Identification of Pacemaker Lead Position Using Fluoroscopy to Avoid Significant Tricuspid Regurgitation
Abstract
1. Introduction
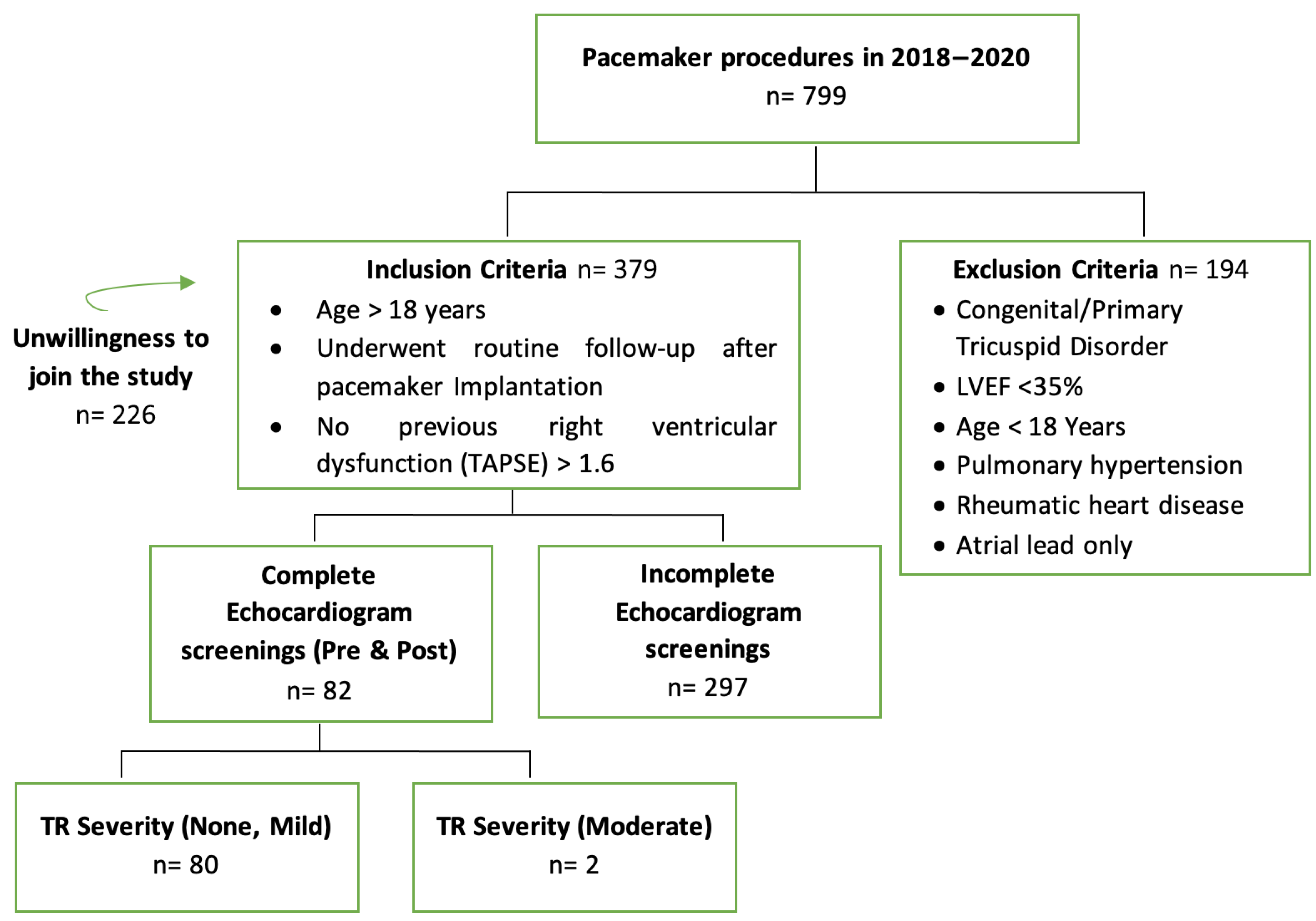
2. Materials and Methods
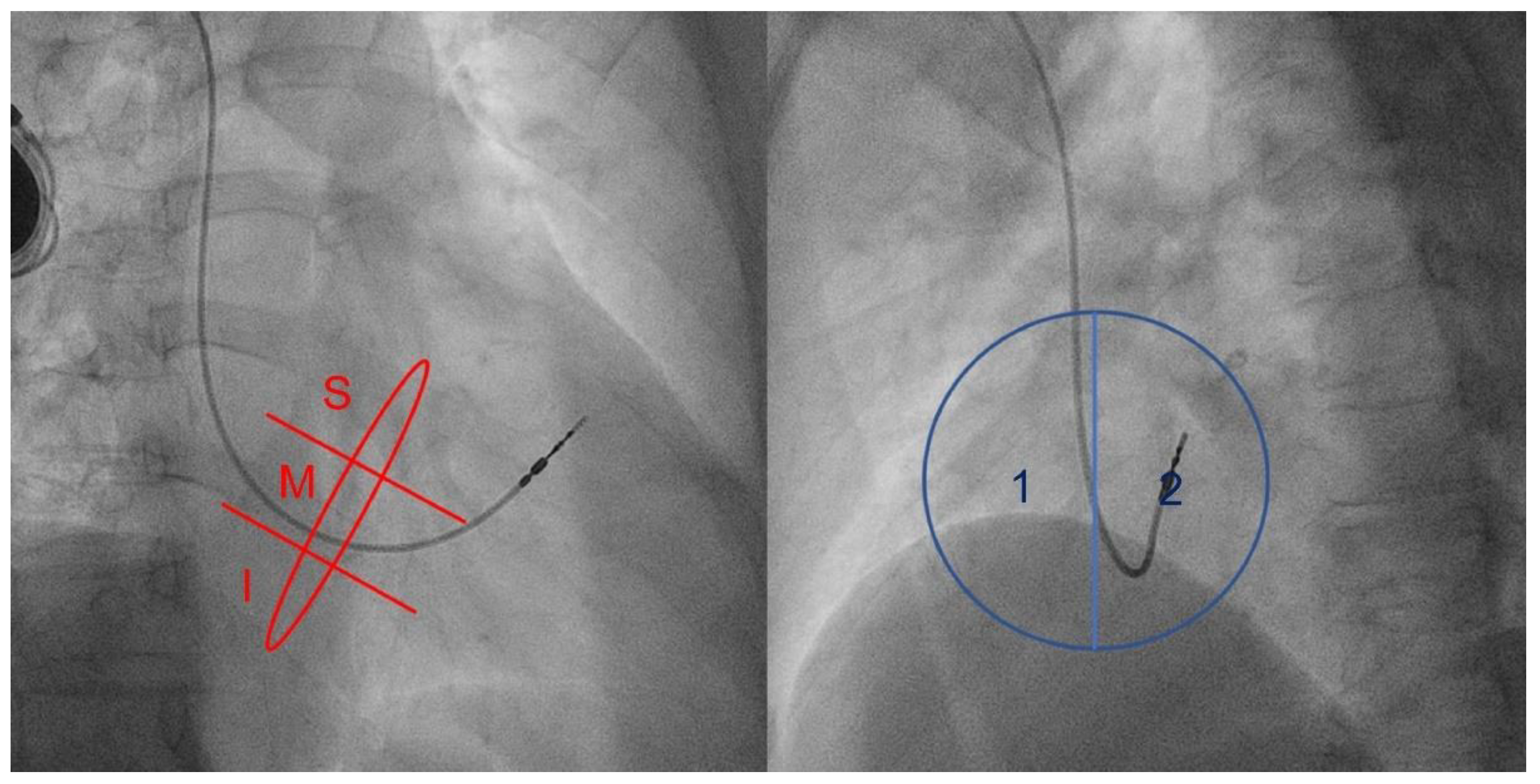
2.1. Fluoroscopy
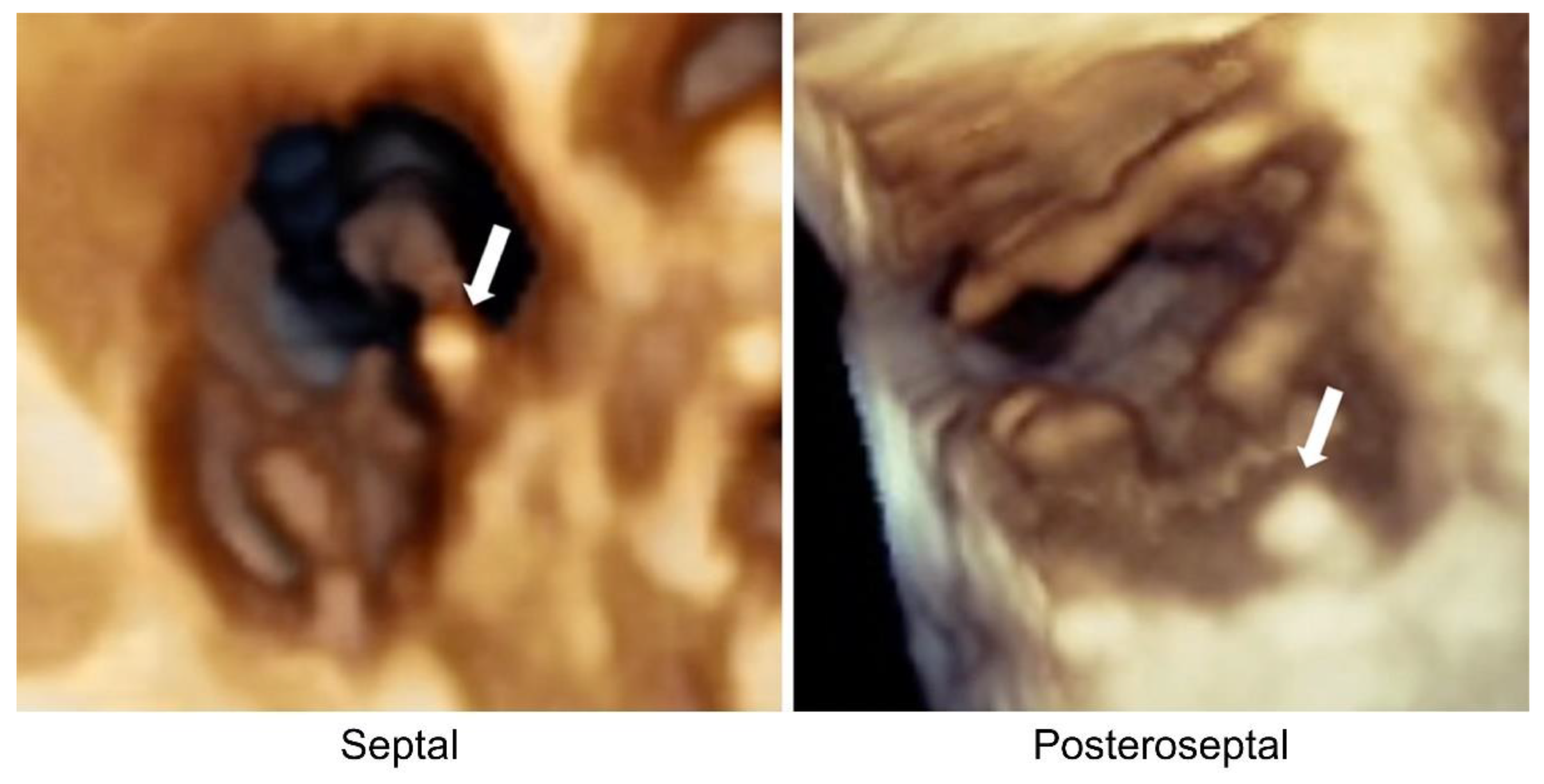
2.2. Transthoracic Echocardiogram
2.3. Study Variables
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Risk Factors for Significant TR
4.2. Association between the Pacemaker Lead Position and Worsening TR
4.3. Association between the Pacemaker Lead Position and Lead Impingement Status
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, J.D.; Manning, W.J.; Ebrille, E.; Zimetbaum, P.J. Tricuspid Valve Dysfunction Following Pacemaker or Cardioverter-Defibrillator Implantation. J. Am. Coll. Cardiol. 2017, 69, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Prihadi, E.A.; van der Bijl, P.; Gursoy, E.; Abou, R.; Mara Vollema, E.; Hahn, R.T.; Stone, G.W.; Leon, M.B.; Ajmone Marsan, N.; Delgado, V.; et al. Development of Significant Tricuspid Regurgitation over Time and Prognostic Implications: New Insights into Natural History. Eur. Heart J. 2018, 39, 3574–3581. [Google Scholar] [CrossRef] [PubMed]
- Delling, F.N.; Hassan, Z.K.; Piatkowski, G.; Tsao, C.W.; Rajabali, A.; Markson, L.J.; Zimetbaum, P.J.; Manning, W.J.; Chang, J.D.; Mukamal, K.J. Tricuspid Regurgitation and Mortality in Patients with Transvenous Permanent Pacemaker Leads. Am. J. Cardiol. 2016, 117, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Nakajima, H.; Ishizu, T.; Iida, N.; Sato, K.; Yamamoto, M.; Machino-Ohtsuka, T.; Nogami, A.; Ohte, N.; Ieda, M. Comparison of Outcomes in Patients with Heart Failure with Versus without Lead-Induced Tricuspid Regurgitation after Cardiac Implantable Electronic Devices Implantations. Am. J. Cardiol. 