Comparison of Drug-Related Problems in COVID-19 and Non-COVID-19 Patients Provided by a German Telepharmacy Service for Rural Intensive Care Units
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Variables
2.3. Data Sources/Measurement
2.4. Quantitative Variables and Statistical Methods
2.5. Special Drug Treatment
2.6. Special Patients on Risk for DRPs
3. Results
3.1. Participants
3.2. Descriptive Data
3.3. Outcome Data
3.4. Main Results
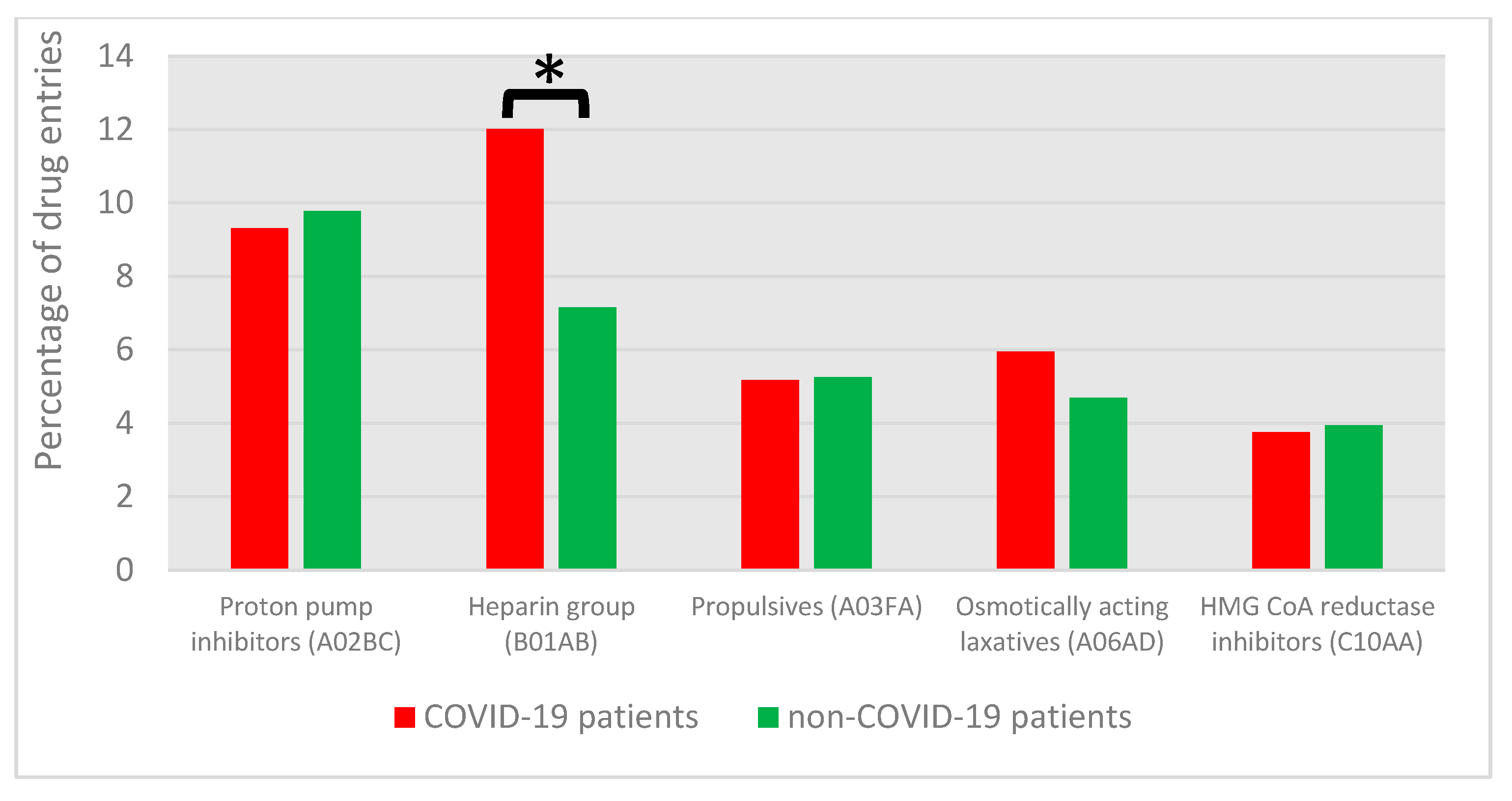
3.4.1. DRP Categories and Associated Drug Classes
3.4.2. DRPs with COVID-19-Specific Medications
3.5. Other Analyses
3.5.1. Patients on a Special Risk for DRPs: Age and Sex
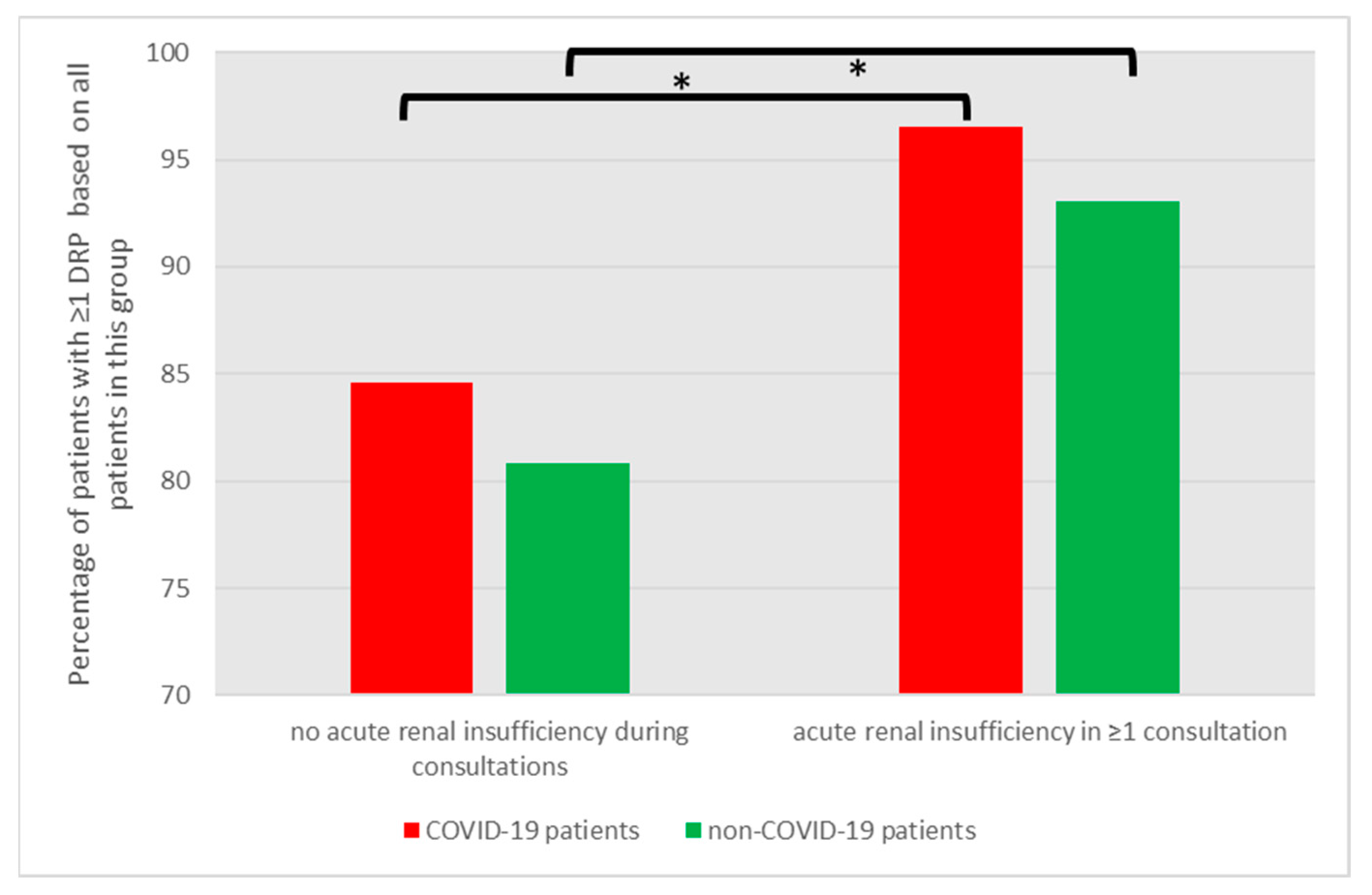
3.5.2. Patients on a Special Risk for DRPs: Acute Renal Insufficiency
3.5.3. Patients on a Special Risk for DRPs: Liver Cirrhosis
3.5.4. Patients on a Special Risk for DRPs: Obesity
4. Discussion
4.1. Key Results
4.2. Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angaran, D.M. Telemedicine and telepharmacy: Current status and future implications. Am. J. Health Syst. Pharm. 1999, 56, 1405–1426. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, M.; Ćulafić, M.; Kovačević, S.V.; Borjanić, S.; Keleč, B.; Miljković, B.; Amidžić, R. Telepharmacy service experience during the COVID-19 pandemic in the Republic of Srpska, Bosnia and Herzegovina. Health Soc. Care Community 2022, 30, e1639–e1650. [Google Scholar] [CrossRef]
- Inch, J.; Notman, F.; Watson, M.; Green, D.; Baird, R.; Ferguson, J.; Hind, C.; McKinstry, B.; Strath, A.; Bond, C. Tele-pharmacy in rural Scotland: A proof of concept study. Int. J. Pharm. Pract. 