Detection of Antibodies against the Acetylcholine Receptor in Patients with Myasthenia Gravis: A Comparison of Two Enzyme Immunoassays and a Fixed Cell-Based Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anti-AChR Antibody Assays
- I.
- cELISA was performed using the commercially available kit RSR AChR Autoantibody (RSR Ltd., Cardiff, UK) according to the manufacturer’s instructions [18]. It is a non-isotopic assay based on the ability of AChR autoantibodies to compete with three different AChR monoclonal antibodies (MAbs 1–3) for binding sites on affinity-purified fetal and adult-type AChR. One MAb (MAb1) is coated onto ELISA plate wells, and the other two are labeled with biotin and used in the assay in the liquid phase. In the absence of serum AChR autoantibodies, a sandwich is formed among MAb1, the AChR and the two biotinylated MAbs, which are subsequently detected by the addition of streptavidin peroxidase, which is bound specifically to biotin. In the presence of serum AChR autoantibodies, the formation of the sandwich fails, and the amount of biotinylated MAbs is reduced. A higher concentration of serum AChR autoantibodies is associated with greater inhibition of MAb-biotin binding. The concentration of AChR autoantibodies is measured in nmol/L, and a raised value above the cut-off (0.5 nmol/L) is considered nearly 100% specific for MG.
- II.
- iELISA was performed using the commercially available Anti-Acetylcholine Receptor ELISA (IgG) kit from Euroimmun (Lübeck, Germany) according to the manufacturer’s instructions. The stabilized antigen is coated onto the surface of the microwells to serve as antigenic substrates. The manufacturer-recommended cut-off values were used as follows: <0.4 nmol/L, negative; 0.4–0.5 nmol/L, borderline; >0.5 nmol/L, positive.
- III.
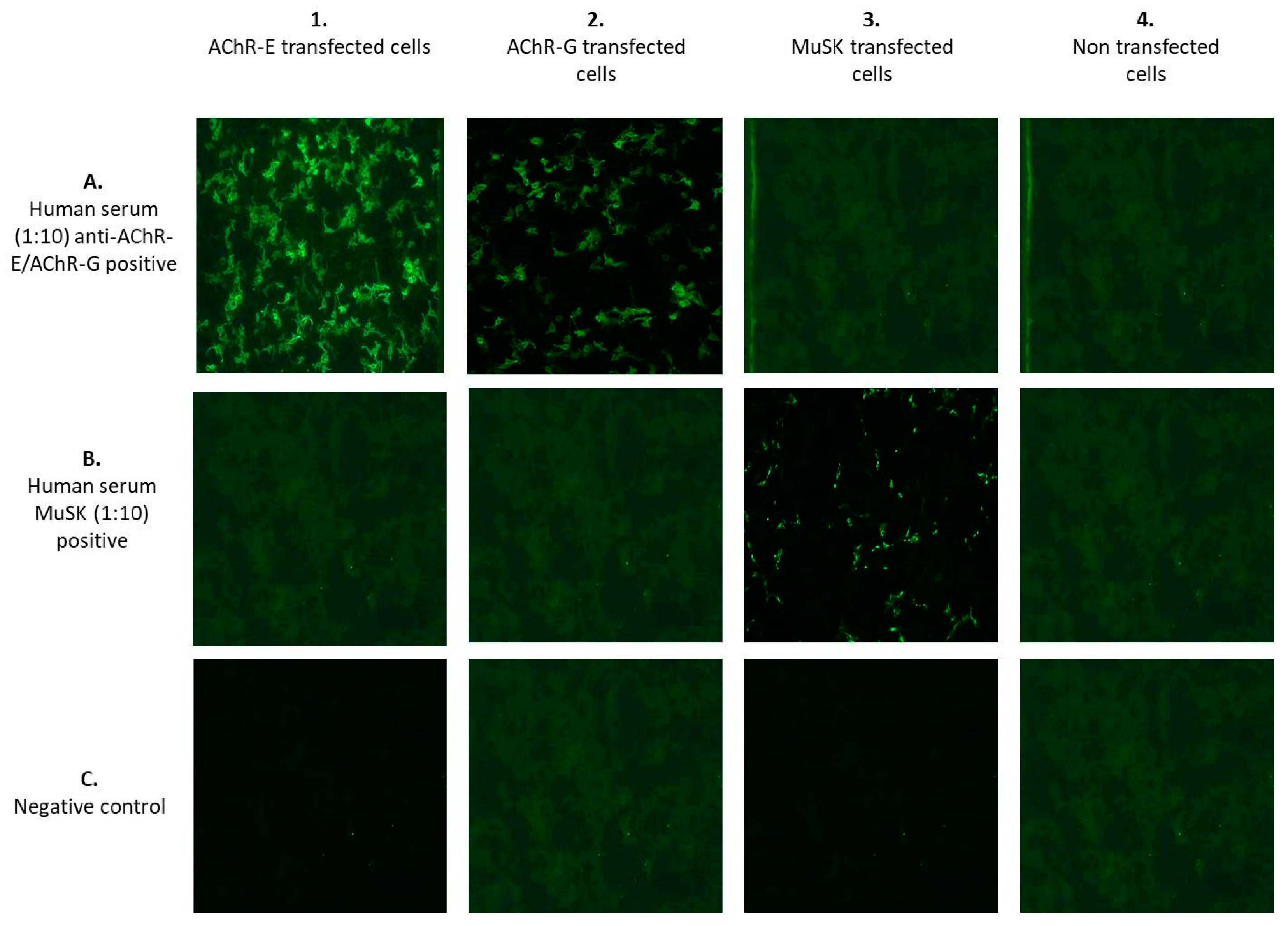
- F-CBA was performed using the commercially available kit MG Mosaics (Euroimmun, Lübeck, Germany) based on the principle of BIOCHIP, which simultaneously detects different antibodies. It is performed by transfecting the fixed HEK cells with complementary DNA expressing human AChR α, β, δ and ε/γ subunits and rapsyn-enhanced green fluorescent protein. The transfected cells are incubated with serum samples diluted with phosphate-buffered saline containing 0.002% Tween 20 in 1:10 dilutions for 30 min at room temperature. Measurement of antibody binding is performed by indirect immunofluorescence. In the second and third steps, the linked antibodies are stained with biotin-labeled anti-human IgG, followed by fluorescein isothiocyanate-labeled avidin and