New Possibilities for Hormonal Vaginal Treatment in Menopausal Women
Abstract
1. Introduction
2. Methods
3. Estrogens
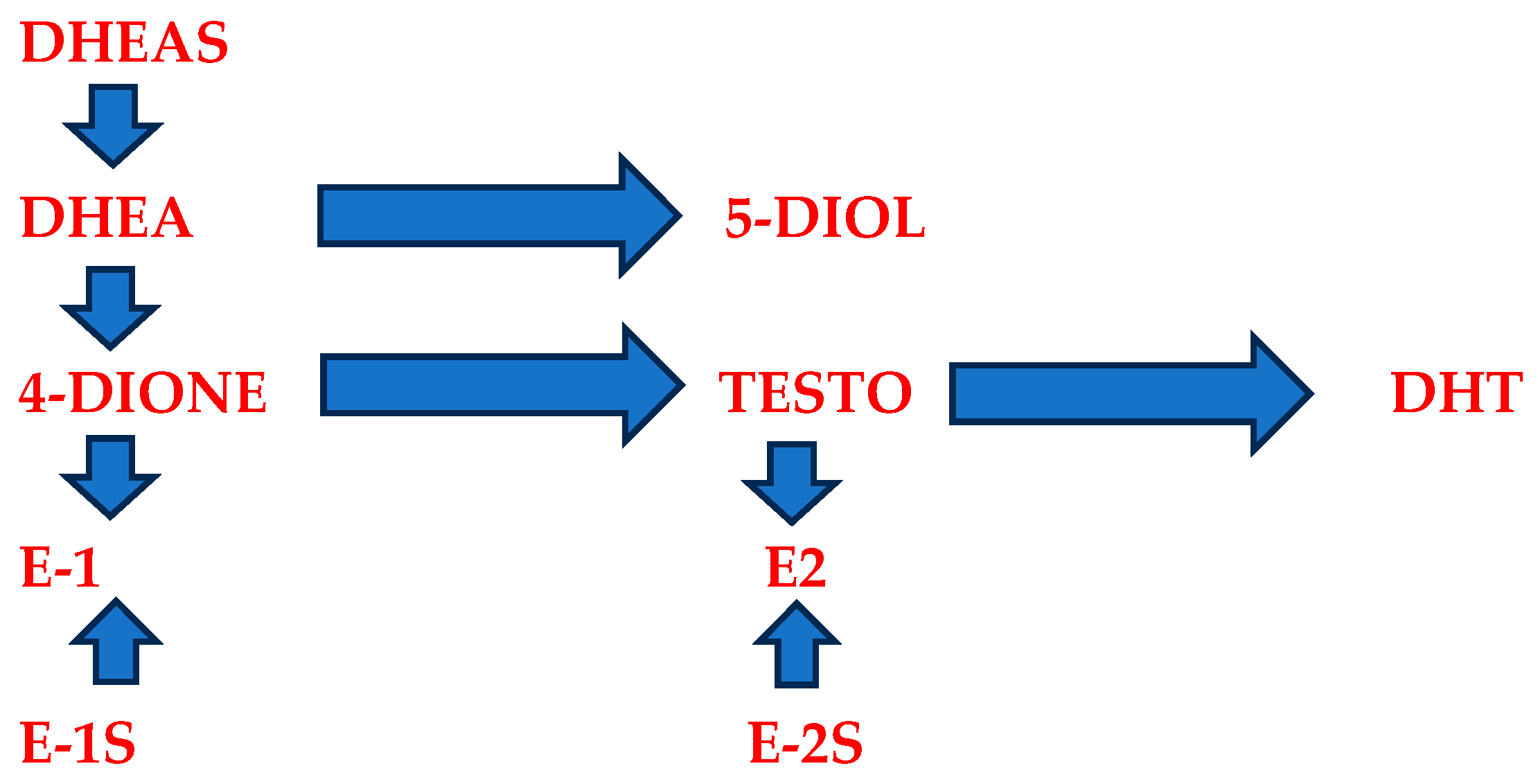
4. Prasterone
5. Vaginal Microbiota and Hormonal Vaginal Therapy
6. Discussion
7. Conclusions
8. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaccaro, C.M.; Mutema, G.K.; Fellner, A.N.; Crisp, C.C.; Estanol, M.V.; Kleeman, S.D.; Pauls, R.N. Histologic and cytologic effects of vaginal estrogen in women with pelvic organ prolapse: A randomized controlled trial. Female Pelvic Med. Reconstr. Surg. 2013, 19, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Portman, D.J.; Gass, M.L. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: New terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014, 21, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.; Volpe, A.; Villa, P.; Cagnacci, A. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: The AGATA study. Maturitas 2016, 83, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Vegunta, S.; Kling, J.M.; Kapoor, E. Androgen Therapy in Women. J. Womens Health (Larchmt) 2020, 29, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kagan, R.; Kellogg-Spadt, S.; Parish, S.J. Practical Treatment Considerations in the Management of Genitourinary Syndrome of Menopause. Drugs Aging 2019, 36, 897–908. [Google Scholar] [CrossRef]
- Krause, M.; Wheeler, T.L.; Snyder, T.E.; Richter, H.E. Local Effects of Vaginally Administered Estrogen Therapy: A Review. J. Pelvic Med. Surg. 2009, 15, 105–114. [Google Scholar] [CrossRef]
- Rutkowski, K.; Sowa, P.; Talipska-Rutkowska, J.; Kuryliszyn-Moskal, A.; Rutkowski, R. Dehydroepiandrosterone (DHEA): Hypes and hopes. Drugs 2014, 74, 1195–1207. [Google Scholar] [CrossRef]
- Felding, C.; Mikkelsen, A.L.; Clausen, H.V.; Loft, A.; Larsen, L.G. Preoperative treatment with oestradiol in women scheduled for vaginal operation for genital prolapse. A randomised, double-blind trial. Maturitas 1992, 15, 241–249. [Google Scholar] [CrossRef]
- Mikkelsen, A.L.; Felding, C.; Clausen, H.V. Clinical effects of preoperative oestradiol treatment before vaginal repair operation. A double-blind, randomized trial. Gynecol. Obs. Obstet. Investig. 1995, 40, 125–128. [Google Scholar] [CrossRef]
- Maher, C.; Feiner, B.; Baessler, K.; Glazener, C.M.A. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst. Rev. 2010, 4. [Google Scholar] [CrossRef]