2020, 130, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Mediratta, A.; Addetia, K.; Yamat, M.; Moss, J.D.; Nayak, H.M.; Burke, M.C.; Weinert, L.; Maffessanti, F.; Jeevanandam, V.; Mor-Avi, V.; et al. 3D Echocardiographic Location of Implantable Device Leads and Mechanism of Associated Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2014, 7, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Trankle, C.R.; Gertz, Z.M.; Koneru, J.N.; Kasirajan, V.; Nicolato, P.; Bhardwaj, H.L.; Ellenbogen, K.A.; Kalahasty, G. Severe Tricuspid Regurgitation Due to Interactions with Right Ventricular Permanent Pacemaker or Defibrillator Leads. Pacing Clin. Electrophysiol. 2018, 41, 845–853. [Google Scholar] [CrossRef]
- Andre, C.; Piver, E.; Perault, R.; Bisson, A.; Pucheux, J.; Vermes, E.; Pierre, B.; Fauchier, L.; Babuty, D.; Clementy, N. Galectin-3 Predicts Response and Outcomes after Cardiac Resynchronization Therapy. J. Transl. Med. 2018, 16, 299. [Google Scholar] [CrossRef]
- Lin, G.; Nishimura, R.A.; Connolly, H.M.; Dearani, J.A.; Sundt, T.M., III.; Hayes, D.L. Severe Symptomatic Tricuspid Valve Regurgitation Due to Permanent Pacemaker or Implantable Cardioverter-Defibrillator Leads. J. Am. Coll. Cardiol. 2005, 45, 1672–1675. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Kim, J.B.; Spevack, D.M.; Tunick, P.A.; Bullinga, J.R.; Kronzon, I.; Chinitz, L.A.; Reynolds, H.R. The Effect of Transvenous Pacemaker and Implantable Cardioverter Defibrillator Lead Placement on Tricuspid Valve Function: An Observational Study. J. Am. Soc. Echocardiogr. 2008, 21, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Klutstein, M.; Balkin, J.; Butnaru, A.; Ilan, M.; Lahad, A.; Rosenmann, D. Tricuspid Incompetence Following Permanent Pacemaker Implantation. Pacing Clin. Electrophysiol. 2009, 32, S135–S137. