Differential Association of Sex Hormones with Metabolic Parameters and Body Composition in Men and Women from the United States
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory and Clinical Data
2.2. Sex Hormone Measurements
2.3. Whole Body DXA
2.4. Statistical Analysis
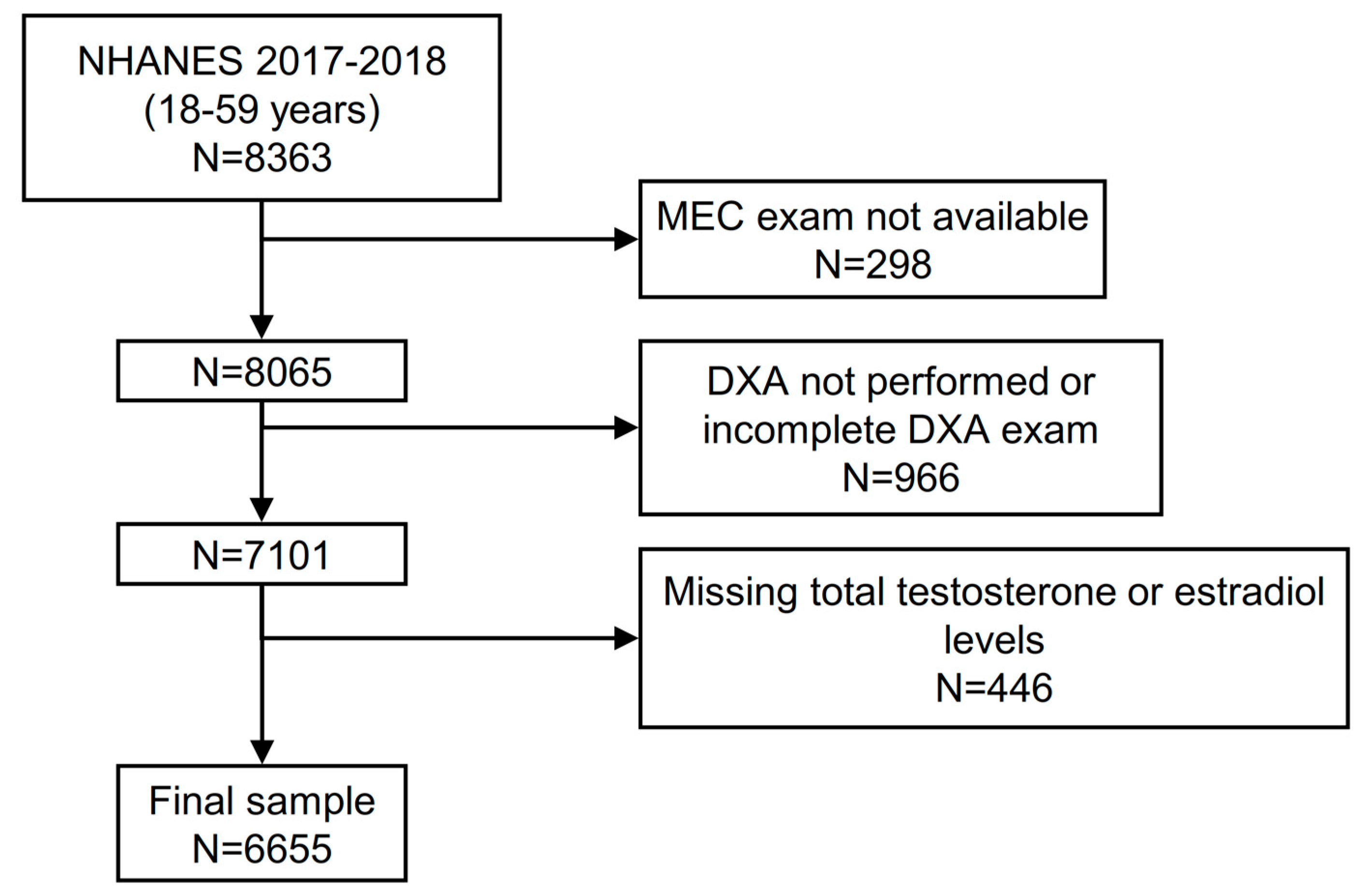
2.5. Analysis Sample
3. Results
3.1. Features of Participants According to Testosterone Levels
3.2. Independent Predictors of Body Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciardullo, S.; Oltolini, A.; Cannistraci, R.; Muraca, E.; Perseghin, G. Sex-related association of NAFLD and liver fibrosis with body fat distribution in the general US population. Am. J. Clin. Nutr. 2022, 115, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, S.; Perseghin, G. Prevalence of elevated liver stiffness in patients with type 1 and type 2 diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2022, 190, 109981. [Google Scholar] [CrossRef] [PubMed]
- Veilleux, A.; Tchernof, A. Sex differences in body fat distribution. In Adipose Tissue Biology; Springer: New York, NY, USA, 2012; pp. 123–166. [Google Scholar]
- Lemieux, S.; Prud’Homme, D.; Bouchard, C.; Tremblay, A.A.; Després, J.P. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am. J. Clin. Nutr. 1993, 58, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Van Loan, M. Total Body Composition: Birth to Old Age. Human Body Composition 1996. Available online: https://us.humankinetics.com/products/human-body-composition-2nd-edition?_pos=1&_sid=c5ddbda9d&_ss=r (accessed on 10 April 2023).
- Wells, J.C. Sexual dimorphism of body composition. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 415–430. [Google Scholar] [CrossRef]
