Psychiatric Treatment in Pregnancy: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Epidemiology of Mental Problems in Pregnancy and Postpartum
4. Etiology of Mental Disorders in the Perinatal Period
5. Diagnostic Criteria
6. Early Detection and Diagnosis
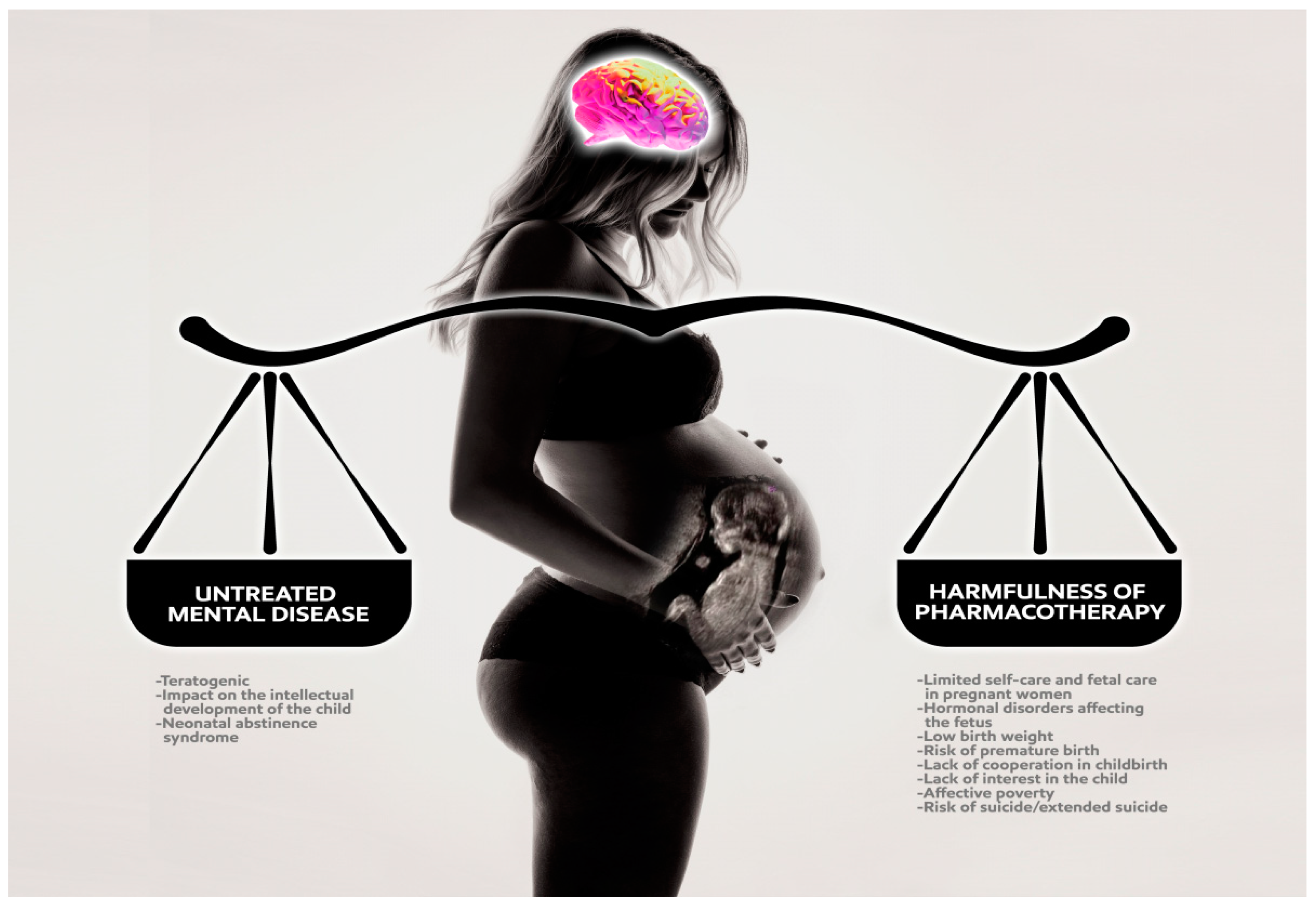
7. Psychopharmacology in Pregnancy
7.1. General Rules of Psychiatric Treatment during Pregnancy
7.2. Antidepressants
A Review of Research on Antidepressants
7.3. Mood Stabilizers
7.3.1. Valproates
7.3.2. Carbamazepine
7.3.3. Lithium Carbonate
7.3.4. Lamotrigine
7.3.5. A Review of Research on Mood Stabilizers
7.4. Antipsychotics/Neuroleptics
7.4.1. Atypical Neuroleptics/Second-Generation Antipsychotics
7.4.2. First-Generation Antipsychotics/Classic Neuroleptics
7.4.3. A Review of Literature on Antipsychotics
7.5. Anxiolytics and Benzodiazepines
7.6. Non-Pharmacological Strategies
8. Discussion
9. Conclusions
- Paroxetine, fluoxetine (congenital heart defects);
- Clomipramine (malformations of the fetal cardiovascular system);
- Valproates (teratogenic);
- Carbamazepine (e.g., spina bifida);
- Lithium (teratogenic, increases the risk of miscarriage);
- Bupropion (birth defects).
