ventricular elastance (Ees) and arterial elastance (Ea), presents a marker of CV efficiency, with an independent diagnostic and prognostic value in CVD and CV risk stratification [83]. VAC reduces with increasing age and in the presence of CV risk factors, such as increased aortic rigidity or left ventricular diastolic dysfunction, especially in the female population [84,85]. PE can reverse these negative adaptations, in a dose-dependent manner, even in the older population, with a potentially more significant effect on women [86,87,88]. Moreover, a differential effect of AET and RET on VAC has been advocated, with AET being associated with increased brachial artery absolute diameter and blood flow during hyperemia and RET inducing changes in vascular reactivity [89].
Besides CV risk factors, even CHT can worsen VAC in BCS, with a reduction in both Ees and left ventricular systolic function [90,91,92]. However, to date, there is no evidence about the effects of PE on VAC in BCS. Our data provide promising results about RT’s role in improving VAC, both in terms of Ea and Ees, in BCS not treated with CHT.
PWV represents the gold standard for assessing arterial stiffness (AS) and is inversely correlated with vascular compliance [93]. AS represents one of the earlier markers of structural and functional vascular degeneration and is associated with a worsening of CV outcomes, regardless of traditional CV risk factors assessed by Framingham Risk Score [94]. The female population experiences an earlier and more severe increase in AS according to age than the male population, with a two-fold higher risk of mortality in women than men [95]. As for VAC, PE can reverse PWV worsening associated with age and CV risk factors [96]. Among PE protocols, AET has been associated with a significant improvement in PWV, for its role in the endothelium-derived nitric oxide function, both in the general population and postmenopausal women [97,98,99]. In the specific setting of BCS, both CHT and RET can determine a significant worsening of PWV, especially in patients treated with aromatase inhibitors [100,101]. To the best of our knowledge, the role of PE on PWV in BCS treated with HT still needs to be clarified. Our data show a potentially relevant role of AET in PWV improvement, regardless of HT.
Several studies have recognized the benefits of PE in HR-QoL in BCS [102]. In particular, a longer exercise session duration, a combination of PE protocol and a supervised intervention could determine the most significant improvement in HR-QoL [103,104,105]. However, according to a meta-analysis by Lahart et al., PE can determine only a small-to-moderate significant improvement in HR-QoL that does not persist longer than 12 weeks [106]. In our cohort, we found a significant improvement in physical function after PE training, but only in the AET subgroup. Moreover, at the follow-up, we found no differences between the PE subgroups regarding CV-QoL and HR-QoL, in line with the data available in the literature. Regarding PA, we observed an increase in METs and VIPAT in AET group and an increase in STEPS/day in RET group; in contrast, we did not document any compensatory increase in ST after PE training. Regarding follow-up data, the IPAQ-score indicated a sufficiently active lifestyle in both the general population and PE subgroups, with a higher percentage of inactivity in RET group. These data underline the need for a call to action to increase motivation for PA in BCS, as many research groups already addressed [107].
The limitations of our study were: the small sample size; the assessment of epicardial fat and sagittal abdominal diameter, respectively, with transthoracic echocardiography and anthropometric evaluation instead of computed tomography; and the evaluation of spontaneous PA with IPAQ score at the follow-up instead of accelerometry.
The latest guidelines on cardio-oncology, recently published by the ESC, strengthened the noteworthy interconnection between CVD and cancer in terms of common modifiable and nonmodifiable risk factors, as well as regarding the importance of maintaining a healthy lifestyle and adequate cardiorespiratory fitness (CRF) after cancer diagnosis and during and after anticancer therapy. In particular, the guidelines recommend maintaining adequate PA and underline that exercise prescription represents a potent multitargeted tool capable of counteracting anticancer treatment side effects, with a potential impact both in primary as well as secondary prevention of CTR-CVT and a paramount role in primary and secondary prevention of CV risk factors [8].
Moreover, the guidelines emphasize that, to date, current evidence does not support a specific protocol of PE, as exercise prescription should be tailored to the specific patient’s fitness level and basal characteristics and systematically improved to enhance physiological adaptation to get the best results both in terms of CRF, prevention, and treatment of anticancer side effects and prevention of cancer relapse as well [25,108,109].
