1. Introduction
Pulmonary vein isolation (PVI)—the cornerstone of catheter-based atrial fibrillation (AF) ablation [
1]—is less successful for persistent AF than for paroxysmal AF, often requiring repeated procedures [
2,
3,
4]. In Europe, approximately one third of AF ablation procedures are performed in patients with persistent AF [
5]. However, ablation strategies based on anatomical and/or electrophysiological approaches are associated with poor outcomes in this specific population [
6], highlighting the need for a safe, efficient and reproducible ablation technique that can be performed within a reasonable amount of time.
Contiguous lesion optimized pre-specified encircling (CLOSE)-guided PVI is an ablation technique aiming at creating contiguous and optimized (Ablation Index-guided) point-by-point radiofrequency (RF) lesions. The efficacy of CLOSE-guided PVI in treating the paroxysmal form of AF has been demonstrated in multicenter studies [
7,
8]. However, the efficacy of CLOSE-guided PVI to treat persistent forms of AF has been poorly evaluated.
In the present study, we aimed to: (1) evaluate 1-year clinical outcomes in a cohort of patients with persistent AF after CLOSE-guided PVI; and (2) describe the different forms of atrial tachyarrhythmia (ATA) recurrence after CLOSE-guided PVI.
2. Materials and Methods
2.1. Study Participants
The workflow of this study is illustrated in
Figure 1. From October 2020 to October 2021, consecutive patients aged 18–80 years who were referred for persistent AF catheter ablation at the two participating institutions (La Timone University Hospital, Marseille, FR, France; Pasteur University Hospital, Nice, FR, France) were evaluated and enrolled by 2 electrophysiologists (PT and SB). To be included in this study, patients were required to (1) have an ongoing uninterrupted episode of symptomatic persistent AF (continuous episode sustained beyond 7 days) documented by 12-lead electrocardiograms at the outpatient clinic visit and (2) be refractory, intolerant or unwilling to take class I or class III anti-arrhythmic drug therapy (ADT). Non-inclusion criteria were as follows: (1) long-standing persistent AF with ongoing uninterrupted AF episodes ≥ 3 years; (2) New York Heart Association functional class IV; (3) left ventricle ejection fraction ≤ 15%; (4) previous left atrium (LA) ablation; (5) previous mitral valve surgery; (6) congenital heart disease; (7) myocardial infarction or cardiac percutaneous intervention in the previous 6 months; (8) stroke or systemic embolism in the previous 6 months. All included patients underwent CLOSE-guided PVI followed by cavotricuspid isthmus (CTI) ablation if typical atrial flutter (AFL) had been documented prior to inclusion. All the patients gave their informed consent for the procedure. The study protocol (PADS 23-69) conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional human research committee.
2.2. Study Design
This was a prospective, single-arm, two-center observational study aimed at investigating the efficacy of CLOSE-guided PVI in subjects eligible for persistent AF ablation. Efficacy was defined as freedom from any recurrence of ATA episodes > 30 s over a 12-month follow-up period after ablation.
2.3. Scheduled Electrical Cardioversion Prior to Ablation
Between inclusion and ablation, scheduled electrical cardioversion (ECV) was encouraged in patients with a European Heart Rhythm Association functional class ≥ II. If scheduled ECV was performed, the results and the time between ECV and the day of ablation were collected.
2.4. Ablation Procedure
Data on rhythm at the beginning of the procedure (SR, AF, AT or AFL) were collected. The procedure was performed under general anesthesia or conscious sedation by two operators (PT, SB) at the two participating institutions. In the case of general anesthesia, esophageal temperature was monitored (SensiTherm
®, St. Jude Medical, St. Paul, MN, USA). The femoral sheaths were introduced using ultrasound-guided venous puncture [
9]. Intravenous heparin (100 IU/kg) was administered immediately after introducing the femoral sheaths and continuously infused (Activated Clotting Time
> 350 s); in cases of uninterrupted direct oral anticoagulant intake, intravenous heparin was administered immediately after transeptal puncture. All patients underwent CLOSE-guided PVI as previously described (
Figure 2) [
10]. Real-time automated display of RF applications (Visitag, Biosense Webster, Inc) was used with predefined settings of catheter stability (3 mm for 3 s) and minimum contact force (CF; 30% of time > 3 g). RF was delivered in power-controlled mode (without ramping, tip temperature < 43 °C) using 35 W (posterior wall) and 45 W (anterior wall and roof) with an irrigation rate of 17 to 30 mL/min. RF was delivered until the Ablation Index (AI) reached ≥450 at the anterior wall and roof and ≥300 at the posterior wall (see
Figure 1). In cases of intraesophageal temperature rising above 38.5 °C during posterior wall ablation or in the vicinity of the esophagus (localized on pre-procedural cardiothoracic tomodensitometry), RF delivery was stopped at an AI of 300. Maximal distance between two neighboring lesions was ≤6 mm (a distance up to 7 mm was tolerated at the posterior wall to limit risk of esophageal injury). If PVI failed to restore SR, ECV was performed. If stable SR was achieved (≥60 s) after PVI and ECV, the procedure was terminated. If stable SR was not achieved following PVI and ECV, left atrial substrate modification (LASM) was performed by creating linear lesions, focal ablation, or both at the discretion of the operator. If linear lesions were created, conduction block of the created lines was required.
