The Controlling Nutritional Status (CONUT) Score for Prediction of Microvascular Flap Complications in Reconstructive Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Anaesthesia and Surgical Protocol
2.3. Data Collection
2.4. Definitions
2.5. Statistical Analysis
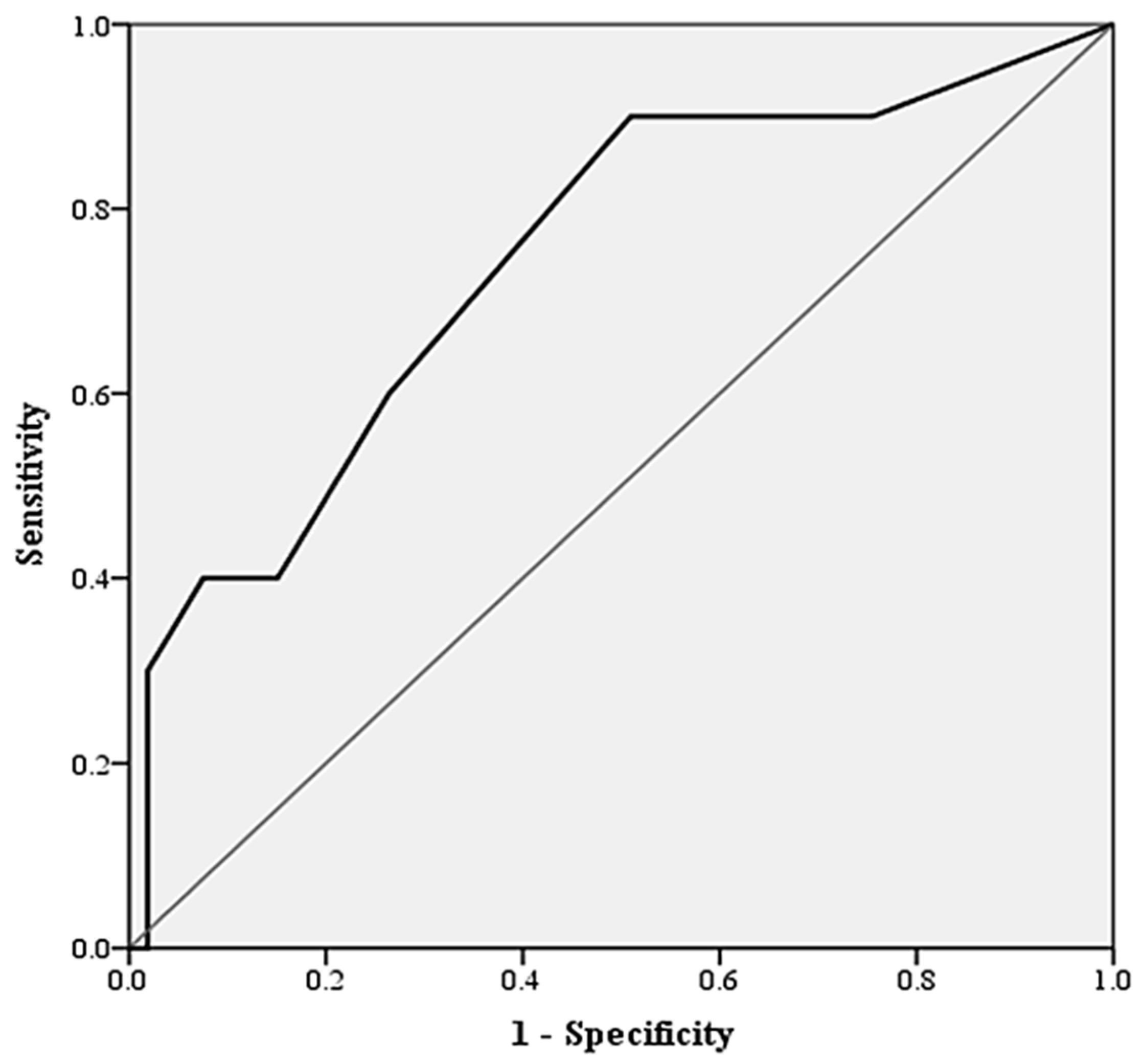
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lese, I.; Biedermann, R.; Constantinescu, M.; Grobbelaar, A.O.; Olariu, R. Predicting risk factors that lead to free flap failure and vascular compromise: A single unit experience with 565 free tissue transfers. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Hanasono, M.M.; Butler, C.E. Prevention and treatment of thrombosis in microvascular surgery. J. Reconstr. Microsurg. 2008, 24, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.L.; Yen, Y.H.; Lee, P.J.; Liu, C.C.; Pu, C.M. Factors Influencing Postoperative Complications in Reconstructive Microsurgery for Head and Neck Cancer. J. Oral Maxillofac. Surg. 2017, 75, 867–873. [Google Scholar] [CrossRef]
- Novakovic, D.; Patel, R.S.; Goldstein, D.P.; Gullane, P.J. Salvage of failed free flaps used in head and neck reconstruction. Head Neck Oncol. 2009, 1, 33. [Google Scholar] [CrossRef] [PubMed]
- Košec, A.; Solter, D.; Ribić, A.; Knežević, M.; Vagić, D.; Pegan, A. Systemic Inflammatory Markers as Predictors of Postoperative Complications and Survival in Patients with Advanced Head and Neck Squamous Cell Carcinoma Undergoing Free-Flap Reconstruction. J. Oral Maxillofac. Surg. 2022, 80, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hong, J.P.; Suh, H.P.; Park, J.Y.; Kim, D.H.; Ha, S.; Lee, J.; Hwang, J.H.; Kim, Y.K. Prognostic Nutritional Index is a Predictor of Free Flap Failure in Extremity Reconstruction. Nutrients 2020, 12, 562. [Google Scholar] [CrossRef]
- Shum, J.; Markiewicz, M.R.; Park, E.; Bui, T.; Lubek, J.; Bell, R.B.; Dierks, E.J. Low prealbumin level is a risk factor for microvascular free flap failure. J. Oral Maxillofac. Surg. 2014, 72, 169–177. [Google Scholar] [CrossRef]
- Takagi, K.; Domagala, P.; Polak, W.G.; Buettner, S.; Wijnhoven, B.P.L.; Ijzermans, J.N.M. Prognostic significance of the controlling nutritional status (CONUT) score in patients undergoing gastrectomy for gastric cancer: A systematic review and meta-analysis. BMC Surg. 2019, 19, 129. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, H.; Pan, J.; Yu, W.; Lv, J.; Yan, J.; Gao, J.; Wang, X.; Ge, X.; Zhou, W. Preoperative Controlling Nutritional Status (CONUT) score predicts short-term outcomes of patients with gastric cancer after laparoscopy-assisted radical gastrectomy. World J. Surg. Oncol. 2021, 19, 25. [Google Scholar] [CrossRef]