- Rees, M.; Pérez-López, F.R.; Ceasuc, I.; Depyperee, H.; Erelf, T.; Lambrinoudaki, I.; Schenck-Gustafssonh, K.; Simoncinii, T.; van der Schouwj, Y.T.; Tremollieres, F. EMAS clinical guide: Low-dose vaginal estrogens for postmenopausal vaginal atrophy. Maturitas 2012, 73, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Lethaby, A.; Ayeleke, R.O.; Roberts, H. Local oestrogen for vaginal atrophy in postmenopausal women (Review). Cochrane Libr. 2016, 31, CD001500. [Google Scholar] [CrossRef]
- Archer, D.F.; Labrie, F.; Bouchard, C.; Portman, D.J.; Koltun, W.; Cusan, L.; Labrie, C.; Côté, I.; Lavoie, L.; Martel, C.; et al. Treatment of pain at sexual activity (dyspareunia) with intravaginal dehydroepiandrosterone (prasterone). Menopause 2015, 22, 950–963. [Google Scholar] [CrossRef] [PubMed]
- Griebling, T.L.; Liao, Z.; Smith, P.G. Systemic and topical hormone therapies reduce vaginal innervation density in postmenopausal women. Menopause 2012, 19, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Rahn, D.D.; Ward, R.M. Vaginal estrogen use in postmenopausal women with pelvic floor disorders: Systematic review and practice guidelines. Int. Urogynecol. J. 2015, 26, 3–13. [Google Scholar] [CrossRef]
- Rahn, D.D.; Good, M.M.; Roshanravan, S.M.; Shi, H.; Schaffer, J.I.; Singh, R.J.; Word, R.A. Effects of preoperative local estrogen in postmenopausal women with prolapse: A randomized trial. J. Clin. Endocrinol. Metab. 2014, 99, 3728–3736. [Google Scholar] [CrossRef]
- Marx, P.; Schade, G.; Wilbourn, S.; Blank, S.; Moyer, D.L.; Nett, R. Low-dose (0.3 mg) synthetic conjugated estrogens a is effective for managing atrophic vaginitis. Maturitas 2004, 47, 47–54. [Google Scholar] [CrossRef]
- Lumbanraja, I.L.; FGSiregar, M.F.G.; Lumbanraja, S.N.; Adenin, I.; Lintang, L.S.; Halim, B. Association of Vaginal Maturation Index and Vaginal pH with the Most Bothersome Symptoms of Genitourinary Syndrome of Menopause. J. South Asian Fed. Obstet. Gynaecol. 2021, 13, 288–291. [Google Scholar] [CrossRef]
- Bahmann, G.; Bouchard, C.; Hoppe, D.; Ranganath, R.; Altomare, C.; Vieweg, A.; Graepel, J.; Helzner, E. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause J. N. Am. Menopause Soc. 2009, 16, 719–727. [Google Scholar] [CrossRef]
- Cano, A.; Estévez, J.; Usandizaga, R.; Gallo, J.L.; Guinot, M.; Delgado, J.L.; Castellanos, E.; Moral, E.; Nieto, C.; del Prado, J.M.; et al. The therapeutic effect of a new ultra low concentration estriol gel formulation (0.005% estriol vaginal gel) on symptoms and signs of postmenopausal vaginal atrophy: Results from a pivotal phase III study. Menopause 2012, 19, 1130–1139. [Google Scholar] [CrossRef]