In the case of preprocedural or periprocedural documentation of a typical AFL, CTI ablation was performed until complete block was achieved [
11,
12]. Primary adverse events occurring within the first month after ablation were recorded.
2.5. Blanking Period
The duration of the blanking period (BP) was set at 3 months, and any recurrence of ATAs (>30 s) during this period was not considered a failure of the ablation procedure [
13] and could be treated if required by introducing or increasing the dose of ADT, by performing a new ECV or a repeat ablation procedure.
If ECV was performed during the BP, the results and time between the ECV and the day of ablation were collected. If a repeat procedure was performed during the BP, the pulmonary vein reconnection (PVR) sites were ablated. At the operator’s discretion, LASM could be performed during the repeat procedure.
2.6. Follow-Up
Complications were recorded during a 12-month follow-up period. After ablation, anticoagulation and previously failed ADT were continued. At 3 months, anticoagulation was continued according to the CHA2DS2VASc score, whereas ADT was continued at the discretion of the treating physician. Clinical evaluation and electrocardiogram were performed at 1, 3, 6 and 12 months, or in cases of symptoms.
Recurrence was defined as any episode of AF, AT or AFL > 30 s on Holter electrocardiographs performed at 3, 6 and 12 months.
After a 3-month BP, 12-month freedom from AF and ATA recurrence was assessed. Freedom from AF and ATA recurrence were defined as freedom from any AF episode > 30 s and from any AF, AT or AFL episodes > 30 s, respectively.
In cases of documented ATA recurrence beyond the BP, the type of arrhythmia (AF, AT, or AFL) and the form of ATA recurrence (paroxysmal or persistent) were collected. Changes in ADT, ECV repetition and repeat ablation were also recorded.
2.7. Statistical Analysis
The normality of data distribution was tested using the Shapiro–Wilk test. Continuous variables were expressed as mean ± SD if normally distributed, medians with interquartile ranges if non-normally distributed, and dichotomous variables as percentages. The Kaplan–Meier curve was used to represent freedom from ATAs after 12 months of follow-up. Statistical significance was set at p < 0.05. All statistical analyses were performed using Microsoft® Excel (Microsoft®, Washington, DC, USA).
3. Results
3.1. Population Selection
From the 55 consecutive patients referred for persistent AF ablation, five met the non-inclusion criteria (previous LA ablation, n = 1; ongoing uninterrupted AF episode ≥ 3 years, n = 1; mitral mechanical valve, n = 1; age > 80 years, n = 1; left ventricle ejection fraction ≤ 15%, n = 1). Finally, 50 patients were included in the study (La Timone University Hospital, n = 35; Pasteur University Hospital, n = 15)
3.2. Patient Characteristics
All patients had persistent AF (baseline clinical characteristics are shown in
Table 1). Thirty-four patients underwent a scheduled ECV (68%) 56 ± 38 days prior to ablation. ECV was successful in 30 out of 34 patients (88%).
3.3. Procedural Characteristics
Patient characteristics are listed in
Table 2. PVI was obtained for all patients in all circles. General anesthesia was used in 34 patients (68%). Mean procedure and fluoroscopy times were 141 ± 33 min and 7 ± 6 min, respectively. Total RF time was 23 ± 7 min. Rhythm at the beginning of the procedure was SR in 26 patients (52%), AF in 23 patients (46%) and CTI-dependent AFL in 1 patient (2%). After PVI, ECV was performed in 21 patients (42%). After PVI and ECV, LASM was required in one patient (2%); this procedure consisted of a posterior box creation followed by ECV (box isolation was confirmed after SR restoration). In six patients (12%), CTI ablation was performed with complete block. At the end of the procedure, stable SR was observed in all patients.
3.4. ECV during and after the BP
During the BP, eight patients (16%) experienced persistent AF recurrence, from which seven patients were receiving class I-III ADT; SR was restored in all eight patients by ECV performed 62 ± 17 days after the Ablation Index procedure. Out of the eight patients who underwent ECV during the BP, only three experienced persistent AF recurrence during the 9-months evaluation period. None of the patients underwent ECV after the BP.