Our data showed, for the first time in the literature, the effects of different PE protocols on cardio-metabolic parameters, QoL, and PA in a BCS population not treated with CHT or HER-2 inhibitors, with data about CV and BC outcomes, PA, CV-QoL, and HR-QoL at a 34-month follow-up. AET and RET can determine specific, positive adaptations on many parameters strongly related to CV risk and outcomes and should be included in every cardio-oncology rehabilitation program. Ideally, each BCS should be involved in a dedicated cardio-oncology rehabilitation program tailored to the specific patient’s cardio-metabolic status, including intense, motivational counseling, to get the best compliance with exercise prescription, maintain an adequate PA level, and achieve the most remarkable effects on both short and long-term BC and CV outcomes and QoL [110].
Author Contributions
Conceptualization, S.G., A.D.B. (Andrea Di Blasio), and V.B.; methodology, A.D.B. (Andrea Di Blasio), T.M., S.G., V.B., F.B. and A.C.; software, F.B.; validation, S.G., E.C., A.D.B. (Angela Di Baldassarre), A.D.B. (Andrea Di Blasio), S.D.S., G.N. and I.B.; formal analysis, V.B. and F.B.; investigation, A.D.B. (Andrea Di Blasio), T.M., V.B., D.T., F.M. and A.C.; resources, S.G., E.C. and A.D.B. (Angela Di Baldassarre); data curation, A.D.B. (Andrea Di Blasio), T.M., V.B., F.B., D.T. and F.M.; writing—original draft preparation, V.B. and D.T.; writing—review and editing, V.B., S.G., F.B., A.D.B. (Andrea Di Blasio), A.D.B. (Angela Di Baldassarre), I.B., G.N. and E.C.; visualization, S.G.; supervision, S.G.; project administration, S.G. and E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of G. d’Annunzio University Chieti-Pescara (#312/2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We want to express our sincere gratitude to all the women who participated in our study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Lima, S.M.; Kehm, R.D.; Terry, M.B. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine 2021, 38, 100985. [Google Scholar] [CrossRef] [PubMed]
- Mehta, L.S.; Watson, K.E.; Barac, A.; Beckie, T.M.; Bittner, V.; Cruz-Flores, S.; Dent, S.; Kondapalli, L.; Ky, B.; Okwuosa, T.; et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e30–e66. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; He, Y.; Hu, X. Cardio-Oncology: Mechanisms, Drug Combinations, and Reverse Cardio-Oncology. Int. J. Mol. Sci. 2022, 23, 10617. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Liang, B. Comparison of American and European guidelines for cardio-oncology of heart failure. Heart Fail. Rev. 2023. [Google Scholar] [CrossRef]
- Cheung, Y.M.; Ramchand, S.K.; Yeo, B.; Grossmann, M. Cardiometabolic Effects of Endocrine Treatment of Estrogen Receptor-Positive Early Breast Cancer. J. Endocr. Soc. 2019, 3, 1283–1301. [Google Scholar] [CrossRef]
- Salerni, S.; Di Francescomarino, S.; Cadeddu, C.; Acquistapace, F.; Maffei, S.; Gallina, S. The different role of sex hormones on female cardiovascular physiology and function: Not only oestrogens. Eur. J. Clin. Investig. 2015, 45, 634–645. [Google Scholar] [CrossRef]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Lahart, I.M.; Metsios, G.S.; Nevill, A.M.; Carmichael, A.R. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015, 54, 635–654. [Google Scholar] [CrossRef]
- Sturgeon, K.M.; Ky, B.; Libonati, J.R.; Schmitz, K.H. The effects of exercise on cardiovascular outcomes before, during, and after treatment for breast cancer. Breast Cancer Res. Treat. 2014, 143, 219–226. [Google Scholar] [CrossRef]
- Di Blasio, A.; Morano, T.; Cianchetti, E.; Gallina, S.; Bucci, I.; Di Santo, S.; Tinari, C.; Di Donato, F.; Izzicupo, P.; Di Baldassarre, A.; et al. Psychophysical health status of breast cancer survivors and effects of 12 weeks of aerobic training. Complement. Ther. Clin. Pract. 2017, 27, 19–26. [Google Scholar] [CrossRef]