- Escitalopram;
- Sertraline;
- Haloperidol;
- Quetiapine.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Classification of Disorders Associated with Pregnancy or Puerperium according to ICD-11. Available online: https://icd.who.int (accessed on 1 May 2023).
- Piotr, G.; Monika, T. Depression in Women. In Depresja u Kobiet; Medical Education: Warszawa, Poland, 2018; ISBN 978-83-65471-51-2. [Google Scholar]
- Recommendation No. 13/2020 of the President of the Agency for Health Technology Assessment and Tarification on Recommended Health Technologies, Activities Carried out within the Framework of Health Policy Programs and Conditions for Implementation of These Programs, Regarding the Health Problem of Postpartum Depression. Available online: https://bipold.aotm.gov.pl/assets/files/ppz/2020/REK/13_2020.pdf (accessed on 13 October 2022).
- Urban-Kowalczyk, M. Mental Disorders in Women. In Zaburzenia Psychiczne u Kobiet Pod Red; Medical Tribune: Warszawa, Poland, 2020; ISBN 978-83-956677-0-1. [Google Scholar]
- Dominiak, M.; Antosik-Wojcinska, A.Z.; Baron, M.; Mierzejewski, P.; Swiecicki, L. Recommendations for the Prevention and Treatment of Postpartum Depression [Online], Polish Gynecology. 2021. Available online: https://journals.viamedica.pl/ginekologia_polska/article/view/69183 (accessed on 23 October 2022).
- Szulc, A.; Gałeckiego, P. Schizophrenia in women. In Schizofrenia u Kobiet Pod Red; Medical Education: Warszawa, Poland, 2020; ISBN 978-83-65471-94-9. [Google Scholar]
- WHO Official Website. Available online: https://www.who.int (accessed on 13 October 2022).
- Piotr, G.; Agata, S. Psychiatry. In Psychiatria; Odra Urban & Partner: Wrocław, Poland, 2018; ISBN 978-83-65835-90-1. [Google Scholar]
- Monika, T.; Piotr, G. Young’s Theory of Early Maladaptive Schemas from the Perspective of Neurodevelopmental Theory of Depression, Teoria Wczesnych Nieadaptacyjnych Schematów Younga z Perspektywy Teorii Neurorozwojowej Depresji, [Online], Termedia. 2017. Available online: https://www.termedia.pl/Teoria-wczesnych-nieadaptacyjnych-schematow-Younga-z-perspektywy-teorii-neurorozwojowej-depresji,46,31086,1,0.html (accessed on 23 October 2022).
- International Classification of Diseases ICD-10. Available online: https://stat.gov.pl/Klasyfikacje/doc/icd10/pdf/ICD10TomI.pdf (accessed on 13 October 2022).