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Ishizu, T.; Nakajima, H.; Sekiguchi, Y.; Watanabe, S.; Aonuma, K. Clinical Utility of 3-Dimensional Echocardiography in the Evaluation of Tricuspid Regurgitation Caused by Pacemaker Leads. Circ. J. 2008, 72, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Riesenhuber, M.; Spannbauer, A.; Gwechenberger, M.; Pezawas, T.; Schukro, C.; Stix, G.; Schneider, M.; Goliasch, G.; Anvari, A.; Wrba, T.; et al. Pacemaker Lead-Associated Tricuspid Regurgitation in Patients with or without Pre-Existing Right Ventricular Dilatation. Clin. Res. Cardiol. 2021, 110, 884–894. [Google Scholar] [CrossRef]
- Seo, J.; Kim, D.-Y.; Cho, I.; Hong, G.-R.; Ha, J.-W.; Shim, C.Y. Prevalence, Predictors, and Prognosis of Tricuspid Regurgitation Following Permanent Pacemaker Implantation. PLoS ONE 2020, 15, e0235230. [Google Scholar] [CrossRef]
- Prihadi, E.A. Tricuspid Valve Regurgitation: No Longer the “Forgotten Valve”. E-J. Cardiol. Pract. 2018, 16, 21–30. [Google Scholar]
- Sysol, J.R.; Machado, R.F. Classification and Pathophysiology of Pulmonary Hypertension. Contin. Cardiol. Educ. 2018, 4, 2–12. [Google Scholar] [CrossRef]
- Nemoto, N.; Lesser, J.R.; Pedersen, W.R.; Sorajja, P.; Spinner, E.; Garberich, R.F.; Vock, D.M.; Schwartz, R.S. Pathogenic Structural Heart Changes in Early Tricuspid Regurgitation. J. Thorac. Cardiovasc. Surg. 2015, 150, 323–330. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Itabashi, Y.; Mihara, H.; Berdejo, J.; Kobayashi, S.; Siegel, R.J.; Shiota, T. Functional Tricuspid Regurgitation Caused by Chronic Atrial Fibrillation. Circ. Cardiovasc. Imaging 2017, 10, e004897. [Google Scholar] [CrossRef]
- Najib, M.Q.; Vinales, K.L.; Vittala, S.S.; Challa, S.; Lee, H.R.; Chaliki, H.P. Predictors for the Development of Severe Tricuspid Regurgitation with Anatomically Normal Valve in Patients with Atrial Fibrillation. Echocardiography 2012, 29, 140–146. [Google Scholar] [CrossRef]