3.5. Follow-Up Compliance
Of the 50 patients enrolled in the study, 50 (100%) completed the 12-month follow-up. Holter monitoring compliance was 90% at the 3-month visit, 90% at the 6-month visit, and 98% at the 12-month visit. In total, Holter monitoring compliance was 92.6% at the scheduled visits.
3.6. 12-Month Freedom from AF and AF/AT/AFL
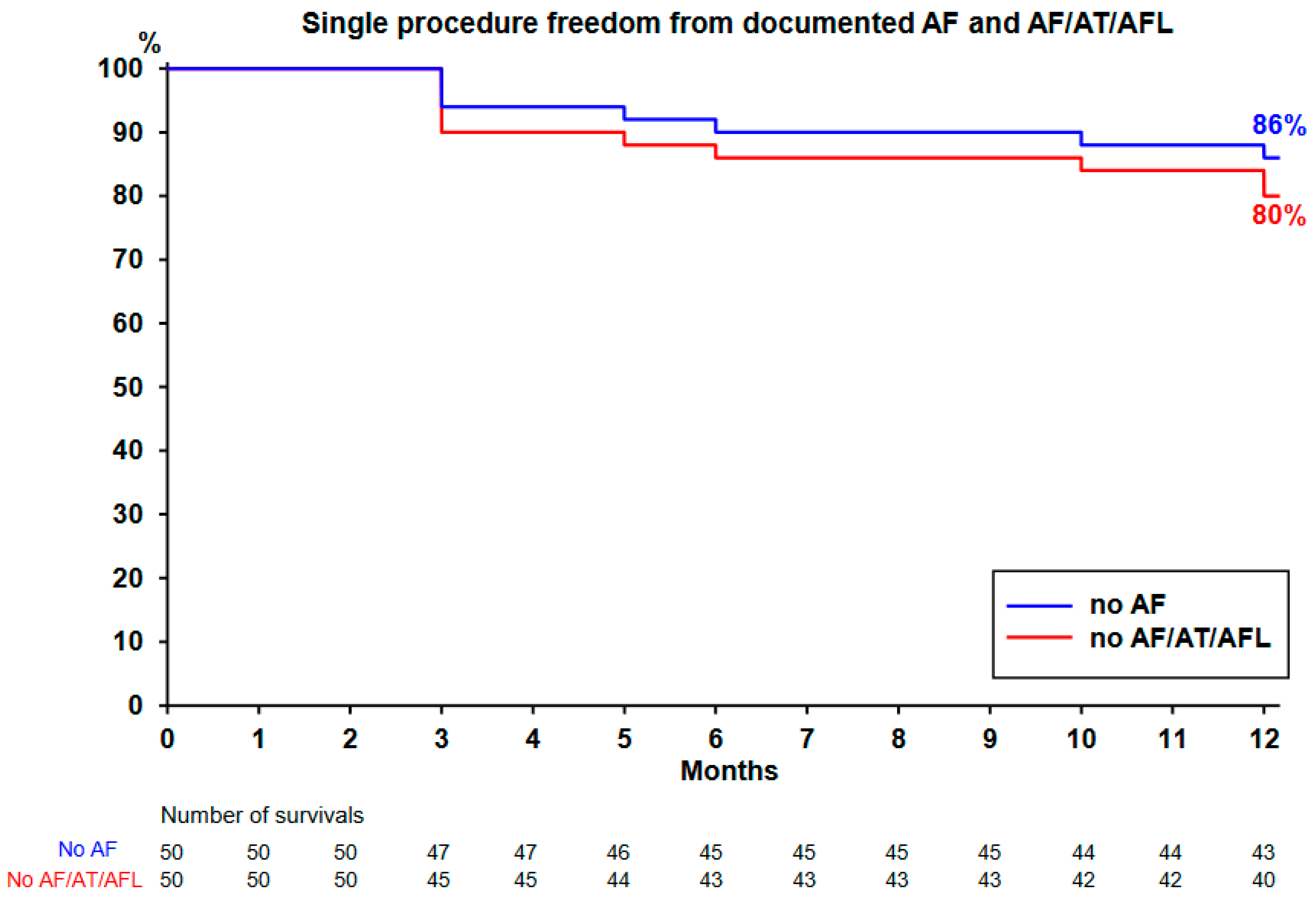
The Kaplan–Meier curve is plotted in
Figure 3 and