- DSM-5 Diagnostic Criteria of Mental Disorders. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders Fifth Edition DSM-5; American Psychiatric Association (APA): Washington, DC, USA, 2013; ISBN 978-0-89042-555-8. [Google Scholar]
- Gałeckiego, P. Mental Status Examination Diagnoses according to ICD-11. In Badanie Stanu Psychicznego Rozpoznania Według ICD-11, Pod Red; Odra Urban & Partner: Wrocław, Poland, 2022; ISBN 978-83-66960-56-5. [Google Scholar]
- Siu, A.L.; Bibbins-Domingo, K.; Grossman, D.C.; Baumann, L.C.; Davidson, K.W.; Ebell, M.; García, F.A.R.; Gillman, M.; Herzstein, J.; Kemper, A.R.; et al. US Preventive Services Task Force (USPSTF). Screening for Depression in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2016, 315, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Appleby, L.; Warner, R.; Whitton, A.; Faragher, B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ 1997, 314, 932–936. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Antenatal and Postnatal Mental Health: Clinical Management and Service Guidance. December 2014. Available online: http://www.nice.org.uk (accessed on 23 October 2022).
- Scottish Intercollegiate Guidelines Network. Management of Perinatal Mood Disorders. March 2012. Available online: http://www.sign.ac.uk (accessed on 23 October 2022).
- Beyondblue. Clinical Practice Guidelines for Depression and Related Disorders—Anxiety, Bipolar Disorder and Puerperal Psychosis—In the Perinatal Period. A Guideline for Primary Care Health Professionals; Beyondblue: Melbourne, Australia, 2011. [Google Scholar]
- Gelenberg, A.J.; Freeman, M.P.; Markowitz, J.C. American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder, 3rd ed.; American Psychiatric Association (APA): Washington, DC, USA, 2010. [Google Scholar]
- Falek, I.; Acri, M.; Dominguez, J.; Havens, J.; McCord, M.; Sisco, S.; Wilcox, W.; Hoagwood, K. Management of depression during the perinatal period: State of the evidence. Int. J. Ment. Health Syst. 2022, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.N.; Lind, J.N.; Simeone, R.M.; Bobo, W.V.; Mitchell, A.A.; Riehle-Colarusso, T.; Polen, K.N.; Reefhuis, J. Maternal Use of Specific Antidepressant Medications During Early Pregnancy and the Risk of Selected Birth Defects. JAMA Psychiatry 2020, 77, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.Y.; Wu, Q.J.; Zhang, T.N.; Shen, Z.Q.; Liu, C.X.; Xu, X.; Ji, C.; Zhao, Y.H. Fluoxetine and congenital malformations: A systematic review and meta-analysis of cohort studies. Br. J. Clin. Pharmacol. 2017, 83, 2134–2147. [Google Scholar] [CrossRef]
- Kolding, L.; Pedersen, L.H.; Petersen, O.B.; Uldbjerg, N.; Sandager, P. Sertraline use during pregnancy and effect on fetal cardiac function. J. Matern.-Fetal Neonatal Med. 2021, 34, 3631–3638. [Google Scholar] [CrossRef]
- Bérard, A.; Zhao, J.P.; Sheehy, O. Sertraline use during pregnancy and the risk of major malformations. Am. J. Obstet. Gynecol. 2015, 212, e1–e795. [Google Scholar] [CrossRef]
- Stahl Stephen, M. Prescriber’s Guide: Stahi’s Essential Psychopharmacology, 7th ed.; Via Medica: Gdańsk, Poland, 2021; ISBN 978-83-66974-80-7. [Google Scholar]
- Huybrechts, K.F.; Palmsten, K.; Avorn, J.; Cohen, L.S.; Holmes, L.B.; Franklin, J.M.; Mogun, H.; Levin, R.; Kowal, M.; Setoguchi, S.; et al. Antidepressant use in pregnancy and the risk of cardiac defects. N. Engl. J. Med. 2014, 370, 2397–2407. [Google Scholar] [CrossRef]
- Cuomo, A.; Maina, G.; Neal, S.M.; De Montis, G.; Rosso, G.; Scheggi, S.; Beccarini Crescenzi, B.; Bolognesi, S.; Goracci, A.; Coluccia, A.; et al. Using ser-traline in postpartum and breastfeeding: Balancing risks and benefits. Expert Opin. Drug Saf. 2018, 17, 719–725. [Google Scholar] [CrossRef]