- Dessole, S.; Rubattu, G.; Ambrosini, G.; Gallo, O.; Capobianco, G.; Cherchi, P.; Marci, R.; Cosmi, E. Efficacy of low-dose intravaginal estradiol on urogenital aging in postmenopausal women. Menopause 2004, 11, 49–56. [Google Scholar] [CrossRef]
- Griesser, H.; Skonietzki, S.; Fischer, T.; Fielder, K.; Suesskind, M. Low dose estriol pessaries for the treatment of vaginal atrophy: A double-blind placebo-controlled trial investigating the efficacy of pessaries containing 0.2 mg and 0.03 mg estriol. Maturitas 2012, 71, 360–368. [Google Scholar] [CrossRef]
- Karp, D.R.; Jean-Michel, M.; Johnston, Y.; Suciu, G.; Aguilar, V.C.; Davila, G.W. A randomized clinical trial of the impact of local estrogen on postoperative tissue quality afer vaginal reconstructive surgery. Female Pelvic Med. Reconstr. Surg. 2012, 18, 211–215. [Google Scholar] [CrossRef]
- Raghunandan, C.; Agrawal, S.; Dubey, P.; Choudhury, M.; Jain, A. A comparative study of the effects of local estrogen with or without local testosterone on vulvovaginal and sexual dysfunction in postmenopausal women. J. Sex. Med. 2010, 7, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Daneshmand, F.; Hosseinzadeh, P.; Ghahiri, A.; Ghasemi, M. A comparative study of vaginal estrogen cream and sustained released estradiol vaginal tablet (Vagifem) in the treatment of atrophic vaginitis among postmenopausal women. Iran. J. Reprod. Med. 2014, 12 (Suppl. S1), 12–13. [Google Scholar]
- Dugal, R.; Hesla, K.; Sordal, T.; Aase, K.H.; Lilleeidet, O.; Wickstrom, E. Comparisons of usefulness of estradiol vaginal tablets and estriol vagitories for treatment of vaginal atrophy. Acta Obstet. Et Gynecol. Scand. 2000, 79, 293–297. [Google Scholar]
- Barentsen, R.; Van de Weijer, P.H.M.; Schram, J.H.N. Continuous low dose estradiol released from a vaginal ring versus estriol vaginal cream for urogenital atrophy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1997, 71, 73–80. [Google Scholar] [CrossRef]
- Casper, F.; Petri, E. Local treatment of urogenital atrophy with an estradiol-releasing vaginal ring: A comparative and placebo controlled multicenter study. Int. Urogynecol. J. 1999, 10, 171–176. [Google Scholar] [CrossRef]
- Iosif, C.S.; Batra, S.; Ek, A.; Astedt, B. Estrogen receptors in the human female lower urinary tract. Am. J. Obstet. Gynecol. 1981, 141, 817–820. [Google Scholar] [CrossRef]
- Al-Baghdadi, O.; Ewies, A.A. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: An up-to-date overview. Climacteric 2009, 12, 91–105. [Google Scholar] [CrossRef]