- Venianaki, M.; Andreou, A.; Nikolouzakis, T.K.; Chrysos, E.; Chalkiadakis, G.; Lasithiotakis, K. Factors Associated with Malnutrition and Its Impact on Postoperative Outcomes in Older Patients. J. Clin. Med. 2021, 10, 2550. [Google Scholar] [CrossRef]
- Abela, G. The potential benefits and harms of early feeding post-surgery: A literature review. Int. Wound J. 2017, 14, 870–873. [Google Scholar] [CrossRef]
- Jones, N.F.; Jarrahy, R.; Song, J.I.; Kaufman, M.R.; Markowitz, B. Postoperative medical complications—Not microsurgical complications—Negatively influence the morbidity, mortality, and true costs after microsurgical reconstruction for head and neck cancer. Plast. Reconstr. Surg. 2007, 119, 2053–2060. [Google Scholar] [CrossRef]
- Jie, B.; Jiang, Z.M.; Nolan, M.T.; Zhu, S.N.; Yu, K.; Kondrup, J. Impact of preoperative nutritional support on clinical outcome in abdominal surgical patients at nutritional risk. Nutrition 2012, 28, 1022–1027. [Google Scholar] [CrossRef]
- Skipper, A.; Coltman, A.; Tomesko, J.; Charney, P.; Porcari, J.; Piemonte, T.A.; Cheng, F.W. Adult Malnutrition (Undernutrition) Screening: An Evidence Analysis Center Systematic Review. J. Acad. Nutr. Diet. 2020, 120, 669–708. [Google Scholar] [CrossRef]
- Smale, B.F.; Mullen, J.L.; Buzby, G.P.; Rosato, E.F. The efficacy of nutritional assessment and support in cancer surgery. Cancer 1981, 47, 2375–2381. [Google Scholar] [CrossRef]
- de Ulíbarri, J.I.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Toyokawa, T.; Kubo, N.; Tamura, T.; Sakurai, K.; Amano, R.; Tanaka, H.; Muguruma, K.; Yashiro, M.; Hirakawa, K.; Ohira, M. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: Results from a retrospective study. BMC Cancer 2016, 16, 722. [Google Scholar] [CrossRef]
- Unal, I. Defining an Optimal Cut-Point Value in ROC Analysis: An Alternative Approach. Comput. Math. Methods Med. 2017, 2017, 3762651. [Google Scholar] [CrossRef]
- Lee, S.C.; Lee, J.G.; Lee, S.H.; Kim, E.Y.; Chang, J.; Kim, D.J.; Paik, H.C.; Chung, K.Y.; Jung, J.Y. Prediction of postoperative pulmonary complications using preoperative controlling nutritional status (CONUT) score in patients with resectable non-small cell lung cancer. Sci. Rep. 2020, 10, 12385. [Google Scholar] [CrossRef]
- Lindeborg, M.M.; Sethi, R.K.V.; Puram, S.V.; Parikh, A.; Yarlagadda, B.; Varvares, M.; Emerick, K.; Lin, D.; Durand, M.L.; Deschler, D.G. Predicting length of stay in head and neck patients who undergo free flap reconstruction. Laryngoscope Investig. Otolaryngol. 2020, 5, 461–467. [Google Scholar] [CrossRef]