2017, 25, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Kester, K.A.; Finck, K.M.; Reehal, P.; Reynolds, D. Telepharmacy services in acute care: Diverse needs within a large health system. Am. J. Health Syst. Pharm. 2022, 79, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Amkreutz, J.; Lenssen, R.; Marx, G.; Deisz, R.; Eisert, A. Medication safety in a German telemedicine centre: Implementation of a telepharmaceutical expert consultation in addition to existing tele-intensive care unit services. J. Telemed. Telecare 2020, 26, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.L.; Kosmisky, D.E.; Everhart, S.S. Characterization of dayshift tele-ICU pharmacist activities. J. Telemed. Telecare 2022, 28, 28–77. [Google Scholar] [CrossRef] [PubMed]
- Kosmisky, D.E.; Everhart, S.S.; Griffiths, C.L. Implementation, Evolution and Impact of ICU Telepharmacy Services Across a Health care System. Hosp. Pharm. 2019, 54, 232–240. [Google Scholar] [CrossRef]
- Hilgarth, H.; Waydhas, C.; Dörje, F.; Sommer, J.; Kluge, S.; Ittner, K.P. Drug therapy safety supported by interprofessional collaboration between ICU physicians and clinical pharmacists in critical care units in Germany: Results of a survey. Med. Klin. Intensivmed. Notfallmedizin 2022, 118, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Ameri, A.; Salmanizadeh, F.; Keshvardoost, S.; Bahaadinbeigy, K. Investigating Pharmacists' Views on Telepharmacy: Prioritizing Key Relationships, Barriers, and Benefits. J. Pharm. Technol. 2020, 36, 171–178. [Google Scholar] [CrossRef]
- Alshakrah, M.A.; Steinke, D.T.; Lewis, P.J. Patient prioritization for pharmaceutical care in hospital: A systematic review of assessment tools. Res. Soc. Adm. Pharm. RSAP 2019, 15, 767–779. [Google Scholar] [CrossRef]