- Vaturi, M.; Kusniec, J.; Shapira, Y.; Nevzorov, R.; Yedidya, I.; Weisenberg, D.; Monakier, D.; Strasberg, B.; Sagie, A. Right Ventricular Pacing Increases Tricuspid Regurgitation Grade Regardless of the Mechanical Interference to the Valve by the Electrode. Eur. J. Echocardiogr. 2010, 11, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, D.; Aldrich, H.R.; Lieberman, E.H.; Lamas, G.A.; Agatston, A.S. Increased Prevalence of Significant Tricuspid Regurgitation in Patients with Transvenous Pacemakers Leads. Am. J. Cardiol. 1998, 82, 1130–1132. [Google Scholar] [CrossRef]
- Al-Mohaissen, M.A.; Chan, K.L. Prevalence and Mechanism of Tricuspid Regurgitation Following Implantation of Endocardial Leads for Pacemaker or Cardioverter-Defibrillator. J. Am. Soc. Echocardiogr. 2012, 25, 245–252. [Google Scholar] [CrossRef]
- Lee, R.C.; Friedman, S.E.; Kono, A.T.; Greenberg, M.L.; Palac, R.T. Tricuspid Regurgitation Following Implantation of Endocardial Leads: Incidence and Predictors. Pacing Clin. Electrophysiol. 2015, 38, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Poorzand, H.; Tayyebi, M.; Hosseini, S.; Heidari, A.; Keihanian, F.; Jarahi, L.; Hamedanchi, A. Effect of Right Ventricular Lead Placement on Tricuspid Valve: Added Value of Post-Procedural Fluoroscopy to Three Dimensional Echocardiography in a Prospective Cohort Study. Authorea Prepr. 2020. [Google Scholar] [CrossRef]
- Addetia, K.; Maffessanti, F.; Mediratta, A.; Yamat, M.; Weinert, L.; Moss, J.D.; Nayak, H.M.; Burke, M.C.; Patel, A.R.; Kruse, E.; et al. Impact of Implantable Transvenous Device Lead Location on Severity of Tricuspid Regurgitation. J. Am. Soc. Echocardiogr. 2014, 27, 1164–1175. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Tricuspid Regurgitation | OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Worsening TR (n = 23) | Not Worsening TR (n = 57) | |||
| Sex, n (%) | ||||