- O’Neill, A.C.; Mughal, M.; Saggaf, M.M.; Wisniewski, A.; Zhong, T.; Hofer, S.O.P. A structured pathway for accelerated postoperative recovery reduces hospital stay and cost of care following microvascular breast reconstruction without increased complications. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Iseki, Y.; Shibutani, M.; Maeda, K.; Nagahara, H.; Ohtani, H.; Sugano, K.; Ikeya, T.; Muguruma, K.; Tanaka, H.; Toyokawa, T.; et al. Impact of the Preoperative Controlling Nutritional Status (CONUT) Score on the Survival after Curative Surgery for Colorectal Cancer. PLoS ONE 2015, 10, e0132488. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Charlton, P. Anaesthesia for microvascular free tissue transfer. BJA CEPD Rev. 2003, 3, 33–37. [Google Scholar] [CrossRef]
- Felekis, D.; Eleftheriadou, A.; Papadakos, G.; Bosinakou, I.; Ferekidou, E.; Kandiloros, D.; Katsaragakis, S.; Charalabopoulos, K.; Manolopoulos, L. Effect of perioperative immuno-enhanced enteral nutrition on inflammatory response, nutritional status, and outcomes in head and neck cancer patients undergoing major surgery. Nutr. Cancer 2010, 62, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Pohlenz, P.; Klatt, J.; Schön, G.; Blessmann, M.; Li, L.; Schmelzle, R. Microvascular free flaps in head and neck surgery: Complications and outcome of 1000 flaps. Int. J. Oral Maxillofac. Surg. 2012, 41, 739–743. [Google Scholar] [CrossRef]
- Chiarelli, M.; Achilli, P.; Tagliabue, F.; Brivio, A.; Airoldi, A.; Guttadauro, A.; Porro, F.; Fumagalli, L. Perioperative lymphocytopenia predicts mortality and severe complications after intestinal surgery. Ann. Transl. Med. 2019, 7, 311. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Choi, C.H.; Sung, C.O.; Do, I.G.; Hub, S.J.; Kim, H.J.; Kim, T.J.; Lee, J.W.; Bae, D.S.; Kim, B.G. Clinical significance of changes in peripheral lymphocyte count after surgery in early cervical cancer. Gynecol. Oncol. 2012, 127, 107–113. [Google Scholar] [CrossRef]
- Vulliamy, P.E.; Perkins, Z.B.; Brohi, K.; Manson, J. Persistent lymphopenia is an independent predictor of mortality in critically ill emergency general surgical patients. Eur. J. Trauma Emerg. Surg. 2016, 42, 755–760. [Google Scholar] [CrossRef]
- Chen, T.; Delano, M.J.; Chen, K.; Sperry, J.L.; Namas, R.A.; Lamparello, A.J.; Deng, M.; Conroy, J.; Moldawer, L.L.; Efron, P.A.; et al. A road map from single-cell transcriptome to patient classification for the immune response to trauma. JCI Insight 2021, 6, e145108. [Google Scholar] [CrossRef]
- Edomskis, P.P.; Dik, W.A.; Sparreboom, C.L.; Nagtzaam, N.M.A.; van Oudenaren, A.; Lambrichts, D.P.V.; Bayon, Y.; van Dongen, N.N.N.; Menon, A.G.; de Graaf, E.J.R.; et al. Monocyte response after colorectal surgery: A prospective cohort study. Front. Immunol. 2022, 13, 1031216. [Google Scholar] [CrossRef]