- Xie, Z.; Shi, H.; Zhou, C.; Xie, Z.; Shi, H.; Zhou, C.; Dong, M.; Hong, L.; Jin, H. Alterations of estrogen receptor-alpha and -beta in the anterior vaginal wall of women with urinary incontinence. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 134, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, L.; Bachmann, G.; McClish, D.; Fonda, D.; Birgerson, L. Meta-analysis of estrogen therapy in the management of urogenital atrophy in postmenopausal women: Second report of the Hormones and Urogenital Therapy Committee. Obstet. Gynecol. 1998, 92, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Jarmy-Di Bella, Z.I.; Girao, M.J.; Sartori, M.F.; Di Bella Junior, V.; Lederman, H.M.; Baracat, E.C.; Lima, G.R. Power Doppler of the urethra in continent or incontinent, pre- and postmenopausal women. Int. Urogynecol. J. Pelvic Floor. Dysfunct 2000, 11, 148–154; discussion 154–155. [Google Scholar] [CrossRef]
- Rud, T.; Andersson, K.E.; Asmussen, M.; Hunting, A.; Ulmsten, U. Factors maintaining the intraurethral pressure in women. Investig. Urol. 1980, 17, 343–347. [Google Scholar]
- Mulholland, S.G.; Qureshi, S.M.; Fritz, R.W.; Silverman, H. Effect of hormonal deprivation on the bladder defense mechanism. J. Urol. 1982, 127, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Ballagh, S.A. Vaginal hormone therapy for urogenital and menopausal symptoms. Semin. Reprod. Med. 2005, 23, 126–140. [Google Scholar] [CrossRef]
- Rioux, J.E.; Devlin, C.; Gelfand, M.M.; Steinberg, W.M.; Hepburn, D.S. 17beta-estradiol vaginal tablet versus conjugated equine estrogen vaginal cream to relieve menopausal atrophic vaginitis. Menopause 2000, 7, 156–161. [Google Scholar] [CrossRef]
- Ewies, A.A.A.; Alfhaily, F. Topical vaginal estrogen therapy in managing postmenopausal urinary symptoms: A reality or a gimmick? Climacteric 2010, 13, 405–418. [Google Scholar] [CrossRef]
- Davis, S.R.; McCloud, P.; Strauss, B.J.; Burger, H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas 1995, 21, 227–236. [Google Scholar] [CrossRef]
- Simpson, E.R. Aromatization of androgens in women: Current concepts and findings. Fertil. Steril. 2002, 77 (Suppl. S4), S6–S10. [Google Scholar] [CrossRef]
- Webb, S.J.; Geoghegan, T.E.; Prough, R.A.; Miller, K.K.M. The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metab. Rev. 2006, 38, 89–116. [Google Scholar] [CrossRef]
- Maninger, N.; Wolkowitz, O.M.; Reus, V.I.; Epel, E.S.; Mellon, S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocrinol. 2009, 30, 65–91. [Google Scholar] [CrossRef]