Figure 4. All the 50 patients completed the 12-month follow-up. Single-procedure 12-month freedom from AF and from AF/AT/AFL was, respectively, 86% and 80% (
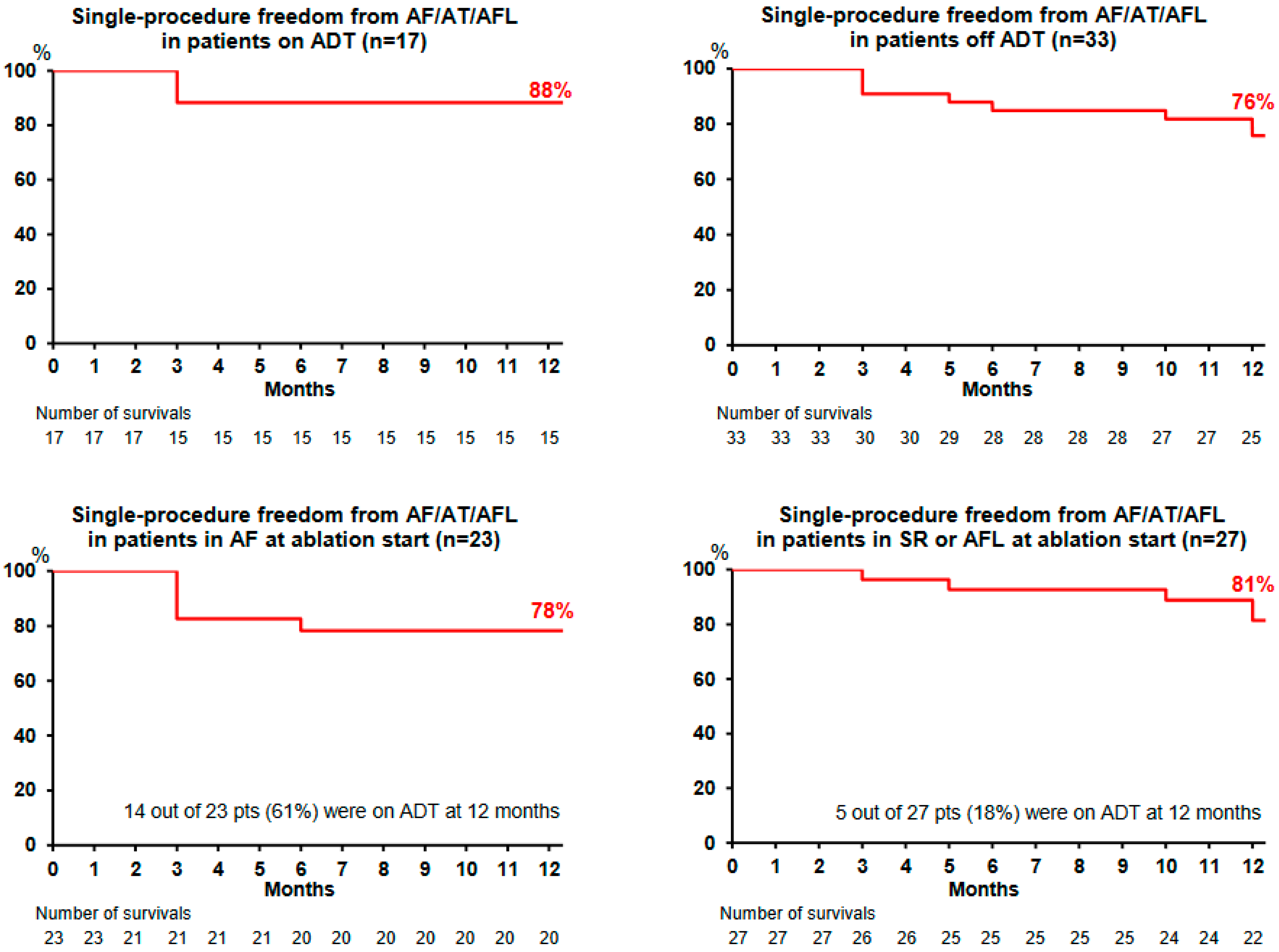
Figure 3). Throughout the course of the study, 19 patients (38%) remained on ADT (maintained in 17 patients and restarted in 2 other patients). Single-procedure freedom values from AF/AT/AFL in patients receiving ADT (
n = 17) and off ADT (
n = 33) were 88% (
Figure 4, upper left) and 76% (
Figure 4, upper right), respectively.