made visible with the fluorescence microscope. A smooth or fine-to-granular green fluorescence signal is detected both in the cytoplasm and at the cell surface membrane. The BIOCHIP slide is composed of combinations of 4 substrates for each patient’s test: (1) recombinant cells transfected with AChR-ε; (2) recombinant cells transfected with AChR-γ; (3) recombinant cells transfected with MuSK; and (4) untransfected recombinant cells used as negative controls (Figure 1). The fluorescence was scored by a DMIRE2 Leica fluorescence microscope (Leica, Milan, Italy) with a 20× lens. Pictures were acquired by a digital camera model DC250 Leica, using the acquisition software Qfluor550 Leica (V7.7.1). Two expert operators, who worked independently and were blinded to the clinical data, interpreted the results. Unclear results were repeated until consensus was achieved.
2.3. Statistical Analysis
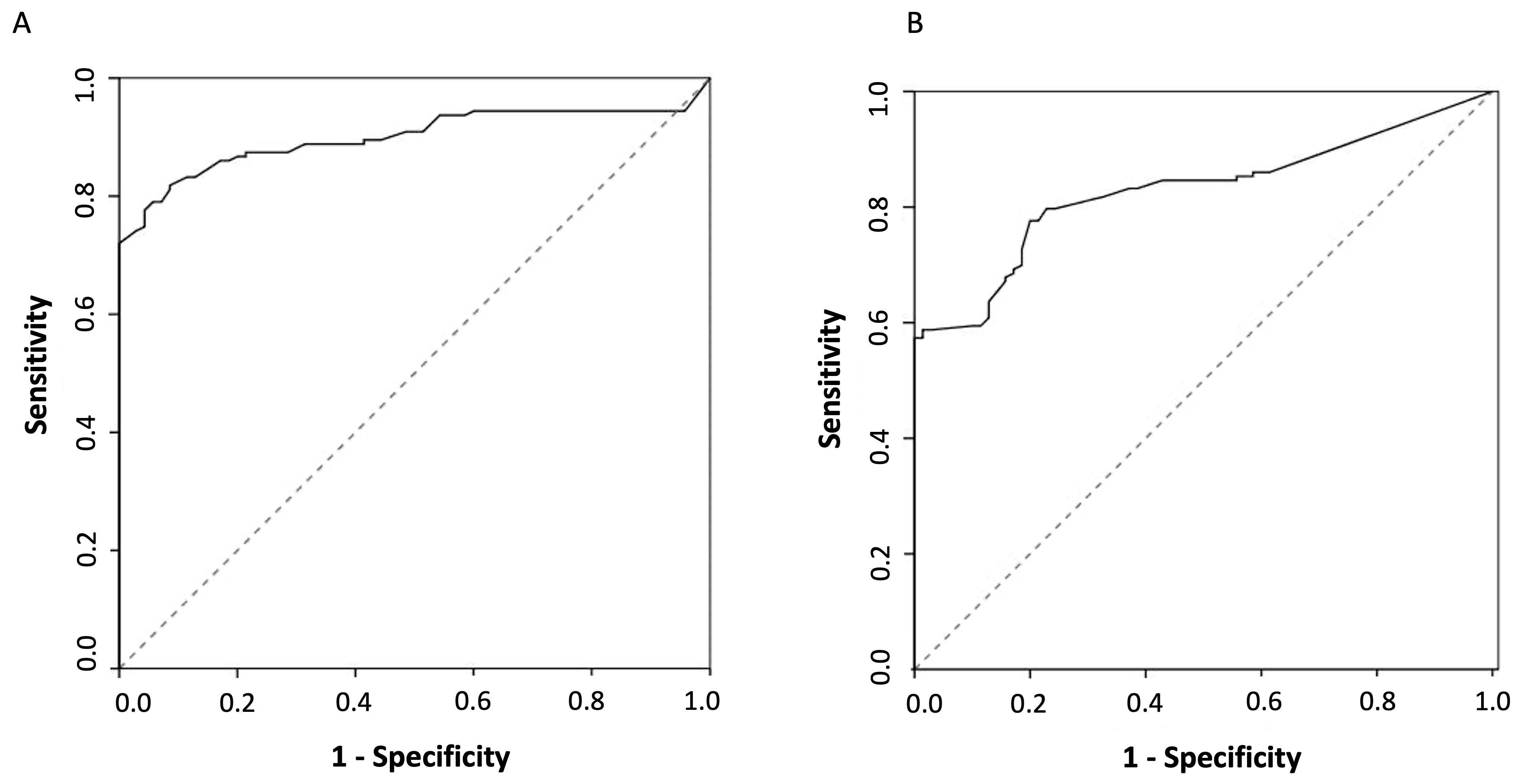
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilhus, N.E.; Tzartos, S.; Evoli, A.; Palace, J.; Burns, T.M.; Verschuuren, J.J.G.M. Myasthenia gravis. Nat. Rev. Dis. Prim. 2019, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.B.; Wolfe, G.I.; Benatar, M.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.; Massey, J.M.; Melms, A.; Murai, H.; et al. International consensus guidance for management of myasthenia gravis: Executive summary. Neurology 2016, 87, 419–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaguchi, H.; Yamashita, S.; Hirano, T.; Nakajima, M.; Kimura, E.; Maeda, Y.; Uchino, M. Myasthenic crisis patients who require intensive care unit management. Muscle Nerve 2012, 46, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Alshekhlee, A.; Miles, J.D.; Katirji, B.; Preston, D.C.; Kaminski, H.J. Incidence and mortality rates of myasthenia gravis and myasthenic crisis in US hospitals. Neurology 2009, 72, 1548–1554. [Google Scholar] [CrossRef]
- Evoli, A.; Antonini, G.; Antozzi, C.; DiMuzio, A.; Habetswallner, F.; Iani, C.; Inghilleri, M.; Liguori, R.; Mantegazza, R.; Massa, R.; et al. Italian recommendations for the diagnosis and treatment of myasthenia gravis. Neurol. Sci. 2019, 40, 1111–1124. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Verschuuren, J.J. Myasthenia gravis: Subgroup classification and therapeutic strategies. Lancet Neurol. 2015, 14, 1023–1036. [Google Scholar] [CrossRef]
- Marx, A.; Pfister, F.; Schalke, B.; Saruhan-Direskeneli, G.; Melms, A.; Ströbel, P. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun. Rev. 2013, 12, 875–884. [Google Scholar] [CrossRef]