- Kang, S.M.; Yoon, J.W.; Ahn, H.Y.; Kim, S.Y.; Lee, K.H.; Shin, H.; Choi, S.H.; Park, K.S.; Jang, H.C.; Lim, S. Android Fat Depot Is More Closely Associated with Metabolic Syndrome than Abdominal Visceral Fat in Elderly People. PLoS ONE 2011, 6, e27694. [Google Scholar] [CrossRef]
- Blouin, K.; Boivin, A.; Tchernof, A. Androgens and body fat distribution. J. Steroid Biochem. Mol. Biol. 2008, 108, 272–280. [Google Scholar] [CrossRef]
- Tchernof, A.; Brochu, D.; Maltais-Payette, I.; Mansour, M.F.; Marchand, G.B.; Carreau, A.; Kapeluto, J. Androgens and the Regulation of Adiposity and Body Fat Distribution in Humans. Compr. Physiol. 2011, 8, 1253–1290. [Google Scholar] [CrossRef]
- Taylor, A.E.; Keevil, B.; Huhtaniemi, I.T. Mass spectrometry and immunoassay: How to measure steroid hormones today and tomorrow. Eur. J. Endocrinol. 2015, 173, D1–D12. [Google Scholar] [CrossRef]
- Büttler, R.M.; Martens, F.; Fanelli, F.; Pham, H.T.; Kushnir, M.M.; Janssen, M.J.; Owen, L.; Taylor, A.E.; Soeborg, T.; Blankenstein, M.A.; et al. Comparison of 7 Published LC-MS/MS Methods for the Simultaneous Measurement of Testosterone, Androstenedione, and Dehydroepiandrosterone in Serum. Clin. Chem. 2015, 61, 1475–1483. [Google Scholar] [CrossRef]
- Gasperino, J. Ethnic differences in body composition and their relation to health and disease in women. Ethn. Health 1996, 1, 337–347. [Google Scholar] [CrossRef]
- Winters, S.J.; Brufsky, A.; Weissfeld, J.; Trump, D.L.; Dyky, M.A.; Hadeed, V. Testosterone, sex hormone-binding globulin, and body composition in young adult African American and Caucasian men. Metabolism 2001, 50, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. 2017: National Health and Nutrition Examination Survey (NHANES). U.S. Department of Health and Human Services. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2017 (accessed on 10 April 2023).