- Di Blasio, A.; Morano, T.; Bucci, I.; Di Santo, S.; D’Arielli, A.; Castro, C.G.; Cugusi, L.; Cianchetti, E.; Napolitano, G. Physical exercises for breast cancer survivors: Effects of 10 weeks of training on upper limb circumferences. J. Phys. Ther. Sci. 2016, 28, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- Paulo, T.R.S.; Rossi, F.E.; Viezel, J.; Tosello, G.T.; Seidinger, S.C.; Simoes, R.R.; de Freitas, R., Jr.; Freitas, I.F., Jr. The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: A randomized controlled trial. Health Qual. Life Outcomes 2019, 17, 17. [Google Scholar] [CrossRef]
- Schutz, S.; Aidar, F.J.; Souza, R.L.M.; Dos Santos, J.L.; Voltarelli, F.A.; Vieira Junior, R.C.; Soares, N.M.M.; Marcal, A.C. Different Methods of Physical Training Applied to Women Breast Cancer Survivors: A Systematic Review. Front. Physiol. 2021, 12, 639406. [Google Scholar] [CrossRef] [PubMed]
- Hartman, S.J.; Marinac, C.R.; Bellettiere, J.; Godbole, S.; Natarajan, L.; Patterson, R.E.; Kerr, J. Objectively measured sedentary behavior and quality of life among survivors of early stage breast cancer. Support Care Cancer 2017, 25, 2495–2503. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.I.; Scherer, R.W.; Geigle, P.M.; Berlanstein, D.R.; Topaloglu, O.; Gotay, C.C.; Snyder, C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst. Rev. 2012, 8, CD007566. [Google Scholar] [CrossRef]
- Fassier, P.; Zelek, L.; Partula, V.; Srour, B.; Bachmann, P.; Touillaud, M.; Druesne-Pecollo, N.; Galan, P.; Cohen, P.; Hoarau, H.; et al. Variations of physical activity and sedentary behavior between before and after cancer diagnosis: Results from the prospective population-based NutriNet-Sante cohort. Medicine 2016, 95, e4629. [Google Scholar] [CrossRef]
- Furmaniak, A.C.; Menig, M.; Markes, M.H. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst. Rev. 2016, 9, CD005001. [Google Scholar] [CrossRef]
- Ibrahim, E.M.; Al-Homaidh, A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med. Oncol. 2011, 28, 753–765. [Google Scholar] [CrossRef]
- Basen-Engquist, K.; Hughes, D.; Perkins, H.; Shinn, E.; Taylor, C.C. Dimensions of physical activity and their relationship to physical and emotional symptoms in breast cancer survivors. J. Cancer Surviv. 2008, 2, 253–261. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, X.; Wang, Y.; Xiong, H.; Zhao, Y.; Sun, F. Effects of exercise intervention in breast cancer survivors: A meta-analysis of 33 randomized controlled trails. OncoTargets Ther. 2016, 9, 2153–2168. [Google Scholar] [CrossRef]
- Segar, M.; Katch, V.; Roth, R.; Garcia, A.; Portner, T.; Glickman, S.; Haslanger, S.; Wilkins, E. The effect of aerobic exercise on self-esteem and depressive and anxiety symptoms among breast cancer survivors. Oncol. Nurs. Forum. 1998, 25, 107–113. [Google Scholar] [PubMed]
- Kirkham, A.A.; Davis, M.K. Exercise Prevention of Cardiovascular Disease in Breast Cancer Survivors. J. Oncol. 2015, 2015, 917606. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Nilsen, T.S.; Gupta, D.; Jones, L.W. Exercise Therapy and Cardiovascular Toxicity in Cancer. Circulation 2018, 137, 1176–1191. [Google Scholar] [CrossRef] [PubMed]
- Gasbarro, V.; Michelini, S.; Antignani, P.L.; Tsolaki, E.; Ricci, M.; Allegra, C. The CEAP-L classification for lymphedemas of the limbs: The Italian experience. Int. Angiol. 2009, 28, 315–324. [Google Scholar] [PubMed]
- Marfell-Jones, M.; Olds, T.; Stewart, A.; Carter, L. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Potchefstroom, South Africa, 2006. [Google Scholar]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. Nutrition 1989, 5, 303–311. [Google Scholar]
- Sampaio, L.R.; Simoes, E.J.; Assis, A.M.; Ramos, L.R. Validity and reliability of the sagittal abdominal diameter as a predictor of visceral abdominal fat. Arq. Bras. Endocrinol. Metabol. 2007, 51, 980–986. [Google Scholar] [CrossRef]