Single-procedure freedom from AF/AT/AFL recurrence in patients presenting with AF at the beginning of the ablation was 78% (
Figure 4, lower left), with 14 out of 23 patients (61%) receiving ADT at 12 months. Single-procedure freedom from AF/AT/AFL recurrence in patients presenting with SR, AT, or AFL at the beginning of the ablation was 81% (
Figure 4, lower right), with 5 out of 27 patients (18%) receiving ADT at the 12-month follow-up visit.
3.7. Arrhythmias Subtypes and ADT Evolution after Ablation
Arrhythmias subtypes and ADT after ablation are shown in
Figure 5.
AF/AT/AFL recurrence was documented in 10 patients (20%) after the 3-month BP. Of the 10 patients with recurrence, 5 still received ADT at the time of recurrence. Paroxysmal ATAs were documented in four patients (8%), with mainly paroxysmal AF (in three patients). Persistent ATAs were documented in six patients (12%) at the 3-month follow-up: AF in four patients, AT in one patient, and typical AFL in one patient. In the two patients with persistent AT/AFL, spontaneous restoration of SR (without changes in ADT) was observed at the next scheduled visit, without any recurrence of ATA on Holter recordings performed at 6 and 12 months. In one patient who experienced persistent AF recurrence 10 months after ablation but did not receive ADT, SR was restored after flecainide reintroduction. For the three patients with persistent AF recurrence, repeat ablation was advised.
A total of 33 patients (66%) were on class I-III ADT at the time of inclusion; 46 patients (92%) were on class I-III ADT at ablation discharge. After the 3-month follow-up visit, the number of patients receiving ADT decreased to 19 (38%) at 12 months, including 2 patients (4%) for whom ADT was reinitiated or the ADT dose was increased due to ATA recurrence; of note, after ADT resumption or dose increase in the two patients mentioned, ATA recurrence was no longer documented during the rest of the follow-up period.
3.8. Ablation Repetition
Repeat ablation was indicated for three patients with persistent AF recurrence, but no repeat procedure was performed. One patient declined and, in the two other patients, repeat ablation was postponed for medical reasons (one patient was waiting for thyroidectomy before amiodarone resumption; the other patient had morbid obesity and maintained excessive alcohol intake requiring specific care before repeat ablation).
3.9. Primary Adverse Events
Two periprocedural complications were observed. One patient with a previous history of sinus node dysfunction presented with transient sinus node dysfunction after SR restoration, which resolved spontaneously within 24 h after ablation without pacemaker implantation (12 months after the Ablation Index procedure, pacemaker implantation was still not needed). One case of non-effusive pericarditis was observed on the day of ablation and was related to the procedure; the patient recovered completely after 4 weeks of oral treatment with colchicine.
No femoral vascular damage, stroke, transient ischemic attack, pericardial effusion, tamponade, atrioesophageal fistula, nor death were observed. Although neither pulmonary vein imaging nor chest radiography were systematically performed after ablation, none of the patients presented with suspicious symptoms indicative of pulmonary vein stenosis or phrenic nerve injury.
4. Discussion
4.1. Main Findings
Our study demonstrates that CLOSE-guided PVI performed in a population with persistent AF monitored using intermittent cardiac rhythm recordings is associated with a high 12-month single-procedure success rate (80%), with one third of the participants still receiving ADT at the end of the follow-up. The two-center nature of our study suggests that this approach may be reproducible using different electrophysiology staff and lab facilities.
In patients with recurrence of AF/AT/AFL after CLOSE-guided PVI, persistent AF and persistent AT/AFL were the dominant forms of recurrence; regardless of paroxysmal or persistent ATA, in 70% of patients with ATA recurrence, SR was restored either spontaneously or after resumption of ADT.
4.2. Candidates for CLOSE-Guided PVI
The studied population was representative of our practice (64-year-old, mostly male participants with obesity; median CHA2DS2VASc score 2.2 ± 1.7), mainly restricted to non-long standing persistent AF, with a relatively short duration of uninterrupted AF episode at the time of inclusion (4 months). Of note, 86% of patients had a history of previous ECV and our cohort presented markedly dilated LAs (50 mL/m
2) related to electro-anatomical remodeling observed in advanced stages of persistent AF and associated with poor outcomes [
14,
15,
16]. In a meta-analysis including populations with persistent AF who underwent various techniques of ablation, single-procedure success without ADT was 43% and increased up to 69% after multiple ablations and/or ADT dose increase, while the addition of extra-pulmonary substrate approaches was associated with declining efficacy when compared to PVI alone [
17]. The high 12-month single-procedure success rate (80%) observed in our study may be explained by factors such as the non-inclusion of patients with severe structural heart disease (mitral valve surgery or severe left ventricle dysfunction) or the use of an optimized PVI approach (CLOSE-guided PVI), including ADT maintenance in one third of our cohort at the end of the follow-up.