- Alsayed, A.R.; Halloush, S.; Hasoun, L.; Alnatour, D.; Al-Dulaimi, A.; Alnajjar, M.S.; Blaibleh, A.; Al-Imam, A.; Alshammari, F.; Khader, H.A. Perspectives of the community in the developing countries toward telemedicine and pharmaceutical care during the COVID-19 pandemic. Pharm. Pract. 2022, 20, 2618. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. JTH 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, I.; Fuster, V.; Lala, A.; Russak, A.J.; Glicksberg, B.S.; Levin, M.A.; Charney, A.W.; Narula, J.; Fayad, Z.A.; Bagiella, E.; et al. Association of Treatment Dose Anticoagulation with In-Hospital Survival Among Hospitalized Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Gevenois, P.A.; Peluso, L.; Pletchette, Z.; Lheureux, O.; Brasseur, A.; Garufi, A.; Talamonti, M.; Motte, S.; Nobile, L.; et al. Higher Intensity Thromboprophylaxis Regimens and Pulmonary Embolism in Critically Ill Coronavirus Disease 2019 Patients. Crit. Care Med. 2020, 48, e1087–e1090. [Google Scholar] [CrossRef] [PubMed]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- The Writing Committee for the REMAP-CAP Investigators. Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. Jama 2020, 324, 1317–1329. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch Institut. Dritte Aktualisierung der Retrospektiven Phaseneinteilung der COVID-19-Pandemie in Deutschland. Epidemiol. Bull. 2022, 38, 3–6. [Google Scholar]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- World Health Organization. ICD-11 for Mortality and Morbidity Statistics. Available online: https://icd.who.int/browse11/l-m/en (accessed on 20 May 2023).
- World Health Organization. ATC/DDD Index 2022. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 20 May 2023).