- Hoch, W.; McConville, J.; Helms, S.; Newsom-Davis, J.; Melms, A.; Vincent, A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat. Med. 2001, 7, 365–368. [Google Scholar] [CrossRef]
- Bartoccioni, E.; Marino, M.; Evoli, A.; Ruegg, M.A.; Scuderi, F.; Provenzano, C. Identification of disease-specific autoantibodies in seronegative myasthenia gravis. Ann. N. Y. Acad. Sci. 2003, 998, 356–358. [Google Scholar] [CrossRef]
- Mori, S.; Kubo, S.; Akiyoshi, T.; Yamada, S.; Miyazaki, T.; Hotta, H.; Desaki, J.; Kishi, M.; Konishi, T.; Nishino, Y.; et al. Antibodies against muscle-specific kinase impair both presynaptic and postsynaptic functions in a murine model of myasthenia gravis. Am. J. Pathol. 2012, 180, 798–810. [Google Scholar] [CrossRef] [Green Version]
- Doppler, K.; Hemprich, A.; Haarmann, A.; Brecht, I.; Franke, M.; Kröger, S.; Villmann, C.; Sommer, C. Autoantibodies to cortactin and agrin in sera of patients with myasthenia gravis. J. Neuroimmunol. 2021, 56, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, C.; Lazaridis, K.; Zouvelou, V.; Tzartos, J.; Mantegazza, R.; Antozzi, C.; Andreetta, F.; Evoli, A.; Deymeer, F.; Saruhan-Direskeneli, G.; et al. Titin antibodies in “seronegative” myasthenia gravis--A new role for an old antigen. J. Neuroimmunol. 2016, 292, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, K.; Tzartos, S.J. Autoantibody Specificities in Myasthenia Gravis; Implications for Improved Diagnostics and Therapeutics. Front. Immunol. 2020, 11, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivner, M.H.; Quarles, B.M.; Pan, J.X.; Yu, Z.; Howard, J.F.; Corse, A., Jr.; Dimachkie, M.M.; Jackson, C.; Vu, T.; Small, G.; et al. Clinical features of LRP4/agrin-antibody-positive myasthenia gravis: A multicenter study. Muscle Nerve 2020, 62, 333–343. [Google Scholar] [CrossRef]
- Phillips, W.D.; Vincent, A. Pathogenesis of myasthenia gravis: Update on disease types, models, and mechanisms. F1000Research 2016, 5, 1513. [Google Scholar] [CrossRef] [Green Version]
- Lindstrom, J. An assay for antibodies to human acetylcholine receptor in serum from patients with myasthenia gravis. Clin. Immunol. Immunopathol. 1977, 7, 36–43. [Google Scholar] [CrossRef]
- Matthews, I.; Chen, S.; Hewer, R.; McGrath, V.; Furmaniak, J.; Rees Smith, B. Muscle-specific receptor tyrosine kinase autoantibodies–A new immunoprecipitation assay. Clin. Chim. Acta 2004, 348, 95–99. [Google Scholar] [CrossRef]
- Hewer, R.; Matthews, I.; Chen, S.; McGrath, V.; Evans, M.; Roberts, E.; Nute, S.; Sanders, J.; Furmaniak, J.; Smith, B.R. A sensitive non-isotopic assay for acetylcholine receptor autoantibodies. Clin. Chim. Acta 2006, 364, 159–166. [Google Scholar] [CrossRef]