4.3. One-Year Freedom from ATA
A summary of previous studies evaluating the effectiveness of CLOSE-guided PVI is shown in
Table 3.
In a population with persistent AF and clinical characteristics comparable to our population who was treated with a similar protocol, Hussein et al. reported 80% 1-year freedom from 30 s AF/AT episodes using Holter recordings and daily electrocardiogram monitoring [
18], in line with our results. However, in contrast to the study by Hussein et al., where patients with long-standing persistent AF were not included and a second ablation performed in 22% of patients, our study evaluated the effectiveness of a single CLOSE-guided PVI procedure in a population with persistent AF, including long-standing persistent AF. In a prospective study evaluating midterm efficacy of AI-guided PVI also using a similar protocol, Solimene and colleagues reported 78% freedom from AF/AT in a subgroup of 24 patients with persistent AF monitored with 24 h Holter recordings [
19], also in line with our results. Nonetheless, patients with long-standing persistent AF were not included in that study, and not all the patients completed a 12-month follow-up period, which may have improved the outcomes. In a retrospective monocentric single-arm study evaluating the effectiveness of an AI-guided PVI strategy in a population with persistent AF including long-standing persistent AF, Yamaguchi et al. reported 70% freedom from AF/AT in patients not receiving ADT [
20]; however, one quarter of the participants was followed up for only 7 months, which may have contributed to improved ablation outcomes.
In a multicenter registry evaluating the effectiveness of CLOSE-guided PVI in a population with persistent AF, Stabile et al. reported 83% freedom from AF/AT in patients receiving or not receiving ADT. However, in contrast to the study by Solimene et al., where patients with long-standing persistent AF were not included and rhythm at the start was AF in less than 13% of the studied population [
21], patients with long-standing persistent AF were included in our study and almost half of the studied population was in AF at the beginning of the procedure, which are factors associated with a higher risk for atrial tachyarrhythmia recurrence.
Finally, our study is the first prospective, bicentric, single-arm study to evaluate the effectiveness of a single CLOSE-guided PVI procedure in a population with persistent AF, including long-standing persistent AF, during a follow-up period of at least 12 months for all patients.
4.4. Safety of CLOSE-Guided PVI
In our study, the rate of adverse events related to the procedure was low, reaching 2% (one case of pericarditis related to the ablation). The procedure time and radiation exposure remained acceptable for daily practice. Recently, a more extensive ablation strategy based on bi-atrial anatomical lesion sets including PVI + ligament of Marshall ablation + mitral line + roof line + CTI ablation was evaluated in a prospective monocentric single-arm study. The authors reported single-procedure 12-month freedom from AF/AT without ADT reaching 72% but, in contrast with our study, complications were observed in 8% of patients (two transient ischemic attack and four pericarditis cases), with prolonged procedure time (more than 4 h) and longer fluoroscopy time [
22].
4.5. ADT throughout the Study
In our study, the use of ADT mildly decreased between time of inclusion (66%) and end of the follow-up period (38% at 12 months). In patients with paroxysmal AF, maintenance of ADT up to 9 and 12 months after RF-based standardized PVI («hybrid» rhythm control strategy) has been shown to improve clinical outcomes in a randomized controlled trial [
23]. In our study of a population with persistent AF, 1-year single-procedure arrhythmia survival was slightly higher in patients receiving ADT. However, the impact of ADT maintenance after ablation on clinical outcomes in patients with persistent AF is still unknown and is under evaluation in a multicenter prospective randomized trial [
24]. Concerns exist about safety in the case of long-term use of class I-III ADT [
25], especially amiodarone [
26], which should be stopped as soon as possible after individualized medical evaluation.
4.6. PVI as Ablation Index Strategy in Persistent AF Population
In the multicenter CRYO4PERSISTENT AF study that evaluated the efficacy of single-cryoballoon PVI-only ablation in a population with persistent AF comparable to our population (mainly non-long-standing persistent AF with mild LA dilation) monitored with Holter recordings, the authors reported ≈60% single-procedure freedom from ATA [
27]. However, in our study, a higher proportion of patients received ADT than that in the CRYO4PERSISTENT AF study, which may explain the differences in the observed outcomes. In a multicenter randomized study comparing laser balloon-guided PVI with non-standardized RF-guided PVI in patients with non-long-standing persistent AF with clinical characteristics comparable to our study population, the authors reported 71% single-procedure freedom from ATA using Holter recordings [
28], slightly inferior to the outcomes observed in our study, which may be related to longer AF episode duration in the population treated with laser balloon-guided PVI.