- Comitato Organizzativo Cardiologico per l’idoneità Allo Sport. Protocolli Cardiologici Per il Giudizio di Idoneità Allo Sport Agonistico; Casa Editrice Scientifica Internazionale: Roma, Italy, 2017. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Chen, C.H.; Fetics, B.; Nevo, E.; Rochitte, C.E.; Chiou, K.R.; Ding, P.A.; Kawaguchi, M.; Kass, D.A. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J. Am. Coll. Cardiol. 2001, 38, 2028–2034. [Google Scholar] [CrossRef]
- Iacobellis, G.; Willens, H.J. Echocardiographic epicardial fat: A review of research and clinical applications. J. Am. Soc. Echocardiogr. 2009, 22, 1311–1319, quiz 1417–1318. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Flore, R.; Ponziani, F.R.; Tinelli, G.; Arena, V.; Fonnesu, C.; Nesci, A.; Santoro, L.; Tondi, P.; Santoliquido, A. New modalities of ultrasound-based intima-media thickness, arterial stiffness and non-coronary vascular calcifications detection to assess cardiovascular risk. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1430–1441. [Google Scholar] [PubMed]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 2008, 21, 93–111, quiz 189–190. [Google Scholar] [CrossRef] [PubMed]
- Zardi, E.M.; Di Geso, L.; Afeltra, A.; Zardi, D.M.; Giorgi, C.; Salaffi, F.; Carotti, M.; Gutierrez, M.; Filippucci, E.; Grassi, W. An ultrasound automated method for non-invasive assessment of carotid artery pulse wave velocity. J. Investig. Med. 2018, 66, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef]
- Beutner, F.; Teren, A.; Gielen, S.; Schuler, G.; Wirkner, K.; Tiller, D.; Loeffler, M.; Scholz, M. Automated photoplethysmography-based determination of ankle-brachial index: A validation study against Doppler sonography. Clin. Res. Cardiol. 2012, 101, 875–883. [Google Scholar] [CrossRef]
- Treanor, C.; Donnelly, M. A methodological review of the Short Form Health Survey 36 (SF-36) and its derivatives among breast cancer survivors. Qual. Life Res. 2015, 24, 339–362. [Google Scholar] [CrossRef]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey: Translation, validation and norming. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef]
- Scheers, T.; Philippaerts, R.; Lefevre, J. SenseWear-determined physical activity and sedentary behavior and metabolic syndrome. Med. Sci. Sports Exerc. 2013, 45, 481–489. [Google Scholar] [CrossRef]
- Rich, C.; Geraci, M.; Griffiths, L.; Sera, F.; Dezateux, C.; Cortina-Borja, M. Quality control methods in accelerometer data processing: Defining minimum wear time. PLoS ONE 2013, 8, e67206. [Google Scholar] [CrossRef] [PubMed]
- van Holland, B.J.; Frings-Dresen, M.H.; Sluiter, J.K. Measuring short-term and long-term physiological stress effects by cortisol reactivity in saliva and hair. Int. Arch. Occup. Environ. Health 2012, 85, 849–852. [Google Scholar] [CrossRef]
- Patel, K.K.; Arnold, S.V.; Chan, P.S.; Tang, Y.; Jones, P.G.; Guo, J.; Buchanan, D.M.; Qintar, M.; Decker, C.; Morrow, D.A.; et al. Validation of the Seattle angina questionnaire in women with ischemic heart disease. Am. Heart J. 2018, 201, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Chan, P.; Gosch, K.; Li, Y.; Reid, K.; Tang, F.; Spertus, J. Abstract 54: The SAQ-7: A Short Version of the Seattle Angina Questionnaire. Circ. Cardiovasc. Qual. Outcomes 2013, 6, A54. [Google Scholar] [CrossRef]
- Chan, P.S.; Jones, P.G.; Arnold, S.A.; Spertus, J.A. Development and validation of a short version of the Seattle angina questionnaire. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Natalucci, V.; Villarini, M.; Emili, R.; Acito, M.; Vallorani, L.; Barbieri, E.; Villarini, A. Special Attention to Physical Activity in Breast Cancer Patients during the First Wave of COVID-19 Pandemic in Italy: The DianaWeb Cohort. J. Pers. Med. 2021, 11, 381. [Google Scholar] [CrossRef]
- Scoring Protocol for the International Physical Activity Questionnaire (IPAQ). Available online: https://sites.google.com/view/ipaq/score (accessed on 10 October 2022).