- Kolding, L.; Henriksen, J.N.; Pedersen, L.H. Fetal cardiac safety of sertraline use dur-ing pregnancy. Expert Opin. Drug Saf. 2023; Epub ahead of print. [Google Scholar] [CrossRef]
- Bellantuono, C.; Bozzi, F.; Orsolini, L.; Catena-Dell’Osso, M. The safety of escital-opram during pregnancy and breastfeeding: A comprehensive review. Hum. Psychopharmacol. 2012, 27, 534–539. [Google Scholar] [CrossRef]
- Bellantuono, C.; Orsolini, L.; Bozzi, F. La sicurezza dell’escitalopram in gravidanza e nell’allattamento [The safety profile of escitalopram in pregnancy and breastfeeding]. Riv. Psichiatr. 2013, 48, 407–414. (In Italian) [Google Scholar] [CrossRef]
- Gentile, S. Tricyclic antidepressants in pregnancy and puerperium. Expert Opin. Drug Saf. 2014, 13, 207–225. [Google Scholar] [CrossRef]
- Vitale, S.G.; Laganà, A.S.; Muscatello, M.R.; La Rosa, V.L.; Currò, V.; Pandolfo, G.; Zoccali, R.A.; Bruno, A. Psychopharmacotherapy in Pregnancy and Breastfeeding. Obstet. Gynecol. Surv. 2016, 71, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.Y.; Wu, Q.J.; Sun, C.; Zhang, T.N.; Shen, Z.Q.; Liu, C.X.; Gong, T.T.; Xu, X.; Ji, C.; Huang, D.H.; et al. Selective serotonin reuptake inhibitor use during early pregnancy and congenital malformations: A systematic review and meta-analysis of cohort studies of more than 9 million births. BMC Med. 2018, 16, 205. [Google Scholar] [CrossRef]
- Womersley, K.; Ripullone, K.; Agius, M. What are the risks associated with different Selective Serotonin Re-uptake Inhibitors (SSRIs) to treat depression and anxiety in pregnancy? An evaluation of current evidence. Psychiatr. Danub. 2017, 29 (Suppl. S3), 629–644. [Google Scholar]
- Nielsen, R.E.; Damkier, P. Pharmacological treatment of unipolar depression during pregnancy and breast-feeding--a clinical overview. Nord. J. Psychiatry 2012, 66, 159–166. [Google Scholar] [CrossRef]
- Kaplan, Y.C.; Demir, O. Use of Phenytoin, Phenobarbital Carbamazepine, Levetiracetam Lamotrigine and Valproate in Pregnancy and Breastfeeding: Risk of Major Malformations, Dose-dependency, Monotherapy vs Polytherapy, Pharmacokinetics and Clinical Implications. Curr. Neuropharmacol. 2021, 19, 1805–1824. [Google Scholar] [CrossRef]
- Mossakowska-Wójcik, J.; Gałecki, P. Mood stabilizing drugs in pregnancy—possible complications, Leki stabilizujące nastrój w ciąży—możliwe powikłania, “Psychiatria po dyplomie”. 2022; 15–20. [Google Scholar]
- Khan, S.J.; Fersh, M.E.; Ernst, C.; Klipstein, K.; Albertini, E.S.; Lusskin, S.I. Bipolar Disorder in Pregnancy and Postpartum: Principles of Management. Curr. Psychiatry Rep. 2016, 18, 13. [Google Scholar] [CrossRef]
- Arfman, I.J.; Wammes-van der Heijden, E.A.; Ter Horst, P.G.J.; Lambrechts, D.A.; Wegner, I.; Touw, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs in Women with Epilepsy Before, During, and After Pregnancy. Clin. Pharmacokinet. 2020, 59, 427–445. [Google Scholar] [CrossRef]
- Błaszczyk, B.; Miziak, B.; Pluta, R.; Czuczwar, S.J. Epilepsy in Pregnancy-Management Principles and Focus on Valproate. Int. J. Mol. Sci. 2022, 23, 1369. [Google Scholar] [CrossRef]
- Fornaro, M.; Maritan, E.; Ferranti, R.; Zaninotto, L.; Miola, A.; Anastasia, A.; Murru, A.; Solé, E.; Stubbs, B.; Carvalho, A.F.; et al. Lithium Exposure During Pregnancy and the Postpartum Period: A Systematic Review and Meta-Analysis of Safety and Efficacy Outcomes. Am. J. Psychiatry 2020, 177, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Poels, E.M.P.; Bijma, H.H.; Galbally, M.; Bergink, V. Lithium during pregnancy and after delivery: A review. Int. J. Bipolar Disord. 2018, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Niethe, M.; Whitfield, K. Psychotropic medication use during pregnancy. J. Pharm. Pract. Res. 2018, 48, 384–391. [Google Scholar] [CrossRef]