- Lenssen, R.; Heidenreich, A.; Schulz, J.B.; Trautwein, C.; Fitzner, C.; Jaehde, U.; Eisert, A. Analysis of drug-related problems in three departments of a German University hospital. Int. J. Clin. Pharm. 2015, 38, 119–126. [Google Scholar] [CrossRef]
- Van Mil, J.W.; Horvat, N.; Westerlund, T. Classification for Drug Related Problems V9.00m; Association PCNE: Zuidlaren, The Netherland, 2019. [Google Scholar]
- Langebrake, C.; Hilgarth, H. Clinical pharmacists' interventions in a German university hospital. Pharm. World Sci. PWS 2010, 32, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Langebrake, C.; Ihbe-Heffinger, A.; Leichenberg, K.; Kaden, S.; Kunkel, M.; Lueb, M.; Hilgarth, H.; Hohmann, C. Nationwide evaluation of day-to-day clinical pharmacists' interventions in German hospitals. Pharmacotherapy 2015, 35, 370–379. [Google Scholar] [CrossRef]
- Shank, B.R.; Zimmerman, D.E. Demystifying Drug Dosing in Obese Patients; American Society of Health System Pharmacists: Bethesda, MD, USA, 2016. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, J.E.; Jaynes, H.A.; Kingery, J.R.; Mourad, N.A.; Trujillo, T.N.; Overholser, B.R.; Kovacs, R.J. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 479–487. [Google Scholar] [CrossRef]
- Consultation, W.H.O. Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Erstad, B.L.; Barletta, J.F. Drug dosing in the critically ill obese patient: A focus on medications for hemodynamic support and prophylaxis. Crit. Care 2021, 25, 77. [Google Scholar] [CrossRef] [PubMed]
- Robert Koch Institut. Epidemiologischer Steckbrief zu SARS-CoV-2 und COVID-19. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Steckbrief.html (accessed on 30 September 2022).
- Silva, C.; Ramalho, C.; Luz, I.; Monteiro, J.; Fresco, P. Drug-related problems in institutionalized, polymedicated elderly patients: Opportunities for pharmacist intervention. Int. J. Clin. Pharm. 2015, 37, 327–334. [Google Scholar] [CrossRef]
- Leendertse, A.J.; Egberts, A.C.; Stoker, L.J.; van den Bemt, P.M.; Group, H.S. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch. Intern. Med. 2008, 168, 1890–1896. [Google Scholar] [CrossRef]
- Krähenbühl-Melcher, A.; Schlienger, R.; Lampert, M.; Haschke, M.; Drewe, J.; Krähenbühl, S. Drug-related problems in hospitals: A review of the recent literature. Drug Saf. 2007, 30, 379–407. [Google Scholar] [CrossRef]
- Quintana-Bárcena, P.; Lord, A.; Lizotte, A.; Berbiche, D.; Lalonde, L. Prevalence and Management of Drug-Related Problems in Chronic Kidney Disease Patients by Severity Level: A Subanalysis of a Cluster Randomized Controlled Trial in Community Pharmacies. J. Manag. Care Spec. Pharm. 2018, 24, 173–181. [Google Scholar] [CrossRef]
- Jasińska-Stroschein, M. The Effectiveness of Pharmacist Interventions in the Management of Patient with Renal Failure: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 11170. [Google Scholar] [CrossRef]
- Hayward, K.L.; Patel, P.J.; Valery, P.C.; Horsfall, L.U.; Li, C.Y.; Wright, P.L.; Tallis, C.J.; Stuart, K.A.; Irvine, K.M.; Cottrell, N.W.; et al. Medication-Related Problems in Outpatients with Decompensated Cirrhosis: Opportunities for Harm Prevention. Hepatol. Commun. 2019, 3, 620–631. [Google Scholar] [CrossRef]
- Aghili, M.; Kasturirangan, M.N. Identifying characteristics of drug-related problems in critically ill patients with decompensated liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1569–1576. [Google Scholar] [CrossRef]