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Persinger, R.; Foster, C.; Gibson, M.; Fater, D.; Porcari, J. Consistency of the talk test for exercise prescription. Med. Sci. Sports Exerc. 2004, 36, 1632–1636. [Google Scholar]
- Pouliot, M.C.; Despres, J.P.; Lemieux, S.; Moorjani, S.; Bouchard, C.; Tremblay, A.; Nadeau, A.; Lupien, P.J. Waist circumference and abdominal sagittal diameter: Best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am. J. Cardiol. 1994, 73, 460–468. [Google Scholar] [CrossRef]
- Ohrvall, M.; Berglund, L.; Vessby, B. Sagittal abdominal diameter compared with other anthropometric measurements in relation to cardiovascular risk. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Huai, P.; Liu, J.; Ye, X.; Li, W.Q. Association of Central Obesity with All Cause and Cause-Specific Mortality in US Adults: A Prospective Cohort Study. Front. Cardiovasc. Med. 2022, 9, 816144. [Google Scholar] [CrossRef] [PubMed]
- An, K.Y.; Kim, S.; Oh, M.; Lee, H.S.; Yang, H.I.; Park, H.; Lee, J.W.; Jeon, J.Y. Cardiopulmonary fitness but not muscular fitness associated with visceral adipose tissue mass. Arch. Physiol. Biochem. 2021, 127, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Boeing, H.; Hoffmann, K.; Bergmann, M.; Schulze, M.B.; Overvad, K.; van der Schouw, Y.T.; Spencer, E.; Moons, K.G.; Tjonneland, A.; et al. General and abdominal adiposity and risk of death in Europe. N. Engl. J. Med. 2008, 359, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- Dyrstad, S.M.; Edvardsen, E.; Hansen, B.H.; Anderssen, S.A. Waist circumference thresholds and cardiorespiratory fitness. J. Sport Health Sci. 2019, 8, 17–22. [Google Scholar] [CrossRef]
- Jones, L.M.; Stoner, L.; Brown, C.; Baldi, J.C.; McLaren, B. Cardiorespiratory fitness predicts cardiovascular health in breast cancer survivors, independent of body composition, age and time post-treatment completion. Breast Cancer 2019, 26, 729–737. [Google Scholar] [CrossRef]
- Moller, G.; Ritz, C.; Kjolbaek, L.; Vuholm, S.; Korndal, S.K.; Larsen, T.M.; Pedersen, O.; Saris, W.; Astrup, A.; Lauritzen, L.; et al. Sagittal abdominal diameter and waist circumference appear to be equally good as identifiers of cardiometabolic risk. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 518–527. [Google Scholar] [CrossRef]
- Saad, M.A.N.; Jorge, A.J.L.; de Avila, D.X.; Martins, W.A.; Dos Santos, M.M.S.; Tedeschi, L.T.; Cavalcanti, I.L.; Rosa, M.L.G.; Filho, R. Sagittal abdominal diameter as a marker of visceral obesity in older primary care patients. J. Geriatr. Cardiol. 2020, 17, 279–283. [Google Scholar] [CrossRef]
- Vasques, A.C.; Souza, J.R.; Yamanaka, A.; de Oliveira Mda, S.; Novaes, F.S.; Pareja, J.C.; Geloneze, B. Sagittal abdominal diameter as a marker for epicardial adipose tissue in premenopausal women. Metabolism 2013, 62, 1032–1036. [Google Scholar] [CrossRef]
- Thomas, G.A.; Cartmel, B.; Harrigan, M.; Fiellin, M.; Capozza, S.; Zhou, Y.; Ercolano, E.; Gross, C.P.; Hershman, D.; Ligibel, J.; et al. The effect of exercise on body composition and bone mineral density in breast cancer survivors taking aromatase inhibitors. Obesity 2017, 25, 346–351. [Google Scholar] [CrossRef]
- Skrypnik, D.; Bogdanski, P.; Madry, E.; Karolkiewicz, J.; Ratajczak, M.; Krysciak, J.; Pupek-Musialik, D.; Walkowiak, J. Effects of Endurance and Endurance Strength Training on Body Composition and Physical Capacity in Women with Abdominal Obesity. Obes. Facts 2015, 8, 175–187. [Google Scholar] [CrossRef] [PubMed]
- de Paulo, T.R.S.; Winters-Stone, K.M.; Viezel, J.; Rossi, F.E.; Aro, B.L.; Trindade, A.; Codogno, J.S.; Freitas Junior, I.F. Comparing exercise responses to aerobic plus resistance training between postmenopausal breast cancer survivors undergoing aromatase inhibitor therapy and healthy women. Disabil. Rehabil. 2019, 41, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Ennour-Idrissi, K.; Maunsell, E.; Diorio, C. Effect of physical activity on sex hormones in women: A systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res. 2015, 17, 139. [Google Scholar] [CrossRef]