- Huybrechts, K.F.; Hernández-Díaz, S.; Patorno, E.; Desai, R.J.; Mogun, H.; Dejene, S.Z.; Cohen, J.M.; Panchaud, A.; Cohen, L.; Bateman, B.T. Antipsychotic Use in Pregnancy and the Risk for Congenital Malformations. JAMA Psychiatry 2016, 73, 938–946. [Google Scholar] [CrossRef]
- Ellfolk, M.; Leinonen, M.K.; Gissler, M.; Kiuru-Kuhlefelt, S.; Saastamoinen, L.; Malm, H. Second-generation antipsychotic use during pregnancy and risk of congenital malformations. Eur. J. Clin. Pharmacol. 2021, 77, 1737–1745. [Google Scholar] [CrossRef]
- Andrade, C. Major Congenital Malformations Associated With Exposure to Second-Generation Antipsychotic Drugs During Pregnancy. J. Clin. Psychiatry 2021, 82, 21f14252. [Google Scholar] [CrossRef]
- Brunner, E.; Falk, D.M.; Jones, M.; Dey, D.K.; Shatapathy, C.C. Olanzapine in pregnancy and breastfeeding: A review of data from global safety surveillance. BMC Pharmacol. Toxicol. 2013, 14, 38. [Google Scholar] [CrossRef]
- Kulkarni, J.; Storch, A.; Baraniuk, A.; Gilbert, H.; Gavrilidis, E.; Worsley, R. Antipsychotic use in pregnancy. Expert Opin. Pharmacother. 2015, 16, 1335–1345. [Google Scholar] [CrossRef]
- Damkier, P.; Videbech, P. The Safety of Second-Generation Antipsychotics During Pregnancy: A Clinically Focused Review. CNS Drugs 2018, 32, 351–366. [Google Scholar] [CrossRef]
- Straub, L.; Hernández-Díaz, S.; Bateman, B.T.; Wisner, K.L.; Gray, K.J.; Pennell, P.B.; Lester, B.; McDougle, C.J.; Suarez, E.A.; Zhu, Y.; et al. Association of Antipsychotic Drug Exposure in Pregnancy With Risk of Neurodevelopmental Disorders: A National Birth Cohort Study. JAMA Intern. Med. 2022, 182, 522–533. [Google Scholar] [CrossRef]
- Lee, H.; Koh, J.W.; Kim, Y.A.; Chun, K.C.; Han, J.Y.; Hwang, J.H.; Choi, J.S.; Joo, S.H.; Kwon, H.Y. Pregnancy and Neonatal Outcomes After Exposure to Alprazolam in Pregnancy. Front. Pharmacol. 2022, 13, 854562. [Google Scholar] [CrossRef] [PubMed]
- Uzun, S.; Kozumplik, O.; Jakovljević, M.; Sedić, B. Side effects of treatment with benzodiazepines. Psychiatr. Danub. 2010, 22, 90–93. [Google Scholar] [PubMed]

| Mental or behavioral disorders associated with pregnancy, childbirth or the puerperium Syndromes associated with pregnancy or the puerperium (commencing within about 6 weeks after delivery) that involve significant mental and behavioral features. If the symptoms meet the diagnostic requirements for a specific mental disorder, that diagnosis should also be assigned. | |
| 6E20 | Mental or behavioral disorders associated with pregnancy, childbirth or the puerperium, without psychotic symptoms. A syndrome associated with pregnancy or the puerperium (commencing within about 6 weeks after delivery) that involves significant mental and behavioral features, most commonly depressive symptoms. The syndrome does not include delusions, hallucinations or other psychotic symptoms. If the symptoms meet the diagnostic requirements for a specific mental disorder, that diagnosis should also be assigned. This designation should not be used to describe mild and transient depressive symptoms that do not meet the diagnostic requirements for a depressive episode, which may occur soon after delivery (so-called postpartum blues). |
| 6E21 | Mental or behavioral disorders associated with pregnancy, childbirth or the puerperium, with psychotic symptoms. A syndrome associated with pregnancy or the puerperium (commencing within about 6 weeks after delivery) that involves significant mental and behavioral features, including delusions, hallucinations or other psychotic symptoms. Mood symptoms (depressive and/or manic) are also typically present. If the symptoms meet the diagnostic requirements for a specific mental disorder, that diagnosis should also be assigned. |