- Kluge, S.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Schälte, G.; Spinner, C.D.; Malin, J.J.; Gastmeier, P.; Langer, F.; Wepler, M.; et al. S2k Guideline—Recommendations for Inpatient Therapy of Patients with COVID-19. Pneumologie 2021, 75, 88–112. [Google Scholar] [CrossRef] [PubMed]
- Kluge, S.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Schälte, G.; Spinner, C.D.; Malin, J.J.; Gastmeier, P.; Langer, F.; Wepler, M.; et al. S3-Leitlinie—Empfehlungen zur stationären Therapie von Patienten mit COVID-19. In Pneumologie; Thieme: Stuttgart, Germany, 2021. [Google Scholar]
- MacFie, C.C.; Baudouin, S.V.; Messer, P.B. An integrative review of drug errors in critical care. J. Intensive Care Soc. 2016, 17, 63–72. [Google Scholar] [CrossRef]
- Lee, H.; Ryu, K.; Sohn, Y.; Kim, J.; Suh, G.Y.; Kim, E. Impact on Patient Outcomes of Pharmacist Participation in Multidisciplinary Critical Care Teams: A Systematic Review and Meta-Analysis*. Crit. Care Med. 2019, 47, 1243–1250. [Google Scholar] [CrossRef]
- Borthwick, M. The role of the pharmacist in the intensive care unit. J. Intensive Care Soc. 2019, 20, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Strnad, K.; Shoulders, B.R.; Smithburger, P.L.; Kane-Gill, S.L. A Systematic Review of ICU and Non-ICU Clinical Pharmacy Services Using Telepharmacy. Ann. Pharmacother. 2018, 52, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Hefti, E.; Wei, B.; Engelen, K. Access to Telepharmacy Services May Reduce Hospital Admissions in Outpatient Populations During the COVID-19 Pandemic. Telemed. J. e-Health Off. J. Am. Telemed. Assoc. 2022, 28, 1324–1331. [Google Scholar] [CrossRef]
- Eickhoff, C.; Griese-Mammen, N.; Mueller, U.; Said, A.; Schulz, M. Primary healthcare policy and vision for community pharmacy and pharmacists in Germany. Pharm. Pract. 2021, 19, 2248. [Google Scholar] [CrossRef]
- Khoshnam-Rad, N.; Gholamzadeh, M.; Gharabaghi, M.A.; Amini, S. Rapid implementation of telepharmacy service to improve patient-centric care and multidisciplinary collaboration across hospitals in a COVID era: A cross-sectional qualitative study. Health Sci. Rep. 2022, 5, e851. [Google Scholar] [CrossRef]
- Isleem, N.; Shoshaa, S.; AbuGhalyoun, A.; Khatib, M.; Naseralallah, L.M.; Danjuma, M.I.; Saad, M. Critical care tele-pharmacy services during COVID-19 pandemic: A qualitative exploration of healthcare practitioners' perceptions. J. Clin. Pharm. Ther. 2022, 47, 1591–1599. [Google Scholar] [CrossRef]
- Kane-Gill, S.L.; Kirisci, L.; Verrico, M.M.; Rothschild, J.M. Analysis of risk factors for adverse drug events in critically ill patients. Crit. Care Med. 2012, 40, 823–828. [Google Scholar] [CrossRef]
- Seiberth, S.; Mannell, H.; Birkenmaier, C.; Neuerburg, C.; Smolka, V.; Andraschko, M.; Strobach, D. Benefit of medication reviews by renal pharmacists in the setting of a computerized physician order entry system with clinical decision support. J. Clin. Pharm. Ther. 2022, 47, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Raymond, C.B.; Wazny, L.D.; Sood, A.R. Standards of clinical practice for renal pharmacists. Can. J. Hosp. Pharm. 2013, 66, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Camiré, E.; Moyen, E.; Stelfox, H.T. Medication errors in critical care: Risk factors, prevention and disclosure. Cmaj 2009, 180, 936–943. [Google Scholar] [CrossRef]
- Institute for Safe Medication Practices (ISMP). ISMP List of High-Alert Medications in Acute Care Setttings. Available online: https://www.ismp.org/recommendations/high-alert-medications-acute-list (accessed on 29 June 2016).