- Ciardullo, S.; Muraca, E.; Zerbini, F.; Manzoni, G.; Perseghin, G. NAFLD and Liver Fibrosis Are Not Associated with Reduced Femoral Bone Mineral Density in the General US Population. J. Clin. Endocrinol. Metab. 2021, 106, e2856–e2865. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43 (Suppl. S1), S14–S31. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. 2017: National Health and Nutrition Examination Survey (NHANES). U.S. Department of Health and Human Services. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals/2017_MEC_Laboratory_Procedures_Manual.pdf (accessed on 31 March 2020).
- Ciardullo, S.; Perseghin, G. Statin use is associated with lower prevalence of advanced liver fibrosis in patients with type 2 diabetes. Metabolism 2021, 121, 154752. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Rosner, W.; Auchus, R.J.; Azziz, R.; Sluss, P.M.; Raff, H. Utility, Limitations, and Pitfalls in Measuring Testosterone: An Endocrine Society Position Statement. J. Clin. Endocrinol. Metab. 2006, 92, 405–413. [Google Scholar] [CrossRef]
- Ly, L.P.; Handelsman, D.J. Empirical estimation of free testosterone from testosterone and sex hormone-binding globulin immunoassays. Eur. J. Endocrinol. 2005, 152, 471–478. [Google Scholar] [CrossRef]
- Ly, L.P.; Sartorius, G.; Hull, L.; Leung, A.; Swerdloff, R.S.; Wang, C.; Handelsman, D.J. ORIGINAL ARTICLE: Accuracy of calculated free testosterone formulae in men. Clin. Endocrinol. 2010, 73, 382–388. [Google Scholar] [CrossRef]
- Kissebah, A.H.; Krakower, G.R. Regional adiposity and morbidity. Physiol. Rev. 1994, 74, 761–811. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.A.; Fan, B.; Lu, Y.; Wu, X.P.; Wacker, W.K.; Ergun, D.L.; Levine, M.A. A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J. Bone Miner. Res. 2012, 27, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Travison, T.G.; Vesper, H.W.; Orwoll, E.; Wu, F.; Kaufman, J.M.; Wang, Y.; Lapauw, B.; Fiers, T.; Matsumoto, A.M.; Bhasin, S. Harmonized Reference Ranges for Circulating Testosterone Levels in Men of Four Cohort Studies in the United States and Europe. J. Clin. Endocrinol. Metab. 2017, 102, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C. Definition of Metabolic Syndrome. Arter. Thromb. Vasc. Biol. 2004, 24, e13–e18. [Google Scholar] [CrossRef] [PubMed]
- Blouin, K.; Després, J.-P.; Couillard, C.; Tremblay, A.; Prud’Homme, D.; Bouchard, C.; Tchernof, A. Contribution of age and declining androgen levels to features of the metabolic syndrome in men. Metabolism 2005, 54, 1034–1040. [Google Scholar] [CrossRef]
- Gapstur, S.M.; Gann, P.H.; Kopp, P.; Colangelo, L.; Longcope, C.; Liu, K. Serum androgen concentrations in young men: A longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1041–1047. [Google Scholar]