- Davison, S.L.; Davis, S.R. Androgens in women. J. Steroid Biochem. Mol. Biol. 2003, 85, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F.; Archer, D.; Bouchard, C.; Fortier, M.; Cusan, L.; Gomez, J.-L.; Girard, G.; Baron, M.; Ayotte, N.; Moreau, M.; et al. High internal consistency and efficacy of intravaginal DHEA for vaginal atrophy. Gynecol. Endocrinol. 2010, 26, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Panjari, M.; Davis, S.R. Vaginal DHEA to treat menopause related atrophy: A review of the evidence. Maturita 2011, 70, 22–25. [Google Scholar] [CrossRef]
- Labrie, F.; Archer, D.F.; Bouchard, C.; Girard, G.; Ayotte, N.; Gallagher, J.C.; Cusan, L.; Baron, M.; Blouin, F.; Waldbaum, A.S.; et al. Prasterone has parallel beneficial effects on the main symptoms of vulvovaginal atrophy: 52-week open-label study. Maturitas 2015, 81, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.; El-Alfy, M.; Martel, C.; Labrie, F. Effects of dehydroepiandrosterone, premarin and acolbifene on histomorphology and sex steroid receptors in the rat vagina. J. Steroid Biochem. Mol. Biol. 2005, 96, 201–215. [Google Scholar] [CrossRef]
- Pelletier, G.; Ouellet, J.; Martel, C.; Labrie, F. Androgenic action of dehydroepiandrosterone (DHEA) on nerve density in the ovariectomized rat vagina. J. Sex. Med. 2013, 10, 1908–1914. [Google Scholar] [CrossRef]
- Labrie, F.; Archer, D.F.; Koltun, W.; Vachon, A.; Young, D.; Frenette, L.; Portman, D.; Montesino, M.; Côté, I.; Parent, J.; et al. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause 2018, 23, 243–256. [Google Scholar] [CrossRef]
- Shufelt, C.L.; Braunstein, G.D. Safety of testosterone use in women. Maturitas 2009, 63, 63–66. [Google Scholar] [CrossRef]
- Zang, H.; Sahlin, L.; Masironi, B.; Eriksson, E.; Linden Hirschberg, A. Effects of testosterone treatment on endometrial proliferation in postmenopausal women. J. Clin. Endocrinol. Metab. 2007, 92, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, T.K.; Braunstein, G.D. Physiological effects of androgens in women. In Androgen Excess Disorders in Women: Polycystic Ovary Syndrome and Other Disorders; Azziz, R., Nestler, J.E., Dewailly, D., Eds.; Contemporary Endocrinology; Humana Press Inc.: Totowa, NJ, USA, 2006; pp. 49–62. [Google Scholar]
- Wierman, M.E.; Arlt, W.; Basson, R.; Davis, S.R.; Miller, K.K.; Murad, M.H.; Rosner, W.; Santoro, N. Androgen therapy in women: A reappraisal: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 3489–3510. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Intrarosa: Summary of Product Characteristics. 2019. Available online: http://www.ema.europa.eu/ (accessed on 20 June 2019).