| Male | 7 (30.4) | 25 (43.9) | 0.56 (0.20–1.57) | 0.267 * |
| Female | 16 (69.6) | 32 (56.1) | ||
| Mean age, years | 63.35 ± 11.24 | 59.93 ± 12.70 | 0.221 † | |
| History of coronary heart disease | ||||
| Yes | 9 (39.1) | 11 (19.3) | 2.69 (0.93–7.80) | 0.064 * |
| No | 14 (60.9) | 46 (80.7) | ||
| Hypertension | ||||
| Yes | 20 (87.0) | 35 (61.4) | 4.19 (1.11–15.77) | 0.026 * |
| No | 3 (13.0) | 22 (38.6) | ||
| Diabetes mellitus, type 2 | ||||
| Yes | 7 (30.4) | 21 (35.6) | 0.75 (0.27– 2.12) | 0.587 * |
| No | 16 (69.6) | 36 (63.2) | ||
| Atrial fibrillation | ||||
| Yes | 8 (34.8) | 11 (19.3) | 2.23 (0.76–6.58) | 0.141 * |
| No | 15 (65.2) | 46 (80.7) | ||
| History of heart failure | ||||
| Yes | 10 (43.5) | 14 (24.6) | 2.36 (0.85–6.56) | 0.095 * |
| No | 13 (56.5) | 43 (75.4) | ||
| Mean PPM implantation duration, months/median | 43.91 ± 40.52/32 (7–151) | 32.51 ± 33.96/24 (8–179) | 0.271 † | |
| Median pacing percentage | 100.0 (2.1–100.0) | 100.0 (0.2–100.0) | 0.591 † | |
| PPM indication | ||||
| Sinus node dysfunction | 7 (30.4) | 16 (28.1) | 0.706 | |
| Complete heart block | 14 (60.9) | 32 (56.1) | ||
| Other AV conduction disturbance | 2 (8.7) | 9 (15.8) | ||
| PPM type | ||||
| Single chamber | 8 (34.8%) | 14 (24.6%) | 1.64 (0.57–4.67) | 0.354 * |
| Dual chamber | 15 (65.2%) | 43 (75.4%) | ||
| Echocardiography | ||||
| LVEF, % | 61.86 ± 14.00 | 65.48 ± 10.87 | 0.257 ‡ | |
| LVIDd, mm | 48.49 ± 5.80 | 47.05 ± 8.95 | 0.481 ‡ | |
| LVIDs, mm | 30.46 ± 7.87 | 28.57 ± 7.86 | 0.395 † | |
| LAVI, mL/mm2 | 40.57 ± 17.13 | 36.12 ± 12.55 | 0.233 † | |
| Left atrial dimension, mm | 39.72 ± 7.03 | 37.90 ± 6.98 | 0.305 † | |
| TAPSE, mm | 21.67 ± 4.07 | 22.56 ± 4.10 | 0.324 † | |
| RA Area, cm2 | 17.70 ± 4.90 | 15.78 ± 3.32 | 0.068 ‡ | |
| RVd basal, cm | 3.59 ± 0.70 | 3.40 ± 0.49 | 0.280 ‡ | |
| RVd mid, cm | 2.42 ± 0.47 | 2.39 ± 0.43 | 0.848 † | |