- Klava, A.; Windsor, A.; Boylston, A.W.; Reynolds, J.V.; Ramsden, C.W.; Guillou, P.J. Monocyte activation after open and laparoscopic surgery. Br. J. Surg. 1997, 84, 1152–1156. [Google Scholar]
- Malietzis, G.; Johns, N.; Al-Hassi, H.O.; Knight, S.C.; Kennedy, R.H.; Fearon, K.C.; Aziz, O.; Jenkins, J.T. Low Muscularity and Myosteatosis Is Related to the Host Systemic Inflammatory Response in Patients Undergoing Surgery for Colorectal Cancer. Ann. Surg. 2016, 263, 320–325. [Google Scholar] [CrossRef]
- Pedrazzani, C.; Conti, C.; Zamboni, G.A.; Chincarini, M.; Turri, G.; Valdegamberi, A.; Guglielmi, A. Impact of visceral obesity and sarcobesity on surgical outcomes and recovery after laparoscopic resection for colorectal cancer. Clin. Nutr. 2020, 39, 3763–3770. [Google Scholar] [CrossRef]
- Ri, M.; Aikou, S.; Seto, Y. Obesity as a surgical risk factor. Ann. Gastroenterol. Surg. 2017, 2, 13–21. [Google Scholar] [CrossRef]
- Fischer, J.P.; Nelson, J.A.; Sieber, B.; Cleveland, E.; Kovach, S.J.; Wu, L.C.; Serletti, J.M.; Kanchwala, S. Free tissue transfer in the obese patient: An outcome and cost analysis in 1258 consecutive abdominally based reconstructions. Plast. Reconstr. Surg. 2013, 131, 681e–692e. [Google Scholar] [CrossRef]
- Sinha, S.; Ruskin, O.; D’Angelo, A.; McCombe, D.; Morrison, W.A.; Webb, A. Are overweight and obese patients who receive autologous free-flap breast reconstruction satisfied with their postoperative outcome? A single-centre study. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 30–36. [Google Scholar] [CrossRef]
- Chang, E.I.; Liu, J. Prospective Evaluation of Obese Patients Undergoing Autologous Abdominal Free Flap Breast Reconstruction. Plast. Reconstr. Surg. 2018, 142, 120e–125e. [Google Scholar] [CrossRef]
- de la Garza, G.; Militsakh, O.; Panwar, A.; Galloway, T.L.; Jorgensen, J.B.; Ledgerwood, L.G.; Kaiser, K.; Kitzerow, C.; Shnayder, Y.; Neumann, C.A.; et al. Obesity and perioperative complications in head and neck free tissue reconstruction. Head Neck 2016, 38 (Suppl. S1), E1188–E1191. [Google Scholar] [CrossRef]
- Crippen, M.M.; Brady, J.S.; Mozeika, A.M.; Eloy, J.A.; Baredes, S.; Park, R.C.W. Impact of Body Mass Index on Operative Outcomes in Head and Neck Free Flap Surgery. Otolaryngol. Head Neck Surg. 2018, 159, 817–823. [Google Scholar] [CrossRef]
- Popkin, B.M.; Corvalan, C.; Grummer-Strawn, L.M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 2020, 395, 65–74. [Google Scholar] [CrossRef]
- Wells, J.C.; Sawaya, A.L.; Wibaek, R.; Mwangome, M.; Poullas, M.S.; Yajnik, C.S.; Demaio, A. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet 2020, 395, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.C.; Corkins, M.R.; Malone, A.; Miller, S.; Mogensen, K.M.; Guenter, P. The Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr. Clin. Pract. 2021, 36, 22–28. [Google Scholar] [CrossRef] [PubMed]