- Labrie, F.; Archer, D.; Bouchard, C.; Fortier, M.; Cusan, L.; Gomez, J.-L.; Girard, G.; Baron, M.; Ayotte, N.; Moreau, M.; et al. Effect of intravaginal dehydroepiandrosterone (Prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause 2009, 16, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Factories 2020, 19, 203. [Google Scholar] [CrossRef]
- Smith, S.B.; Ravel, J. The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef]
- Gupta, S.; Kakkar, V.; Bhushan, I. Crosstalk between vaginal microbiome and female health: A review. Microb. Pathog. 2019, 136, 103696. [Google Scholar] [CrossRef]
- Torcia, M.G. Interplay among vaginal microbiome, immune response and sexually transmitted viral infections. Int. J. Mol. Sci. 2019, 20, 266. [Google Scholar] [CrossRef]
- Aldunate, M.; Srbinovski, D.; Hearps, A.C.; Latham, C.F.; Ramsland, P.A.; Gugasyan, R.; Cone, R.A.; Tachedjian, G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 2015, 6, 164. [Google Scholar] [CrossRef]
- Barrientos-Durán, A.; Fuentes-López, A.; de Salazar, A.; Plaza-Díaz, J.; García, F. Reviewing the Composition of Vaginal Microbiota: Inclusion of Nutrition and Probiotic Factors in the Maintenance of Eubiosis. Nutrients 2020, 12, 419. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.; Chen, T. Role of Vaginal Microbiota Dysbiosis in Gynecological Diseases and the Potential Interventions. Front. Microbiol. 2021, 12, 643422. [Google Scholar] [CrossRef] [PubMed]
- Lewis, F.M.; Bernstein, K.T.; Aral, S.O. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet. Gynecol. 2017, 129, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Naumova, I.; Castelo-Branco, C. Current treatment options for postmenopausal vaginal atrophy. Int. J. Women’s Health 2018, 10, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Muhleisen, A.L.; Herbst-Kralovetz, M.M. Menopause and the vaginal microbiome. Maturitas 2016, 91, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Gómez, G.; Del Prado-Audelo, M.L.; Ortega-Peña, S.; Mendoza-Muñoz, N.; Urbán-Morlán, Z.; González-Torres, M.; González-Del Carmen, M.; Figueroa-González, G.; Reyes-Hernández, O.D.; Cortés, H. Modifications in Vaginal Microbiota and Their Influence on Drug Release. Chall. Oppor. Pharm. 2019, 11, 217. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Srinivasan, S.; Zhan, X.; Wu, M.C.; Reed, S.D.; Guthrie, K.A.; LaCroix, A.Z.; Fiedler, T.; Munch, M.; Liu, C.; et al. Vaginal microbiota and genitourinary menopausal symptoms: A cross sectional analysis. Menopause 2017, 24, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef]
- Domoney, C. Treatment of vaginal atrophy. Women’s Health 2014, 10, 191–200. [Google Scholar] [CrossRef]
- Marschalek, M.-L.; Bodner, K.; Kimberger, O.; Zehetmayer, S.; Morgenbesser, R.; Dietrich, W.; Obruca, C.; Husslein, H.; Umek, H.; Koelbl, H.; et al. Does preoperative locally applied estrogen treatment facilitate prolapse-associated symptoms in postmenopausal women with symptomatic pelvic organ prolapse? A randomised controlled double-masked, placebo-controlled, multicenter study. Int. J. Obstet. Ang Gynecol. 2021, 128, 2200–2208. [Google Scholar] [CrossRef]
- Zhixing, S.; Zhu, L.; Xu, T.; Shi, X.; Lang, J. Effects of preoperative vaginal estrogen therapy for the incidence of mesh complication after pelvic organ prolapse surgery in postmenopausal women: Is it helpful or a myth? A 1-year randomized controlled trial. Menopause 2016, 23, 740–748. [Google Scholar]
- Yu, X.; He, L.; Wang, Y.; Wang, L.; Yang, Z.; Lin, Y. Local Estrogen Therapy for Pelvic Organ Prolapse in Postmenopausal Women: A Systematic Review and Meta-Analysis. Iran. J. Public Health 2022, 51, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Loze Onwude, J. Stress incontinence. BMJ Clin. Evid. 2009, 14, 0808. [Google Scholar]