| RVd long, cm | 6.24 ± 0.82 | 6.37 ± 0.70 | 0.496 ‡ | |
| FAC, % | 43.32 ± 7.14 | 45.39 ± 6.38 | 0.253 ‡ | |
| Fluoroscopy | Worsening TR (n = 23) | Not Worsening TR (n = 57) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| RAO, n (%) | ||||
| Mid | 14 (60.9) | 15 (26.3) | 4.36 (1.56–12.13) | 0.04 |
| Inferior | 9 (39.1) | 42 (73.7) | ||
| LAO, n (%) | ||||
| Septal | 21 (91.3) | 41 (71.9) | 4.10 (0.86–19.52) | 0.06 |
| Lateral | 2 (8.7) | 16 (28.1) | ||
| RAO–LAO, n (%) | ||||
| Mid-lateral | 0 | 6 (10.5) | 0.001 | |
| Mid-septal | 14 (60.9) | 9 (15.8) | ||
| Inferior-lateral | 2 (8.7) | 10 (17.5) | ||
| Inferior-septal | 7 (30.4) | 32 (56.1) | ||
| RAO–LAO, n (%) | ||||
| Mid-septal | 14 (60.9) | 9 (15.8) | 8.30 (2.76–24.90) | <0.001 |
| Others | 9 (39.1) | 48 (84.2) |
| Fluoroscopy | Impinging (n = 21) | Non-Impinging (n = 59) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| RAO, n (%) | ||||
| Mid | 16 (76.2) | 13 (22.0) | 11.32 (3.49–36.77) | <0.001 |
| Inferior | 5 (23.8) | 46 (78.0) | ||
| LAO, n (%) | ||||
| Septal | 19 (90.5) | 43 (72.9) | 3.54 (0.74–16.92) | 0.097 |
| Lateral | 2 (9.5) | 16 (27.1) | ||
| RAO-LAO, n (%) | ||||
| Mid-Septal | 16 (76.2) | 7 (11.9) | 23.77 (6.63–85.25) | <0.001 |
| Other | 5 (23.8) | 52 (88.1) |
| Lead Impingement | Tricuspid Regurgitation | OR (95% CI) | |
|---|---|---|---|
| Worsening (n = 23) | Not Worsening (n = 57) | ||
| Impinging, n (%) | 18 (78.3) | 3 (5.3) | 64.8 (14.06–298.52) |
| Non-impinging, n (%) | 5 (21.7) | 54 (94.7) | |
| Characteristic | β | SE | ORadj (95% CI) | p-Value | |
|---|---|---|---|---|---|
| PPM lead position | |||||
| Inferior-septal (ref) | - | - | - | ||
| First Step | Mid-septal | 2.558 | 0.735 | 12.913 (3.056–54.554) | 0.001 |
| Sex | 0.638 | 0.749 | 1.892 (0.436–8.212) | 0.038 | |
| Age (years) | −0.006 | 0.028 | 0.994 (9.942–1.049) | 0.831 | |