- Leite, M.I.; Jacob, S.; Viegas, S.; Cossins, J.; Clover, L.; Morgan, B.P.; Beeson, D.; Willcox, N.; Vincent, A. IgG1 antibodies to acetylcholine receptors in ‘seronegative’ myasthenia gravis. Brain 2008, 131, 1940–1952. [Google Scholar] [CrossRef] [Green Version]
- Zisimopoulou, P.; Lagoumintzis, G.; Kostelidou, K.; Bitzopoulou, K.; Kordas, G.; Trakas, N.; Poulas, K.; Tzartos, S.J. Towards antigen-specific apheresis of pathogenic autoantibodies as a further step in the treatment of myasthenia gravis by plasmapheresis. J. Neuroimmunol. 2008, 201, 95–103. [Google Scholar] [CrossRef]
- Bokoliya, S.; Patil, S.; Nagappa, M.; Taly, A. A Simple, Rapid and Non-Radiolabeled Immune Assay to Detect Anti-AChR Antibodies in Myasthenia Gravis. Lab. Med. 2019, 50, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Han, L.; Zhu, D.; Peng, J.; Li, J.; Ding, J.; Luo, J.; Hong, R.; Wang, K.; Wan, W.; et al. A Stable Cell Line Expressing Clustered AChR: A Novel Cell-Based Assay for Anti-AChR Antibody Detection in Myasthenia Gravis. Front. Immunol. 2021, 12, 666046. [Google Scholar] [CrossRef] [PubMed]
- Mirian, A.; Nicolle, M.W.; Edmond, P.; Budhram, A. Comparison of fixed cell-based assay to radioimmunoprecipitation assay for acetylcholine receptor antibody detection in myasthenia gravis. J. Neurol. Sci. 2022, 432, 120084. [Google Scholar] [CrossRef]
- Narayanaswami, P.; Sanders, D.B.; Wolfe, G.; Benatar, M.; Cea, G.; Evoli, A.; Gilhus, N.E.; Illa, I.; Kuntz, N.L.; Massey, J.; et al. International Consensus Guidance for Management of Myasthenia Gravis: 2020 Update. Neurology 2021, 96, 114–122. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Owe, J.F.; Hoff, J.M.; Romi, F.; Skeie, G.O.; Aarli, J.A. Myasthenia gravis: A review of available treatment approaches. Autoimmune Dis. 2011, 2011, 847393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambino, C.M.; Agnello, L.; Lo Sasso, B.; Scazzone, C.; Giglio, R.V.; Candore, G.; Ciaccio, A.M.; Di Stefano, V.; Brighina, F.; Vidali, M.; et al. Comparative Analysis of BIOCHIP Mosaic-Based Indirect Immunofluorescence with Enzyme-Linked Immunosorbent Assay for Diagnosing Myasthenia Gravis. Diagnostics 2021, 11, 2098. [Google Scholar] [CrossRef]
- Strijbos, E.; Verschuuren, J.J.G.M.; Kuks, J.B.M. Serum Acetylcholine Receptor Antibodies Before the Clinical Onset of Myasthenia Gravis. J. Neuromuscul. Dis. 2018, 5, 261–264. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.W.; Kim, M.; Choi, Y.C.; Kim, S.M.; Shin, H.Y. Evaluating an In-House Cell-Based Assay for Detecting Antibodies Against Muscle-Specific Tyrosine Kinase in Myasthenia Gravis. J. Clin. Neurol. 2021, 17, 400–408. [Google Scholar] [CrossRef]