- Collà Ruvolo, C.; Gabrielli, O.; Formisano, C.; Califano, G.; Manna, P.; Venturella, R.; Di Carlo, C. Prasterone in the treatment of mild to moderate urge incontinence: An observational study. Menopause 2022, 29, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Hummelen, R.; Macklaim, J.M.; Bisanz, J.E.; Hammond, J.A.; McMillan, A.; Vongsa, R.; Koenig, D.; Gloor, G.B.; Reid, G. Vaginal microbiome and epithelial gene array in postmenopausal women with moderate to severe dryness. PLoS ONE 2011, 6, e26602. [Google Scholar] [CrossRef]
| Paperwork | Dose | Time of Treatment | n | VMI | Vaginal pH | Lubrication | Dyspareunia | UTI |
|---|---|---|---|---|---|---|---|---|
| Bauchmann 2008 [19] | 0.3 mg estrogen (21 days on/7 days off or twice weekly) | 12 weeks | 423 | + | + | + | ? | ? |
| Cano 2012 [20] | 50 mcg estriol Daily for 3 weeks and then twice weekly | 12 weeks | 114 | + | + | + | ? | ? |
| Dessole 2004 [21] | Intravaginal oestradiol ovules (1 mg) once daily for 2 weeks and then two ovules once weekly for 6 months | 6 months | 44 | ? | + | + | + | + |
| Griesser 2012 [22] | Estriol pessary 0.2 mg. Once daily for 20 days and twice weekly for 9 weeks. | 12 weeks | 142 | + | + | + | ? | ? |
| Estriol pessary 0.03 mg. Once daily for 20 days and twice weekly for 9 weeks. | 147 | + | + | + | ? | ? | ||
| Karp 2012 [23] | Oestradiol-releasing vaginal ring | 12 weeks | 22 | + | + | + | ? | ? |
| Raghunandan 2010 [24] | 0.625 mg of conjugated equine estrogen for 2 weeks and twice weekly for 10 weeks | 12 weeks | 25 | + | + | + | + | ? |
| Daneshmand 2014 [25] | Vaginal estrogen cream 1 tube per night for 14 nights, then 1 tube 2 nights in 1 week for 10 weeks | 12 weeks | 80 | ? | ? | + | + | |
| Estrogen tablet 25 mcg tablets | 12 weeks | 80 | ? | ? | + | + | ? | |
| Dugal 2000 [26] | Oestradiol vaginal tablet 25 mcg 17β oestradiol | 12 weeks | 85 | ? | ? | + | + | ? |
| Barentses 1997 [27] | Oestradiol ring containing 2 mg micronized 17β oestradiol with a constant release of 7.5 mcg oestradiol/24 h or estriol cream containing 1 mg estriol/G of cream 0.5 mg daily for 2 weeks followed by maintenance dose 0.5 mg | 12 weeks | 165 | + | + | + | + | ? |
| Casper 1999 [28] | Oestradiol-releasing silicone ring | 24 weeks | 33 | + | + | + | + | ? |
| Paperwork | Dose | Time of Treatment | n | VMI | Vaginal Ph | Lubrication | Sexual Function | UTI |
|---|---|---|---|---|---|---|---|---|
| Labrie 2009 [55] | Intravaginal prasterone (0.25, 0.5, 1.0%) | 12 weeks | 216 | + | + | + | + | ? |
| Archer [13] | Intravaginal prasterone 0.50% | 12 weeks | 253 | + | + | + | + | ? |
| Labrie [49] | Intravaginal prasterone 0.50% | 12 weeks | 325 | + | + | + | + | ? |
| Labrie [46] | Intravaginal prasterone 0.50% | Up to 52 weeks | 530 | + | + | ? | ? | ? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczyk, K.; Chmaj-Wierzchowska, K.; Wszołek, K.; Wilczak, M. New Possibilities for Hormonal Vaginal Treatment in Menopausal Women. J. Clin. Med. 2023, 12, 4740. https://doi.org/10.3390/jcm12144740
Tomczyk K, Chmaj-Wierzchowska K, Wszołek K, Wilczak M. New Possibilities for Hormonal Vaginal Treatment in Menopausal Women. Journal of Clinical Medicine. 2023; 12(14):4740. https://doi.org/10.3390/jcm12144740
Chicago/Turabian StyleTomczyk, Katarzyna, Karolina Chmaj-Wierzchowska, Katarzyna Wszołek, and Maciej Wilczak. 2023. "New Possibilities for Hormonal Vaginal Treatment in Menopausal Women" Journal of Clinical Medicine 12, no. 14: 4740. https://doi.org/10.3390/jcm12144740
APA StyleTomczyk, K., Chmaj-Wierzchowska, K., Wszołek, K., & Wilczak, M. (2023). New Possibilities for Hormonal Vaginal Treatment in Menopausal Women. Journal of Clinical Medicine, 12(14), 4740. https://doi.org/10.3390/jcm12144740