4.7. Limitation of Radiation Exposure
Owing to the use of an electro-anatomical mapping system, the fluoroscopy time was relatively low in our study (7 min), at least half of that reported in previously published studies that evaluated the efficacy of cryoballoon-based PVI in a persistent AF population [
29,
30]. Andreassi et al. reported that the odds ratios for developing cancer and cataracts were 3 and 6.3, respectively, in a study including 218 electrophysiologists compared to non-exposed professionals [
31]. More recently, it has been reported that the lifetime attributable risk for all cancer incidence is 0.4% for male and 1.5% for female electrophysiologists, while the risk for cancer mortality is 0.22% for male and 0.83% for female electrophysiologists [
32]. When considering the lifetime exposure of an electrophysiologist, this may be a critical concern for reducing the risk of X-ray irradiation-related diseases. Thus, among the available AF ablation technologies presenting similar safety and efficacy, those associated with lower radiation exposure should be preferred for the benefit of the patients as well as for the exposed professionals.
4.8. Impact on Outcomes of Rhythm at the Beginning of the Ablation Procedure
Single-procedure freedom from AF/AT/AFL recurrence was similar between patients with AF at the beginning of the ablation procedure and those in SR, AT or AFL (78% vs. 81%), in contrast to a previous persistent AF ablation study where the authors reported that SR at the beginning of the procedure was associated with better outcomes compared to AF [
33]. In our study, patients presenting with AF at the beginning of the ablation procedure had a 3-fold higher rate of ADT use at the end of the follow-up compared to those in SR or with AT or AFL, which may have contributed to the lack of difference in observed outcomes. Whether rhythm at the beginning of the ablation procedure is a prognostic marker of success needs to be assessed in further studies on ADT withdrawal at the end of the BP.
Finally, our strategy consisting of a single CLOSE-guided PVI procedure in a population with mainly non-long-standing persistent AF was efficient in maintaining stable SR.
5. Study Limitations
This study had a single-arm design and included only a limited number of patients. However, this study prospectively evaluated the largest population of patients with persistent AF, including long-standing persistent AF, with a complete 12-month follow-up period, treated using an ablation strategy limited to CLOSE-guided PVI.
In our study, arrhythmia recurrence was assessed using 24 h Holter recordings, which led to undiagnosed ATA recurrence, and thus overestimation of success [
34].
Although representative of the patients referred to our centers for ablation, the patients included in this study mainly presented non-long-standing persistent AF, which precludes generalization for patients with long-standing persistent AF.
These limitations could be addressed by a large-scale randomized controlled trial, with 30 day Holter monitoring or an insertable cardiac monitor.
6. Conclusions
In a population with persistent AF monitored with intermittent cardiac rhythm recordings, CLOSE-guided PVI was associated with a high 1-year single-procedure arrhythmia survival rate. In this population, one out of six patients required ECV during the BP to terminate persistent AF recurrence and ADT was maintained in one third of the cases. Large-scale multicenter studies focusing on ATA burden monitored using insertable cardiac monitors are required.
7. Contribution to the Field
CLOSE-guided PVI may be a first-line ablation strategy for treating persistent AF, particularly in patients with non-long-standing persistent AF. CLOSE-guided PVI is a non-extensive, safe, and reproducible ablation approach that provides good clinical outcomes 1 year after ablation in a population with persistent AF.
Author Contributions
P.T.: conceptualization, methodology, validation, formal analysis, investigation, writing—original draft, project administration; J.-C.D.: conceptualization, methodology, validation, formal analysis, investigation, writing—review and editing, supervision; S.A.: conceptualization, methodology, validation, formal analysis, investigation, writing—review and editing, supervision; S.-S.B.: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing—review and editing, visualization, supervision, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol (PADS 23-69) conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional human research committee.