| Variable | Undernutrition Degree | |||
|---|---|---|---|---|
| Normal | Mild | Moderate | Severe | |
| Serum albumin (g/dL) | ≥3.50 | 3.00–3.49 | 2.50–2.99 | <2.50 |
| Score | 0 | 2 | 4 | 6 |
| Total lymphocyte count (/mm3) | ≥1600 | 1200–1599 | 800–1199 | <800 |
| Score | 0 | 1 | 2 | 3 |
| Total cholesterol (mg/dL) | ≥180 | 140–179 | 100–139 | <100 |
| Score | 0 | 1 | 2 | 3 |
| Patient Group | Overall n = 72 | No Complications n = 61 | True Flap Loss n = 4 | Any Flap Complications n = 11 | p-Value |
|---|---|---|---|---|---|
| Demographical data | |||||
| Mean age, years | 55.3 (51.5–59.1) | 56.9 (61.0–65.4) | 65.0 (63.5–66.5) | 49.6 (37.7–56.1) | 0.057 |
| Sex (female), n (%) | 32 (44.4%) | 25 (40.1%) | 2 (50.0%) | 5 (45.5%) | 0.418 |
| Area of reconstruction | |||||
| Extremity, n (%) | 15 (20.8%) | 12 (19.6%) | - | 3 (27.3%) | 0.289 |
| ENT, n (%) | 26 (36.1%) | 22 (36.1%) | 2 (50.0%) | 4 (36.4%) | 0.496 |
| Head and neck, n (%) | 16 (22.2%) | 14 (30.0%) | 1 (25.0%) | 2 (18.2%) | 0.322 |
| Breast, n (%) | 15 (20.8%) | 13 (21.3%) | 1 (25.0%) | 2 (18.2%) | 0.457 |
| Microvascular flap type | |||||
| ALT, (%) | 32 (44.4%) | 27 (44.3%) | 2 (50.0%) | 5 (45.5%) | 0.828 |
| Fibular flap, (%) | 9 (12.5%) | 8 (13.1%) | 1 (25.0%) | 1 (9.1%) | 0.478 |
| DIEP, n (%) | 9 (12.5%) | 7 (11.5%) | - | 2 (18.2%) | 0.528 |
| Radial artery flap, n (%) | 6 (8.3%) | 6 (9.8%) | - | - | - |
| Other, n (%) | 16 (22.2%) | 13 (21.3%) | 1 (25.0%) | 3 (27.3%) | 0.413 |
| Indication for surgery | |||||
| Trauma, n (%) | 8 (11.1%) | 6 (10.1%) | - | 1 (9.1%) | 0.918 |
| Oncology, n (%) | 40 (55.6%) | 32 (58.2%) | 3 (75.0%) | 6 (54.5%) | 0.469 |
| Defect, n (%) | 19 (26.4%) | 11 (20.0%) | 1 (25.0%) | 4 (36.4%) | 0.511 |
| Infection, n (%) | 5 (6.9%) | 5 (8.2%) | - | - | - |
| Comorbidities | |||||
| Coronary artery disease, n (%) | 4 (5.6%) | 3 (4.9%) | 1 (25.0%) | 1 (9.1%) | 0.059 |
| Diabetes mellitus, n (%) | 5 (6.9%) | 4 (6.6%) | - | 1 (9.1%) | 0.691 |
| Hypertension, n (%) | 28 (38.8%) | 19 (31.1%) | 3 (75.0%) | 6 (54.5%) | 0.133 |
| Dyslipidemia, n (%) | 16 (22.2%) | 13 (21.3%) | 1 (25.0%) | 3 (27.3%) | 0.624 |
| Smoking history, n (%) | 13 (18.1%) | 11 (18.0%) | 1 (25.0%) | 2 (18.2%) | 0.249 |
| Obesity (BMI > 30 kg/m2), n (%) | 12 (16.6%) | 8 (13.1%) | 2 (50.0%) | 5 (45.5%) | 0.010 ** |
| Cerebrovascular accident, n (%) | 4 (5.6%) | 4 (6.6%) | - | - | 0.620 |
| Patient Group | Overall n = 72 | No Complications n = 61 | True Flap Loss n = 4 | Any Flap Complications n = 11 | p-Value |
|---|---|---|---|---|---|
| Duration of surgery, hours | 6.39 (5.75–7.02) | 6.33 (5.59–7.07) | 7.63 (5.86–9.39) | 6.66 (5.29–8.04) | 0.235 |