- Spagni, G.; Gastaldi, M.; Businaro, P.; Chemkhi, Z.; Carrozza, C.; Mascagna, G.; Falso, S.; Scaranzin, S.; Franciotta, D.; Evoli, A.; et al. Comparison of Fixed and Live Cell-Based Assay for the Detection of AChR and MuSK Antibodies in Myasthenia Gravis. Neurol. Neuroimmunol. Neuroinflamm. 2022, 10, e200038. [Google Scholar] [CrossRef]
- Alkabie, S.; Budhram, A. Testing for Antibodies Against Aquaporin-4 and Myelin Oligodendrocyte Glycoprotein in the Diagnosis of Patients with Suspected Autoimmune Myelopathy. Front. Neurol. 2022, 13, 912050. [Google Scholar] [CrossRef]
- Frykman, H.; Kumar, P.; Oger, J. Immunopathology of Autoimmune Myasthenia Gravis: Implications for Improved Testing Algorithms and Treatment Strategies. Front. Neurol. 2020, 11, 596621. [Google Scholar] [CrossRef] [PubMed]


| Assays | Competitive ELISA | Sandwich ELISA | F-CBA |
|---|---|---|---|
| Status | CE-IVD | CE-IVD | CE-IVD |
| Antibody isotype | IgG | IgG | IgG |
| Test format | 96-well microplate | 96-well microplate | 10 × 5 slides |
| Sample type | Serum | Serum and plasma | Serum and plasma |
| Sample dilution | Non-dilution | 1:26 | 1:10 |
| Conjugate | Streptavidin-HRP | HRP-rabbit anti-human IgG | Biotin-labeled anti-human IgG, FITC-labeled avidin |
| Incubation time (hours) | 24 | 3 | 2 |
| No calibrators | 4 | 5 | NA |
| Calibration range | 0.5–20 nmol/L (0.5, 1, 6.5, 20) | 0–8 nmol/L (0, 0.25, 0.75, 2.5, 8) | NA |
| Cut-off value | Negative: <0.45 nmol/L Positive: ≥0.45 nmol/L | Negative: <0.40 nmol/L Borderline: ≥0.40 < 0.50 nmol/L Positive: ≥0.50 nmol/L | No reaction at 1:10 Positive reaction at 1:10 |
| Limit of detection | 0.25 nmol/L | 0.11 nmol/L | NA |
| Variable | Descriptive Statistics |
|---|---|
| Demographic | |
| N | 143 |
| Sex, M (%) | 46% |
| Age, years | 61 |
| Clinical | |
| Age at onset, years | 52 (41–62) |
| Type, generalized:ocular | 67%:33% |
| MGFA at onset | |
| I | 29% |
| II | 48% |
| III | 16% |
| IV | 6% |
| V | 1% |
| MGFA at follow-up | |
| I | 31% |
| II | 47% |
| III | 21% |
| IV | 1% |
| V | 0% |
| Thymoma | 18% |
| Thymic hyperplasia | 12% |
| Thyreopathy | 23% |
| Autoimmune disease | 21% |
| Kidney disease | 8% |
| Neuropathy | 14% |
| Hypertension | 38% |
| Cardiovascular disease | 15% |
| Osteoporosis | 26% |
| Eye disease | 12% |
| Gastrointestinal disease | 15% |
| Diabetes | 12% |
| Hematological disease | 8% |
| Cancer disease | 7% |
| Psychiatric disorder | 14% |
| Respiratory disease | 11% |