- Lee, T.; Davis, E.; Kielly, J. Clinical impact of a pharmacist-led inpatient anticoagulation service: A review of the literature. Integr. Pharm. Res. Pr. 2016, 5, 53–63. [Google Scholar] [CrossRef]
- Al Ammari, M.; AlThiab, K.; AlJohani, M.; Sultana, K.; Maklhafi, N.; AlOnazi, H.; Maringa, A. Tele-pharmacy Anticoagulation Clinic During COVID-19 Pandemic: Patient Outcomes. Front. Pharmacol. 2021, 12, 652482. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, A.R.; Al-Dulaimi, A.; Alnatour, D.; Awajan, D.; Alshammari, B. Validation of an assessment, medical problem-oriented plan, and care plan tools for demonstrating the clinical pharmacist’s activities. Saudi Pharm. J. 2022, 30, 1464–1472. [Google Scholar] [CrossRef]



| COVID-19 Patients (n = 191) | Non-COVID-19 Patients (n = 323) | p Value | |
|---|---|---|---|
| Age | |||
| Median (IQR) | 66 (57–76) | 70 (59–79) | |
| <65 years [n (%)] | 88 (46) | 121 (37) | 0.055 |
| 65–74 years [n (%)] | 47 (25) | 84 (26) | 0.725 |
| 75–84 years [n (%)] | 47 (25) | 93 (29) | 0.303 |
| >85 years [n (%)] | 9 (5) | 25 (8) | 0.182 |
| Sex | |||
| Male [n (%)] | 130 (68) | 187 (58) | 0.022 |
| Female [n (%)] | 61 (32) | 136 (42) | 0.022 |
| BMI | |||
| Median (IQR) | 28 (25–31) | 27 (24–31) | 0.001 |
| Underweight to preobese patients [n (%)] | 119 (62) | 230 (71) | 0.037 |
| Underweight (<18.5) [n (%)] | 1 (1) | 16 (5) | 0.007 |
| Normal range (18.5–24.9) [n (%)] | 77 (40) | 114 (35) | 0.255 |
| Preobese (25.0–29.9) [n (%)] | 41 (21) | 100 (31) | 0.020 |
| Obese patients [n (%)] | 72 (38) | 93 (29) | 0.037 |
| Obese class I (30.0–34.9) [n (%)] | 39 (20) | 52 (16) | 0.215 |
| Obese class II (35.0–39.9) [n (%)] | 18 (9) | 16 (5) | 0.049 |
| Obese class III (>40.0) [n (%)] | 15 (8) | 25 (8) | 0.963 |
| Organ insufficiencies: | |||
| Patients with liver cirrhosis of chronic kidney disease of any type [n (%)] | 30 (16) | 73 (23) | 0.059 |
| Liver cirrhosis of any type [n (%)] | 4 (2) | 17 (5) | 0.040 |
| Chronic kidney disease of any type [n (%)] | 26 (14) | 59 (18) | 0.170 |
| Time period of first telemedicine consultation | |||
| Period 1: first wave (2 March 2020–17 May 2020) [n (%)] | 10 (5) | 22 (7) | NA |
| Period 2: summer plateau (18 May 2020–27 September 2020) [n (%)] | 6 (3) | 83 (26) | NA |
| Period 3: second wave (28 September 2020–28 February 2021) [n (%)] | 58 (30) | 42 (13) | NA |
| Period 4: third wave (1 March 2021–13 June 2021) [n (%)] | 45 (24) | 28 (9) | NA |
| Period 5: summer plateau (14 June 2021–1 August 2021) [n (%)] | 2 (1) | 16 (5) | NA |
| Period 6: fourth wave (2 August 2021–31 December 2021) [n (%)] | 43 (23) | 54 (17) | NA |
| Period 7: fifth wave (1 January 2022–29 May 2022) [n (%)] | 25 (13) | 48 (15) | NA |
| Period 8: sixth wave (30 May 2022–31 August 2022) [n (%)] | 2 (1) | 30 (9) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koeck, J.A.; Dohmen, S.M.; Marx, G.; Eisert, A. Comparison of Drug-Related Problems in COVID-19 and Non-COVID-19 Patients Provided by a German Telepharmacy Service for Rural Intensive Care Units. J. Clin. Med. 2023, 12, 4739. https://doi.org/10.3390/jcm12144739
Koeck JA, Dohmen SM, Marx G, Eisert A. Comparison of Drug-Related Problems in COVID-19 and Non-COVID-19 Patients Provided by a German Telepharmacy Service for Rural Intensive Care Units. Journal of Clinical Medicine. 2023; 12(14):4739. https://doi.org/10.3390/jcm12144739
Chicago/Turabian StyleKoeck, Joachim Andreas, Sandra Maria Dohmen, Gernot Marx, and Albrecht Eisert. 2023. "Comparison of Drug-Related Problems in COVID-19 and Non-COVID-19 Patients Provided by a German Telepharmacy Service for Rural Intensive Care Units" Journal of Clinical Medicine 12, no. 14: 4739. https://doi.org/10.3390/jcm12144739
APA StyleKoeck, J. A., Dohmen, S. M., Marx, G., & Eisert, A. (2023). Comparison of Drug-Related Problems in COVID-19 and Non-COVID-19 Patients Provided by a German Telepharmacy Service for Rural Intensive Care Units. Journal of Clinical Medicine, 12(14), 4739. https://doi.org/10.3390/jcm12144739