| 6E2Z | Mental or behavioral disorders associated with pregnancy, childbirth or the puerperium, unspecified. |
| 6E40 | Psychological or behavioral factors affecting disorders or diseases classified elsewhere. Psychological and behavioral factors affecting disorders or diseases classified elsewhere are those that may adversely affect the manifestation, treatment or course of a condition classified in another chapter of the ICD. These factors may adversely affect the manifestation, treatment or course of the disorder or disease classified in another chapter by interfering with the treatment of the disorder or disease by affecting treatment adherence or care seeking; constituting an additional health risk or influencing the underlying pathophysiology to precipitate or exacerbate symptoms or otherwise necessitate medical attention. This diagnosis should be assigned only when the factors increase the risk of suffering, disability or death and represent a focus of clinical attention and should be assigned together with the diagnosis for the relevant other condition. |
| ICD 11 | ICD 10 | DSM 5 |
|---|---|---|
| A brand-new chapter is included in the classification. It includes mental disorders related to pregnancy, childbirth and the postpartum period. It determines whether the main features of the disorder are accompanied by psychotic symptoms or not. In the absence of psychotic symptoms, we most often deal with mood disorders. When psychotic symptoms appear, we diagnose mood disorders and primary psychoses, for example from the schizophrenia group. | No identical chapter to ICD11. The diagnosis of postpartum depression applies only to the puerperium (beginning up to 6 weeks after delivery) and exclusion criteria for other diagnoses. We use the code F53.0: Mild mental and behavioral disorders associated with the puerperium and not otherwise specified: postnatal depression, postpartum depression. | DSM 4: For the first time in the classification, the term “mental disorders with onset after childbirth” is introduced. Postpartum depression develops within 4 weeks of giving birth. DSM 5: In depressive disorders, episodes are distinguished in the perinatal period up to 4 weeks after delivery. When diagnosing psychosis, it should be determined whether it was related to the onset of puerperium up to 4 weeks after delivery. |
| Publication | Medication | Conclusion |
|---|---|---|
| Gao et al., 2017 [21] | fluoxetine | Maternal fluoxetine use is associated with a slightly increased risk of cardiovascular malformations in infants |
| Kolding et al., 2021 [22] | sertraline | No significant differences in fetal cardiac function in pregnancies exposed to sertraline compared to the unexposed |
| Bérard A., Zhao J.P., Sheehy O., 2015 [23] | sertraline | Sertraline use during the first trimester of pregnancy was associated with an increased risk of atrial/ventricular defects and craniosynostosis |
| Huybrechts et al., 2014 [25] | all types of antidepressants | No substantial increase in the risk of cardiac malformations attributable to antidepressant use during the first trimester |
| Cuomo et al., 2018 [26] | sertraline | Sertraline is one of the safest antidepressants during breastfeeding (limitation: expert opinion only) |
| Kolding L., Henriksen J.N., Pedersen L.H., 2023 [27] | sertraline | Sertraline is associated with septal heart malformations, but not with more severe heart malformations (limitation: expert opinion only) |
| Bellantuono et al., 2012 [28] | escitalopram | Escitalopram might be considered safe during pregnancy, in particular as far as major malformations is concerned |
| Gentile S., 2014 [30] | tricyclic antidepressants (TCAs) | Prenatal clomipramine exposure may increase the risk of cardiac defects. TCA are connected with risk of prenatal antidepressant exposure syndrome. There is a slight increase in safety if TCAs (except clomipramine) are used in late pregnancy |