- Laaksonen, D.E.; Niskanen, L.; Punnonen, K.; Nyyssönen, K.; Tuomainen, T.-P.; Valkonen, V.-P.; Salonen, R.; Salonen, J.T. Testosterone and Sex Hormone–Binding Globulin Predict the Metabolic Syndrome and Diabetes in Middle-Aged Men. Diabetes Care 2004, 27, 1036–1041. [Google Scholar] [CrossRef]
- Pasquali, R.; Casimirri, F.; Cantobelli, S.; Melchionda, N.; Labate, A.M.M.; Fabbri, R.; Capelli, M.; Bortoluzzi, L. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism 1991, 40, 101–104. [Google Scholar] [CrossRef]
- Grossmann, M.; Fui, M.T.; Dupuis, P. Lowered testosterone in male obesity: Mechanisms, morbidity and management. Asian J. Androl. 2014, 16, 223–231. [Google Scholar] [CrossRef]
- Wang, C.; Eyre, D.R.; Clark, R.; Kleinberg, D.; Newman, C.; Iranmanesh, A.; Veldhuis, J.; Dudley, R.E.; Berman, N.; Davidson, T.; et al. Sublingual testosterone replacement improves muscle mass and strength, decreases bone resorption, and increases bone formation markers in hypogonadal men—A clinical research center study. J. Clin. Endocrinol. Metab. 1996, 81, 3654–3662. [Google Scholar] [CrossRef]
- Corona, G.; Giagulli, V.A.; Maseroli, E.; Vignozzi, L.; Aversa, A.; Zitzmann, M.; Saad, F.; Mannucci, E.; Maggi, M. Therapy of Endocrine Disease: Testosterone supplementation and body composition: Results from a meta-analysis study. Eur. J. Endocrinol. 2016, 174, R99–R116. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.; Beebe, J.; McConnell, D.; Randolph, J.; Jannausch, M. Testosterone concentrations in women aged 25–50 years: Associations with lifestyle, body composition, and ovarian status. Am. J. Epidemiol. 2001, 153, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Walker, K.Z.; Strauss, B.J.G. Effects of estradiol with and without testosterone on body composition and relationships with lipids in postmenopausal women. Menopause 2000, 7, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Rađenović, S.S.; Pupovac, M.; Andjić, M.; Bila, J.; Srećković, S.; Gudović, A.; Dragaš, B.; Radunović, N. Prevalence, Risk Factors, and Pathophysiology of Nonalcoholic Fatty Liver Disease (NAFLD) in Women with Polycystic Ovary Syndrome (PCOS). Biomedicines 2022, 10, 131. [Google Scholar] [CrossRef]
- Arefhosseini, S.; Ebrahimi-Mameghani, M.; Najafipour, F.; Tutunchi, H. Non-alcoholic fatty liver disease across endocrinopathies: Interaction with sex hormones. Front. Endocrinol. 2022, 13, 1032361. [Google Scholar] [CrossRef]
- Marchand, G.B.; Carreau, A.-M.; Weisnagel, S.J.; Bergeron, J.; Labrie, F.; Lemieux, S.; Tchernof, A. Increased body fat mass explains the positive association between circulating estradiol and insulin resistance in postmenopausal women. Am. J. Physiol. Metab. 2018, 314, E448–E456. [Google Scholar] [CrossRef]