- Swain, C.T.V.; Drummond, A.E.; Boing, L.; Milne, R.L.; English, D.R.; Brown, K.A.; van Roekel, E.H.; Dixon-Suen, S.C.; Lynch, M.J.; Moore, M.M.; et al. Linking Physical Activity to Breast Cancer via Sex Hormones, Part 1: The Effect of Physical Activity on Sex Steroid Hormones. Cancer Epidemiol. Biomark. Prev. 2022, 31, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Tosti, K.P.; Hackney, A.C.; Battaglini, C.L.; Evans, E.S.; Groff, D. Exercise in patients with breast cancer and healthy controls: Energy substrate oxidation and blood lactate responses. Integr. Cancer Ther. 2011, 10, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Adraskela, K.; Veisaki, E.; Koutsilieris, M.; Philippou, A. Physical Exercise Positively Influences Breast Cancer Evolution. Clin. Breast Cancer 2017, 17, 408–417. [Google Scholar] [CrossRef]
- Lambert, M.; Brunet, J.; Couture-Lalande, M.E.; Bielajew, C. Aerobic physical activity and salivary cortisol levels among women with a history of breast cancer. Complement. Ther. Med. 2019, 42, 12–18. [Google Scholar] [CrossRef]
- Di Blasio, A.; Morano, T.; Lancia, F.; Viscioni, G.; Bucci, I.; Grossi, S.; Cimini, A.; Cianchetti, E.; Verrocchio, S.; Izzcupo, P.; et al. The Role of the Environment and Type of Exercise on Acute Adrenal Modulation and Perceived Distress of Breast Cancer Survivors Practising Light-Intensity Physical Exercise. Arch. Breast Cancer 2022, 9, 152–161. [Google Scholar] [CrossRef]
- Evans, E.S.; Hackney, A.C.; Pebole, M.M.; McMurray, R.G.; Muss, H.B.; Deal, A.M.; Battaglini, C.L. Adrenal Hormone and Metabolic Biomarker Responses to 30 min of Intermittent Cycling Exercise in Breast Cancer Survivors. Int. J. Sports Med. 2016, 37, 921–929. [Google Scholar] [CrossRef]
- Giovannelli, P.; Di Donato, M.; Galasso, G.; Di Zazzo, E.; Bilancio, A.; Migliaccio, A. The Androgen Receptor in Breast Cancer. Front. Endocrinol. 2018, 9, 492. [Google Scholar] [CrossRef]
- Upmanyu, N.; Bulldan, A.; Failing, K.; Scheiner-Bobis, G. DHEAS prevents pro-metastatic and proliferative effects of 17ss-estradiol on MCF-7 breast cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118600. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- de Sousa, E.C.; Abrahin, O.; Ferreira, A.L.L.; Rodrigues, R.P.; Alves, E.A.C.; Vieira, R.P. Resistance training alone reduces systolic and diastolic blood pressure in prehypertensive and hypertensive individuals: Meta-analysis. Hypertens. Res. 2017, 40, 927–931. [Google Scholar] [CrossRef]
- Nascimento, D.D.C.; da Silva, C.R.; Valduga, R.; Saraiva, B.; de Sousa Neto, I.V.; Vieira, A.; Funghetto, S.S.; Silva, A.O.; Oliveira, S.D.C.; Pereira, G.B.; et al. Blood pressure response to resistance training in hypertensive and normotensive older women. Clin. Interv. Aging 2018, 13, 541–553. [Google Scholar] [CrossRef]
- Lee, K.; Mortimer, J.; Spicer, D.; Tripathy, D.; Dieli-Conwright, C. Ps 17-28 the Effect of a Combined Aerobic and Resistance Exercise Training on Blood Pressure in Breast Cancer Survivors with Hypertension. J. Hypertens. 2016, 34, e481. [Google Scholar] [CrossRef]
- Umpierre, D.; Stein, R. Hemodynamic and vascular effects of resistance training: Implications for cardiovascular disease. Arq. Bras. Cardiol. 2007, 89, 256–262. [Google Scholar] [CrossRef]
- Lee, K.; Tripathy, D.; Demark-Wahnefried, W.; Courneya, K.S.; Sami, N.; Bernstein, L.; Spicer, D.; Buchanan, T.A.; Mortimer, J.E.; Dieli-Conwright, C.M. Effect of Aerobic and Resistance Exercise Intervention on Cardiovascular Disease Risk in Women with Early-Stage Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2019, 5, 710–714. [Google Scholar] [CrossRef]