| Duration of implantation (months) | 0.011 | 0.008 | 1.011 (0.005–1.027) | 0.175 | |
| Hypertension | 1.871 | 0.903 | 6.493 (1.106–38.125) | 0.038 | |
| Atrial fibrillation | 1.513 | 0.762 | 4.541 (1.021–20.207) | 0.047 | |
| Coronary heart disease | −1.415 | 0.850 | 0.243 (0.046–1.285) | 0.096 | |
| Heart failure | 0.474 | 0.668 | 1.607 (0.434–5.951) | 0.478 | |
| Constant | −0.942 | ||||
| Hypertension | 1.878 | 0.858 | 6.543 (1.218–35.140) | 0.029 | |
| Final Step | Atrial fibrillation | 1.105 | 0.640 | 3.019 (0.862–10.575) | 0.084 |
| Mid-septal position | 2.123 | 0.613 | 8.352 (2.514–27.746) | 0.001 | |
| Constant | −3.563 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanafy, D.A.; Soesanto, A.M.; Setianto, B.; Immanuel, S.; Raharjo, S.B.; Herqutanto; Amir, M.; Yuniadi, Y. Identification of Pacemaker Lead Position Using Fluoroscopy to Avoid Significant Tricuspid Regurgitation. J. Clin. Med. 2023, 12, 4782. https://doi.org/10.3390/jcm12144782
Hanafy DA, Soesanto AM, Setianto B, Immanuel S, Raharjo SB, Herqutanto, Amir M, Yuniadi Y. Identification of Pacemaker Lead Position Using Fluoroscopy to Avoid Significant Tricuspid Regurgitation. Journal of Clinical Medicine. 2023; 12(14):4782. https://doi.org/10.3390/jcm12144782
Chicago/Turabian StyleHanafy, Dicky A., Amiliana M. Soesanto, Budhi Setianto, Suzanna Immanuel, Sunu B. Raharjo, Herqutanto, Muzakkir Amir, and Yoga Yuniadi. 2023. "Identification of Pacemaker Lead Position Using Fluoroscopy to Avoid Significant Tricuspid Regurgitation" Journal of Clinical Medicine 12, no. 14: 4782. https://doi.org/10.3390/jcm12144782
APA StyleHanafy, D. A., Soesanto, A. M., Setianto, B., Immanuel, S., Raharjo, S. B., Herqutanto, Amir, M., & Yuniadi, Y. (2023). Identification of Pacemaker Lead Position Using Fluoroscopy to Avoid Significant Tricuspid Regurgitation. Journal of Clinical Medicine, 12(14), 4782. https://doi.org/10.3390/jcm12144782