- Del Ghianda, S.; Tonacchera, M.; Vitti, P. Thyroid and menopause. Climacteric 2014, 17, 225–234. [Google Scholar] [CrossRef]
- Marlatt, K.L.; Redman, L.M.; Beyl, R.A.; Smith, S.R.; Champagne, C.M.; Yi, F.; Lovejoy, J.C. Racial differences in body composition and cardiometabolic risk during the menopause transition: A prospective, observational cohort study. Am. J. Obstet. Gynecol. 2020, 222, 365.e1–365.e18. [Google Scholar] [CrossRef]
| Total Testosterone Quartiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 (<310 ng/dL) | Q2 (<403 ng/dL) | Q3 (<530 ng/dL) | Q4 (≥530 ng/dL) | p-Trend | |||||
| n or Mean | % or SE | n or Mean | % or SE | n or Mean | % or SE | n or Mean | % or SE | ||
| Age (years) | 40.6 | (0.5) | 39.2 | (0.5) | 38.0 | (0.6) | 35.6 | (0.6) | <0.001 |
| BMI (kg/m2) | 32.0 | (0.3) | 29.4 | (0.2) | 27.6 | (0.2) | 25.6 | (0.2) | <0.001 |
| Diabetes | 100 | 12.1% | 57 | 6.9% | 54 | 6.6% | 43 | 5.2% | <0.001 |
| Race–ethnicity (%) | 0.091 | ||||||||
| Non-Hispanic white | 523 | 63.0% | 518 | 62.8% | 526 | 63.5% | 482 | 58.3% | |
| Hispanic | 156 | 18.8% | 155 | 18.8% | 144 | 17.4% | 148 | 18.0% | |
| Non-Hispanic black | 72 | 8.6% | 83 | 10.0% | 86 | 10.4% | 113 | 13.7% | |
| Non-Hispanic Asian | 46 | 5.5% | 45 | 5.4% | 47 | 5.7% | 42 | 5.1% | |
| Other | 34 | 4.1% | 24 | 3.0% | 25 | 3.0% | 41 | 5.0% | |
| Fatty liver index | <0.001 | ||||||||
| <30 | 77 | 9.4% | 163 | 19.9% | 277 | 33.8% | 446 | 54.1% | |
| 30–60 | 108 | 13.1% | 175 | 21.4% | 219 | 26.7% | 179 | 21.7% | |
| >60 | 635 | 77.4% | 480 | 58.7% | 323 | 39.5% | 199 | 24.2% | |
| Cigarette smoker | <0.001 | ||||||||
| Never | 456 | 54.9% | 484 | 58.7% | 459 | 55.4% | 397 | 48.0% | |
| Former | 223 | 26.9% | 172 | 20.9% | 171 | 20.6% | 173 | 21.0% | |
| Current | 151 | 18.2% | 169 | 20.4% | 199 | 24.0% | 256 | 31.0% | |
| Testosterone (ng/dL) | 241.1 | (2.2) | 356.1 | (1.1) | 464.3 | (1.6) | 680.6 | (6.2) | <0.001 |
| SHBG (nmol/L) | 25.4 | (0.4) | 32.4 | (0.7) | 40.0 | (0.8) | 52.8 | (1.0) | <0.001 |
| Free testosterone (pmol/L) | 115.4 | (1.6) | 185.8 | (1.3) | 245.8 | (1.7) | 351.6 | (4.5) | <0.001 |
| Estradiol (pg/mL) | 21.5 | (0.4) | 22.6 | (0.3) | 24.9 | (0.3) | 29.9 | (0.5) | <0.001 |
| SBP (mmHg) | 124.4 | (0.6) | 122.4 | (0.6) | 119.7 | (0.6) | 119.8 | (0.6) | <0.001 |
| DBP (mmHg) | 74.2 | (0.5) | 72.8 | (0.4) | 71.3 | (0.4) | 70.3 | (0.5) | <0.001 |
| FPG (mg/dL) | 115.2 | (0.9) | 106.9 | (0.8) | 104.4 | (0.8) | 102.5 | (0.8) | <0.001 |
| Insulin (uU/mL) | 20.4 | (0.5) | 16.9 | (0.7) | 11.5 | (0.5) | 8.5 | (0.3) | <0.001 |
| HOMA-IR | 6.2 | (0.2) | 4.9 | (0.3) | 3.1 | (0.1) | 2.2 | (0.1) | <0.001 |
| Total cholesterol (mg/dL) | 196.2 | (1.8) | 191.3 | (2.3) | 187.6 | (1.7) | 183.9 | (1.7) | <0.001 |