- Wang, S.; Yang, T.; Qiang, W.; Shen, A.; Zhao, Z.; Chen, X.; Xi, C.; Liu, H.; Guo, F. Effectiveness of physical exercise on the cardiovascular system in breast cancer patients: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Clin. Pract. 2021, 44, 101426. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Aboyans, V.; Blacher, J.; Brodmann, M.; Brutsaert, D.L.; Chirinos, J.A.; De Carlo, M.; Delgado, V.; Lancellotti, P.; Lekakis, J.; et al. The role of ventricular-arterial coupling in cardiac disease and heart failure: Assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur. J. Heart Fail. 2019, 21, 402–424. [Google Scholar] [CrossRef]
- Wohlfahrt, P.; Redfield, M.M.; Lopez-Jimenez, F.; Melenovsky, V.; Kane, G.C.; Rodeheffer, R.J.; Borlaug, B.A. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart Fail. 2014, 2, 489–499. [Google Scholar] [CrossRef]
- Coutinho, T.; Borlaug, B.A.; Pellikka, P.A.; Turner, S.T.; Kullo, I.J. Sex differences in arterial stiffness and ventricular-arterial interactions. J. Am. Coll. Cardiol. 2013, 61, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Chantler, P.D. Arterial Ventricular Uncoupling with Age and Disease and Recoupling with Exercise. Exerc. Sport Sci. Rev. 2017, 45, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Hieda, M.; Howden, E.; Shibata, S.; Fujimoto, N.; Bhella, P.S.; Hastings, J.L.; Tarumi, T.; Sarma, S.; Fu, Q.; Zhang, R.; et al. Impact of Lifelong Exercise Training Dose on Ventricular-Arterial Coupling. Circulation 2018, 138, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.D.; Yan, H.; Ranadive, S.M.; Kappus, R.M.; Sun, P.; Cook, M.D.; Harvey, I.; Woods, J.; Wilund, K.; Fernhall, B. Sex differences in ventricular-vascular coupling following endurance training. Eur. J. Appl. Physiol. 2014, 114, 2597–2606. [Google Scholar] [CrossRef]
- Lekavich, C.L.; Allen, J.D.; Bensimhon, D.R.; Bateman, L.A.; Slentz, C.A.; Samsa, G.P.; Kenjale, A.A.; Duscha, B.D.; Douglas, P.S.; Kraus, W.E. Aerobic Versus Resistance Training Effects on Ventricular-Arterial Coupling and Vascular Function in the STRRIDE-AT/RT Trial. Front. Cardiovasc. Med. 2021, 8, 638929. [Google Scholar] [CrossRef]
- Narayan, H.K.; Finkelman, B.; French, B.; Plappert, T.; Hyman, D.; Smith, A.M.; Margulies, K.B.; Ky, B. Detailed Echocardiographic Phenotyping in Breast Cancer Patients: Associations with Ejection Fraction Decline, Recovery, and Heart Failure Symptoms over 3 Years of Follow-up. Circulation 2017, 135, 1397–1412. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Lewis, N.C.; Ellard, S.L.; Jones, L.W.; Gelinas, J.C.; Rolf, J.D.; Melzer, B.; Thomas, S.M.; Douglas, P.S.; Khouri, M.G.; et al. Ventricular-Arterial Coupling in Breast Cancer Patients After Treatment with Anthracycline-Containing Adjuvant Chemotherapy. Oncologist 2016, 21, 141–149. [Google Scholar] [CrossRef]
- Narayan, H.K.; French, B.; Khan, A.M.; Plappert, T.; Hyman, D.; Bajulaiye, A.; Domchek, S.; DeMichele, A.; Clark, A.; Matro, J.; et al. Noninvasive Measures of Ventricular-Arterial Coupling and Circumferential Strain Predict Cancer Therapeutics-Related Cardiac Dysfunction. JACC Cardiovasc. Imaging 2016, 9, 1131–1141. [Google Scholar] [CrossRef]
- Cavalcante, J.L.; Lima, J.A.; Redheuil, A.; Al-Mallah, M.H. Aortic stiffness: Current understanding and future directions. J. Am. Coll. Cardiol. 2011, 57, 1511–1522. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Terentes-Printzios, D.; Laurent, S.; Nilsson, P.M.; Protogerou, A.D.; Aznaouridis, K.; Xaplanteris, P.; Koutagiar, I.; Tomiyama, H.; Yamashina, A.; et al. Association of Estimated Pulse Wave Velocity with Survival: A Secondary Analysis of SPRINT. JAMA Netw. Open 2019, 2, e1912831. [Google Scholar] [CrossRef]
- DuPont, J.J.; Kenney, R.M.; Patel, A.R.; Jaffe, I.Z. Sex differences in mechanisms of arterial stiffness. Br. J. Pharmacol. 2019, 176, 4208–4225. [Google Scholar] [CrossRef]