| Neurological comorbidities | 22% |
| Pyridostigmine | 74% |
| Prednisone | 73% |
| Method | iELISA Pos | iELISA Neg | Total |
|---|---|---|---|
| cELISA pos | 68 | 26 | 94 |
| cELISA neg | 7 | 42 | 49 |
| Total | 75 | 68 | 143 |
| Method | IFA CBA Pos | IFA CBA Neg | Total |
|---|---|---|---|
| cELISA pos | 60 | 34 | 94 |
| cELISA neg | 1 | 48 | 49 |
| Total | 61 | 82 | 143 |
| Method | IFA CBA Pos | IFA CBA Neg | Total |
|---|---|---|---|
| iELISA pos | 57 | 18 | 75 |
| iELISA neg | 4 | 64 | 68 |
| Total | 61 | 82 | 143 |
| Analytical Method | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| cELISA | 66% | 100% | 100% | 59% |
| iELISA | 52% | 100% | 100% | 51% |
| F-CBA | 43% | 100% | 100% | 46% |
| cELISA + iELISA | 71% | 100% | 100% | 63% |
| cELISA + F-CBA | 66% | 100% | 100% | 59% |
| iELISA + F-CBA | 55% | 100% | 100% | 52% |
| cELISA + iELISA+ F-CBA | 71% | 100% | 100% | 63% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambino, C.M.; Agnello, L.; Ciaccio, A.M.; Scazzone, C.; Vidali, M.; Di Stefano, V.; Milano, S.; Brighina, F.; Candore, G.; Lo Sasso, B.; et al. Detection of Antibodies against the Acetylcholine Receptor in Patients with Myasthenia Gravis: A Comparison of Two Enzyme Immunoassays and a Fixed Cell-Based Assay. J. Clin. Med. 2023, 12, 4781. https://doi.org/10.3390/jcm12144781
Gambino CM, Agnello L, Ciaccio AM, Scazzone C, Vidali M, Di Stefano V, Milano S, Brighina F, Candore G, Lo Sasso B, et al. Detection of Antibodies against the Acetylcholine Receptor in Patients with Myasthenia Gravis: A Comparison of Two Enzyme Immunoassays and a Fixed Cell-Based Assay. Journal of Clinical Medicine. 2023; 12(14):4781. https://doi.org/10.3390/jcm12144781
Chicago/Turabian StyleGambino, Caterina Maria, Luisa Agnello, Anna Maria Ciaccio, Concetta Scazzone, Matteo Vidali, Vincenzo Di Stefano, Salvatore Milano, Filippo Brighina, Giuseppina Candore, Bruna Lo Sasso, and et al. 2023. "Detection of Antibodies against the Acetylcholine Receptor in Patients with Myasthenia Gravis: A Comparison of Two Enzyme Immunoassays and a Fixed Cell-Based Assay" Journal of Clinical Medicine 12, no. 14: 4781. https://doi.org/10.3390/jcm12144781
APA StyleGambino, C. M., Agnello, L., Ciaccio, A. M., Scazzone, C., Vidali, M., Di Stefano, V., Milano, S., Brighina, F., Candore, G., Lo Sasso, B., & Ciaccio, M. (2023). Detection of Antibodies against the Acetylcholine Receptor in Patients with Myasthenia Gravis: A Comparison of Two Enzyme Immunoassays and a Fixed Cell-Based Assay. Journal of Clinical Medicine, 12(14), 4781. https://doi.org/10.3390/jcm12144781