| Triglycerides (mg/dL) | 238.7 | (7.3) | 195.9 | (13.9) | 149.0 | (4.4) | 117.6 | (5.0) | <0.001 |
| DXA parameters | |||||||||
| Trunk percent fat | 31.7 | (0.3) | 29.1 | (0.3) | 26.2 | (0.3) | 23.1 | (0.3) | <0.001 |
| Lean mass (g) | 61,977.8 | (433.9) | 57,983.8 | (384.5) | 55,659.1 | (327.6) | 53,939.1 | (417.3) | <0.001 |
| Percent fat | 30.9 | (0.2) | 28.5 | (0.2) | 26.1 | (0.2) | 23.7 | (0.3) | <0.001 |
| Android fat mass (g) | 3276.0 | (66.3) | 2621.9 | (55.1) | 2162.6 | (57.6) | 1710.7 | (49.0) | <0.001 |
| Gynoid fat mass (g) | 4921.3 | (82.0) | 4247.6 | (60.6) | 3792.5 | (64.9) | 3311.8 | (63.6) | <0.001 |
| Android to gynoid ratio | 1.2 | (0.0) | 1.1 | (0.0) | 1.1 | (0.0) | 1.0 | (0.0) | <0.001 |
| Android percent fat | 36.2 | (0.3) | 33.4 | (0.3) | 30.1 | (0.4) | 26.4 | (0.4) | <0.001 |
| Gynoid percent fat | 31.2 | (0.2) | 29.7 | (0.2) | 28.1 | (0.2) | 26.1 | (0.3) | <0.001 |
| Visceral adipose tissue volume | 754.0 | (14.4) | 614.4 | (13.4) | 529.3 | (12.9) | 435.9 | (12.3) | <0.001 |
| Subcutaneous fat volume | 1885.2 | (35.3) | 1574.5 | (30.0) | 1293.4 | (31.4) | 1019.3 | (28.3) | <0.001 |
| Percent lean | 66.8 | (0.2) | 69.0 | (0.2) | 71.2 | (0.2) | 73.5 | (0.3) | <0.001 |
| Total Testosterone Quartiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 (<14.7 ng/dL) | Q2 (<21.1 ng/dL) | Q3 (<29.4 ng/dL) | Q4 (≥29.4 ng/dL) | p-Trend | |||||
| n or Mean | % or SD | n or Mean | % or SD | n or Mean | % or SD | n or Mean | % or SD | ||
| Age (years) | 44.7 | (0.5) | 41.1 | (0.5) | 37.5 | (0.5) | 33.8 | (0.5) | <0.001 |
| BMI (kg/m2) | 29.1 | (0.3) | 29.7 | (0.3) | 29.3 | (0.3) | 29.1 | (0.3) | 0.735 |
| Diabetes | 106 | 12.6% | 59 | 7.1% | 47 | 5.6% | 24 | 2.9% | <0.001 |
| Race–ethnicity | 0.208 | ||||||||
| Non-Hispanic white | 496 | 58.8% | 523 | 62.7% | 514 | 61.5% | 519 | 62.4% | |
| Hispanic | 149 | 17.7% | 149 | 17.9% | 154 | 18.4% | 130 | 15.6% | |
| Non-Hispanic black | 109 | 12.9% | 89 | 10.7% | 96 | 11.5% | 106 | 12.7% | |
| Non-Hispanic Asian | 60 | 7.2% | 47 | 5.6% | 48 | 5.8% | 37 | 4.4% | |
| Other | 29 | 3.4% | 26 | 3.1% | 24 | 2.9% | 40 | 4.8% | |
| Fatty liver index | 0.019 | ||||||||
| <30 | 309 | 37.5% | 344 | 41.6% | 374 | 45.1% | 406 | 49.6% | |
| 30–60 | 160 | 19.5% | 141 | 17.1% | 141 | 17.0% | 135 | 16.5% | |
| >60 | 355 | 43.1% | 340 | 41.2% | 315 | 37.9% | 278 | 33.9% | |
| Cigarette smoker | 0.007 | ||||||||
| Never | 557 | 66.1% | 545 | 65.3% | 558 | 66.7% | 507 | 60.9% | |
| Former | 138 | 16.4% | 146 | 17.5% | 120 | 14.4% | 102 | 12.3% | |
| Current | 148 | 17.5% | 143 | 17.2% | 158 | 18.9% | 223 | 26.8% | |
| Testosterone (ng/dL) | 10.8 | (0.1) | 18.0 | (0.1) | 24.8 | (0.1) | 47.4 | (2.2) | <0.001 |
| SHBG (nmol/L) | 62.6 | (2.0) | 74.2 | (2.6) | 78.2 | (2.5) | 88.0 | (2.8) | <0.001 |