- Tanaka, H. Antiaging Effects of Aerobic Exercise on Systemic Arteries. Hypertension 2019, 74, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Lara, J.; Siervo, M.; Celis-Morales, C.; Mathers, J.C. Effects of exercise modalities on arterial stiffness and wave reflection: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e110034. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Maiorana, A.; O’Driscoll, G.; Taylor, R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J. Physiol. 2004, 561 Pt 1, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Molisz, A.; Schmederer, Z.; Siebert, J.; Kadamani, T.; Glasner, P.; Roslonkiewicz, K.; Nowicka-Sauer, K.; Gutknecht, P.; Trzeciak, B.; Suchanowski, A. Haemodynamic parameters in postmenopausal women—Beneficial effect of moderate continuous exercise training. Ann. Agric. Environ. Med. 2019, 26, 425–428. [Google Scholar] [CrossRef]
- Yersal, O.; Eryilmaz, U.; Akdam, H.; Meydan, N.; Barutca, S. Arterial Stiffness in Breast Cancer Patients Treated with Anthracycline and Trastuzumab-Based Regimens. Cardiol. Res. Pract. 2018, 2018, 5352914. [Google Scholar] [CrossRef]
- Vallerio, P.; Sarno, L.; Stucchi, M.; Musca, F.; Casadei, F.; Maloberti, A.; Lestuzzi, C.; Mancia, G.; Moreo, A.; Palazzi, M.; et al. Long-Term Effects of Radiotherapy on Arterial Stiffness in Breast Cancer Women. Am. J. Cardiol. 2016, 118, 771–776. [Google Scholar] [CrossRef]
- Joaquim, A.; Leao, I.; Antunes, P.; Capela, A.; Viamonte, S.; Alves, A.J.; Helguero, L.A.; Macedo, A. Impact of physical exercise programs in breast cancer survivors on health-related quality of life, physical fitness, and body composition: Evidence from systematic reviews and meta-analyses. Front. Oncol. 2022, 12, 955505. [Google Scholar] [CrossRef]
- Hong, F.; Ye, W.; Kuo, C.H.; Zhang, Y.; Qian, Y.; Korivi, M. Exercise Intervention Improves Clinical Outcomes, but the “Time of Session” is Crucial for Better Quality of Life in Breast Cancer Survivors: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 706. [Google Scholar] [CrossRef]
- Singh, B.; Spence, R.R.; Steele, M.L.; Sandler, C.X.; Peake, J.M.; Hayes, S.C. A Systematic Review and Meta-Analysis of the Safety, Feasibility, and Effect of Exercise in Women with Stage II+ Breast Cancer. Arch. Phys. Med. Rehabil. 2018, 99, 2621–2636. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Liu, D. Effects of exercise on the quality of life in breast cancer patients: A systematic review of randomized controlled trials. Support Care Cancer 2019, 27, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lahart, I.M.; Metsios, G.S.; Nevill, A.M.; Carmichael, A.R. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst. Rev. 2018, 1, CD011292. [Google Scholar] [CrossRef] [PubMed]
- Frikkel, J.; Gotte, M.; Beckmann, M.; Kasper, S.; Hense, J.; Teufel, M.; Schuler, M.; Tewes, M. Fatigue, barriers to physical activity and predictors for motivation to exercise in advanced Cancer patients. BMC Palliat. Care 2020, 19, 43. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Anselmi, F.; Fiorentini, C.; Mannucci, R.; Bonifazi, M.; Mondillo, S. The benefits of exercise in cancer patients and the criteria for exercise prescription in cardio-oncology. Eur. J. Prev. Cardiol. 2021, 28, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Zabor, E.C.; Schwitzer, E.; Koelwyn, G.J.; Adams, S.C.; Nilsen, T.S.; Moskowitz, C.S.; Matsoukas, K.; Iyengar, N.M.; Dang, C.T.; et al. Efficacy of Exercise Therapy on Cardiorespiratory Fitness in Patients with Cancer: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2018, 36, 2297–2305. [Google Scholar] [CrossRef]
- Sasso, J.P.; Eves, N.D.; Christensen, J.F.; Koelwyn, G.J.; Scott, J.; Jones, L.W. A framework for prescription in exercise-oncology research. J. Cachexia Sarcopenia Muscle 2015, 6, 115–124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).