| Vitale et al., 2016 [31] | all types of antidepressants | Regarding antidepressants, only paroxetine seems to lead to an increased risk of malformations, whereas fluoxetine, fluvoxamine, sertraline, citalopram, escitalopram and venlafaxine do not appear to increase this risk |
| Gao S.Y. et al., 2018 [32] | SSRIs | The authors suggest a generally small risk of congenital malformations and argue against a substantial teratogenic effect of SSRIs. Caution is advisable in making decisions about treatment with SSRIs during pregnancy |
| Womersley K., Ripullone K., Agius M., 2017 [33] | SSRIs | The literature shows that paroxetine and fluoxetine have the strongest association with negative outcomes (significant malformations). The associations between sertraline and citalopram with negative outcomes remain mixed and generally unsubstantiated |
| Nielsen R.E., Damkier P., 2012 [34] | all types of antidepressants | Citalopram and sertraline can be used during pregnancy, while some controversy remains over in utero exposure to paroxetine and fluoxetine, which might be associated with an increased risk of fetal cardiovascular malformation |
| Birth Defects Caused by Valproate Use in Pregnancy |
|---|
| Neural tube defects (spinal bifida, anencephaly) Craniofacial abnormalities Intrauterine growth retardation Microcephaly Cardiac defects (septal defect) Hypospadias Valproate syndrome: Facial hypoplasia Ceanth wrinkles Short nose with inverted nostrils, flat nasal bridge Long philtrum Long, narrow superior lip, thick lower scale Microstomy, downturned corners of the mouth Hypertelorism Cardiovascular malformations Psychomotor retardation |
| First trimester The greatest risk-teratogenic potential. May cause cardiac complications (Ebstein syndrome). |
| Second and third trimester Risk of reversible hypothyroidism, non-toxic goiter, neurogenic diabetes insipidus or hypoglycemia. |
| 3rd trimester Risk of neonatal adaptation syndrome (floppy infant syndrome). In order to prevent this, lithium administration should be stopped 1–2 days before delivery by caesarean section, returning to it immediately after delivery. Perinatal period. Risk of blood pressure drops in the newborn; if possible, lithium administration should be withheld. |
| Mild and Moderate Postpartum Depression | Severe Postpartum Depression |
|---|---|
| Computer programs based on the assumptions of CBT therapy | CBT or interpersonal psychotherapy |
| Exercise | Pharmacotherapy if psychotherapy is not sufficient |
| Psychosocial interventions | |
| Non-directive counseling (active listening) | |
| CBT psychotherapy | |
| Interpersonal psychotherapy | |
| Pharmacotherapy when other methods fail |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruszczyńska-Sińczak, I.; Wachowska, K.; Bliźniewska-Kowalska, K.; Gałecki, P. Psychiatric Treatment in Pregnancy: A Narrative Review. J. Clin. Med. 2023, 12, 4746. https://doi.org/10.3390/jcm12144746
Gruszczyńska-Sińczak I, Wachowska K, Bliźniewska-Kowalska K, Gałecki P. Psychiatric Treatment in Pregnancy: A Narrative Review. Journal of Clinical Medicine. 2023; 12(14):4746. https://doi.org/10.3390/jcm12144746
Chicago/Turabian StyleGruszczyńska-Sińczak, Iga, Katarzyna Wachowska, Katarzyna Bliźniewska-Kowalska, and Piotr Gałecki. 2023. "Psychiatric Treatment in Pregnancy: A Narrative Review" Journal of Clinical Medicine 12, no. 14: 4746. https://doi.org/10.3390/jcm12144746
APA StyleGruszczyńska-Sińczak, I., Wachowska, K., Bliźniewska-Kowalska, K., & Gałecki, P. (2023). Psychiatric Treatment in Pregnancy: A Narrative Review. Journal of Clinical Medicine, 12(14), 4746. https://doi.org/10.3390/jcm12144746