| Volume of intraoperative crystalloid, mL | 2345.83 (2141.39–2550.28) | 2352.50 (2133.31–2571.69) | 2875.00 (1681.58–4068.42) | 2312.50 (1608.14–3016.86) | 0.145 |
| Volume of intraoperative colloid, mL | 506.25 (401.74–610.76) | 482.50 (367.10–597.90) | 500.00 (-) | 625.00 (329.42–920.58) | 0.471 |
| Intraoperative colloid to crystalloid ratio | 0.22 (0.17–0.27) | 0.20 (0.15–0.25) | 0.18 (0.10–0.27) | 0.33 (0.09–0.56) | 0.306 |
| Intraoperative hematocrit, % | 30.60 (29.20–32.00) | 29.58 (27.70–31.45) | 31.50 (25.15–37.85) | 34.40 (30.32–38.48) | 0.009 * |
| Use of vasopressors/sympathomimetics, n (%) | 41 (56.90%) | 36 (59.00%) | 2 (50.00%) | 6 (54.50%) | 0.549 |
| Patient Group | Overall n = 72 | No Complications n = 61 | Any Flap Complications n = 11 | p-Value |
|---|---|---|---|---|
| Biomarkers | ||||
| Lymphocyte count 109/L | 1.59 (1.39–1.79) | 1.71 (1.49–1.92) | 0.97 (0.67–1.26) | 0.001 * |
| Monocyte count 109/L | 0.55 (0.48–0.62) | 0.58 (0.51–0.66) | 0.37 (0.22–0.51) | 0.021 * |
| Lymphocyte/monocyte ratio | 3.46 (2.91–4.02) | 3.55 (2.90–4.20) | 2.97 (2.28–3.65) | 0.830 |
| Mean plasma albumin, g/dL | 3.94 (3.81–4.06) | 3.96 (3.84–4.09) | 3.79 (3.28–4.30) | 0.631 |
| Mean total plasma cholesterol, mg/dL | 196.58 (185.21–207.95) | 198.44 (186.43–210.45) | 186.73 (147.93–225.53) | 0.310 |
| Nutritional assessment systems | ||||
| CONUT score | 2(2) | 2 (3) | 3 (6) | 0.013 * |
| CONUT ≤ 2 | 50 (69.4%) | 46 (75.4%) | 4 (36.4%) | 0.009 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocans, R.P.; Zarins, J.; Bine, E.; Deksnis, R.; Citovica, M.; Donina, S.; Mamaja, B. The Controlling Nutritional Status (CONUT) Score for Prediction of Microvascular Flap Complications in Reconstructive Surgery. J. Clin. Med. 2023, 12, 4794. https://doi.org/10.3390/jcm12144794
Rocans RP, Zarins J, Bine E, Deksnis R, Citovica M, Donina S, Mamaja B. The Controlling Nutritional Status (CONUT) Score for Prediction of Microvascular Flap Complications in Reconstructive Surgery. Journal of Clinical Medicine. 2023; 12(14):4794. https://doi.org/10.3390/jcm12144794
Chicago/Turabian StyleRocans, Rihards P., Janis Zarins, Evita Bine, Renars Deksnis, Margarita Citovica, Simona Donina, and Biruta Mamaja. 2023. "The Controlling Nutritional Status (CONUT) Score for Prediction of Microvascular Flap Complications in Reconstructive Surgery" Journal of Clinical Medicine 12, no. 14: 4794. https://doi.org/10.3390/jcm12144794
APA StyleRocans, R. P., Zarins, J., Bine, E., Deksnis, R., Citovica, M., Donina, S., & Mamaja, B. (2023). The Controlling Nutritional Status (CONUT) Score for Prediction of Microvascular Flap Complications in Reconstructive Surgery. Journal of Clinical Medicine, 12(14), 4794. https://doi.org/10.3390/jcm12144794