| Free testosterone (pmol/L) | 1.9 | (0.1) | 5.1 | (0.1) | 7.9 | (0.1) | 15.0 | (0.7) | <0.001 |
| Estradiol (pg/mL) | 36.1 | (2.1) | 62.7 | (3.2) | 88.6 | (3.9) | 113.4 | (5.7) | <0.001 |
| SBP (mmHg) | 119.2 | (0.7) | 116.9 | (0.6) | 114.6 | (0.6) | 113.8 | (0.6) | <0.001 |
| DBP (mmHg) | 72.0 | (0.4) | 70.3 | (0.4) | 69.2 | (0.4) | 68.1 | (0.4) | <0.001 |
| FPG (mg/dL) | 110.4 | (1.2) | 101.8 | (0.6) | 100.5 | (0.7) | 95.7 | (0.4) | <0.001 |
| Insulin (uU/mL) | 12.7 | (0.3) | 12.4 | (0.3) | 11.6 | (0.4) | 10.8 | (0.2) | 0.040 |
| HOMA-IR | 3.8 | (0.1) | 3.3 | (0.1) | 3.1 | (0.2) | 2.6 | (0.1) | 0.009 |
| Total cholesterol (mg/dL) | 195.0 | (2.2) | 193.6 | (1.6) | 184.8 | (1.8) | 187.7 | (1.7) | 0.002 |
| Triglycerides (mg/dL) | 153.8 | (4.7) | 131.4 | (3.7) | 122.3 | (6.7) | 112.7 | (3.4) | <0.001 |
| DXA parameters | |||||||||
| Trunk percent fat | 36.8 | (0.3) | 37.1 | (0.3) | 36.2 | (0.4) | 35.8 | (0.3) | 0.019 |
| Lean mass (g) | 40,828.0 | (356.6) | 41,641.4 | (347.0) | 41,463.6 | (346.4) | 41,434.4 | (327.9) | 0.195 |
| Percent fat | 39.5 | (0.3) | 40.1 | (0.3) | 39.4 | (0.3) | 39.2 | (0.3) | 0.228 |
| Android fat mass (g) | 2529.7 | (62.4) | 2608.0 | (58.7) | 2502.6 | (62.2) | 2491.6 | (59.2) | 0.419 |
| Gynoid fat mass (g) | 5206.1 | (83.0) | 5543.4 | (85.9) | 5559.4 | (87.9) | 5523.8 | (83.7) | 0.018 |
| Android to gynoid ratio | 0.9 | (0.0) | 0.9 | (0.0) | 0.9 | (0.0) | 0.9 | (0.0) | 0.040 |
| Android percent fat | 38.4 | (0.4) | 39.3 | (0.4) | 38.4 | (0.4) | 38.4 | (0.4) | 0.614 |
| Gynoid percent fat | 41.8 | (0.2) | 42.7 | (0.2) | 42.5 | (0.2) | 42.3 | (0.2) | 0.393 |
| Visceral adipose tissue volume | 574.0 | (15.8) | 543.2 | (14.9) | 489.9 | (14.4) | 456.3 | (12.5) | <0.001 |
| Subcutaneous fat volume | 2055.2 | (36.3) | 2158.7 | (36.3) | 2111.4 | (39.3) | 2134.0 | (38.8) | 0.331 |
| Percent lean | 58.2 | (0.3) | 57.6 | (0.2) | 58.3 | (0.3) | 58.5 | (0.3) | 0.272 |
| Lean Mass (%) | Fat Mass (%) | Android/Gynoid Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | p-Value | B | 95% CI | p-Value | B | 95% CI | p-Value | |
| Age (years) | −0.04 | −0.05–−0.02 | <0.001 | 0.04 | 0.02–0.06 | <0.001 | 0.00 | 0.00–0.01 | <0.001 |
| BMI (kg/m2) | −0.65 | −0.70–−0.61 | <0.001 | 0.71 | 0.67–0.76 | <0.001 | 0.01 | 0.01–0.01 | <0.001 |
| Race–ethnicity | |||||||||
| Non-Hispanic white | Ref | Ref | Ref | ||||||
| Hispanic | 0.03 | −0.34–0.40 | 0.871 | −0.01 | −0.41–0.39 | 0.969 | 0.05 | 0.03–0.06 | <0.001 |
| Non-Hispanic black | 2.77 | 2.30–3.24 | <0.001 | −3.00 | −3.48–−2.52 | <0.001 | −0.03 | −0.05–−0.01 | 0.005 |
| Non-Hispanic Asian | −1.26 | −1.84–−0.68 | <0.001 | 1.36 | 0.76–1.95 | <0.001 | 0.07 | 0.04–0.09 | <0.001 |
| Other | 0.73 | −0.19–1.65 | 0.118 | −0.79 | −1.76–0.17 | 0.102 | −0.02 | −0.05–0.01 | 0.185 |
| Testosterone (ng/dL) | 0.01 | 0.01–0.01 | <0.001 | −0.01 | −0.01–−0.01 | <0.001 | −0.00 | −0.00–−0.00 | <0.001 |
| Estradiol (pg/mL) | −0.06 | −0.09–−0.04 | <0.001 | 0.06 | 0.03–0.09 | <0.001 | 0.00 | 0.00–0.00 | 0.003 |
| R2 | 0.59 | 0.62 | 0.38 | ||||||
| Lean Mass (%) | Fat Mass (%) | Android/Gynoid Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95% CI | p-Value | B | 95% CI | p-Value | B | 95% CI | p-Value | |
| Age (years) | −0.05 | −0.07–−0.04 | <0.001 | 0.06 | 0.04–0.08 | <0.001 | 0.00 | 0.00–0.00 | 0.003 |
| BMI (kg/m2) | −0.62 | −0.65–−0.59 | <0.001 | 0.68 | 0.65–0.71 | <0.001 | 0.01 | 0.01–0.01 | <0.001 |
| Race–ethnicity | |||||||||
| Non-Hispanic white | Ref | Ref | Ref | ||||||
| Hispanic | −0.63 | −1.15–−0.10 | 0.021 | 0.70 | 0.16–1.25 | 0.012 | 0.04 | 0.03–0.05 | <0.001 |
| Non-Hispanic black | 0.97 | 0.47–1.46 | <0.001 | −1.07 | −1.58–−0.56 | <0.001 | −0.01 | −0.02–0.01 | 0.316 |
| Non-Hispanic Asian | −1.00 | −1.56–−0.43 | 0.001 | 1.07 | 0.48–1.65 | 0.001 | 0.07 | 0.05–0.09 | <0.001 |
| Other | 0.57 | −0.42–1.57 | 0.250 | −0.61 | −1.65–0.44 | 0.244 | 0.02 | −0.00–0.05 | 0.050 |
| Testosterone, total (ng/dL) | 0.00 | −0.00–0.01 | 0.369 | −0.00 | −0.01–0.01 | 0.408 | 0.00 | −0.00–0.00 | 0.486 |
| Estradiol (pg/mL) | 0.00 | 0.00–0.01 | 0.003 | −0.00 | −0.01–−0.00 | 0.001 | −0.00 | −0.00–0.00 | 0.183 |
| R2 | 0.59 | 0.62 | 0.39 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciardullo, S.; Zerbini, F.; Cannistraci, R.; Muraca, E.; Perra, S.; Oltolini, A.; Perseghin, G. Differential Association of Sex Hormones with Metabolic Parameters and Body Composition in Men and Women from the United States. J. Clin. Med. 2023, 12, 4783. https://doi.org/10.3390/jcm12144783
Ciardullo S, Zerbini F, Cannistraci R, Muraca E, Perra S, Oltolini A, Perseghin G. Differential Association of Sex Hormones with Metabolic Parameters and Body Composition in Men and Women from the United States. Journal of Clinical Medicine. 2023; 12(14):4783. https://doi.org/10.3390/jcm12144783
Chicago/Turabian StyleCiardullo, Stefano, Francesca Zerbini, Rosa Cannistraci, Emanuele Muraca, Silvia Perra, Alice Oltolini, and Gianluca Perseghin. 2023. "Differential Association of Sex Hormones with Metabolic Parameters and Body Composition in Men and Women from the United States" Journal of Clinical Medicine 12, no. 14: 4783. https://doi.org/10.3390/jcm12144783
APA StyleCiardullo, S., Zerbini, F., Cannistraci, R., Muraca, E., Perra, S., Oltolini, A., & Perseghin, G. (2023). Differential Association of Sex Hormones with Metabolic Parameters and Body Composition in Men and Women from the United States. Journal of Clinical Medicine, 12(14), 4783. https://doi.org/10.3390/jcm12144783