Informed Consent Statement
All the patients gave their informed consent for the procedure.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
The authors thank Joseph Ajoury, Julia Legrand, Adrien Mange, Candice Manole, Céline Marra, Alexandre Masse, Yen Nguyen, and Bilel Rezig for technical assistance.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| AAD | anti-arrhythmic drug |
| ADT | anti-arrhythmic drug therapy |
| AF | atrial fibrillation |
| AFL | atrial flutter |
| AI | Ablation Index |
| ASM | atrial substrate modification |
| AT | atrial tachycardia |
| ATA | atrial tachyarrhythmia |
| CCW | counterclockwise |
| CF | contact force |
| CFAE | complex fractionated atrial electrograms |
| CIED | cardiac implantable electronic device |
| CTI | cavotricuspid isthmus |
| ECG | electrocardiogram |
| ECV | electrical cardioversion |
| EGM | electrogram |
| ICM | insertable cardiac monitor |
| LA | left atrium/atrial |
| LOM | ligament of Marshall |
| LVEF | left ventricle ejection fraction |
| PV | pulmonary vein |
| PVI | pulmonary vein isolation |
| PVR | pulmonary vein reconnection |
| RF | radiofrequency |
| SR | sinus rhythm |
| TIA | transient ischemic attack |
References
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.-H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017, 14, e275–e444. [Google Scholar] [CrossRef]
- Arbelo, E.; Brugada, J.; Lundqvist, C.B.; on the behalf of the ESC-EHRA Atrial Fibrillation Ablation Long-term Registry Investigators. Contemporary management of patients undergoing atrial fibrillation ablation: In-hospital and 1-year follow-up findings from the ESC-EHRA atrial fibrillation ablation long-term registry. Eur. Heart J. 2017, 38, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Scherr, D.; Khairy, P.; Miyazaki, S.; Aurillac-Lavignolle, V.; Pascale, P.; Wilton, S.B.; Ramoul, K.; Komatsu, Y.; Roten, L.; Jadidi, A. Five-year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ. Arrhythmia Electrophysiol. 2015, 8, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Tilz, R.R.; Rillig, A.; Thum, A.-M.; Arya, A.; Wohlmuth, P.; Metzner, A.; Mathew, S.; Yoshiga, Y.; Wissner, E.; Kuck, K.-H.; et al. Catheter Ablation of Long-Standing Persistent Atrial Fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J. Am. Coll. Cardiol. 2012, 60, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Brugada, J.; Hindricks, G.; Maggioni, A.P.; Tavazzi, L.; Vardas, P.; Laroche, C.; Anselme, F.; Inama, G.; Jais, P.; et al. The Atrial Fibrillation Ablation Pilot Study: An European Survey on Methodology and results of catheter ablation for atrial fibrillation conducted by the European Heart Rhythm Association. Eur. Heart J. 2014, 35, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Dagres, N.; Bongiorni, M.G.; Larsen, T.B.; Hernández-Madrid, A.; Pison, L.; Blomström-Lundqvist, C.; Scientific Initiatives Committee, European Heart Rhythm Association. Current ablation techniques for persistent atrial fibrillation: Results of the European Heart Rhythm Association Survey. Europace 2015, 17, 1596–1600. [Google Scholar] [CrossRef]
- Duytschaever, M.; Vijgen, J.; De Potter, T.; Scherr, D.; Van Herendael, H.; Knecht, S.; Kobza, R.; Berte, B.; Sandgaard, N.; Albenque, J.-P.; et al. Standardized pulmonary vein isolation workflow to enclose veins with contiguous lesions: The multicentre VISTAX trial. Europace 2020, 22, 1645–1652. [Google Scholar] [CrossRef]
- Di Biase, L.; Monir, G.; Melby, D.; Tabereaux, P.; Natale, A.; Manyam, H.; Athill, C.; Delaughter, C.; Patel, A.; Gentlesk, P.; et al. Composite Index Tagging for PVI in Paroxysmal AF: A Prospective, Multicenter Postapproval Study. JACC Clin. Electrophysiol. 2022, 8, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Errahmouni, A.; Bun, S.-S.; Latcu, D.G.; Saoudi, N. Ultrasound-Guided Venous Puncture in Electrophysiological Procedures: A Safe Method, Rapidly Learned. Pacing Clin. Electrophysiol. 2014, 37, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Taghji, P.; El Haddad, M.; Phlips, T.; Wolf, M.; Knecht, S.; Vandekerckhove, Y.; Tavernier, R.; Nakagawa, H.; Duytschaever, M. Evaluation of a strategy aiming to enclose the pulmonary veins with contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: A pilot study. J. Am. Coll. Cardiol. EP 2018, 4, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Saoudi, N.; Ricard, P.; Rinaldi, J.P.; Yaici, K.; Darmon, J.P.; Anselme, F. Methods to Determine Bidirectional Block of the Cavotricuspid Isthmus in Radiofrequency Ablation of Typical Atrial Flutter. J. Cardiovasc. Electrophysiol. 2005, 16, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Latcu, D.G.; Saoudi, N. A simple and definite proof of complete cavotricuspid isthmus block. Europace 2010, 12, 1211–1212. [Google Scholar] [CrossRef]
- Bertaglia, E.; Stabile, G.; Senatore, G.; Zoppo, F.; Turco, P.; Amellone, C.; De Simone, A.; Fazzari, M.; Pascotto, P. Predictive value of early atrial fibrillation recurrence after circumferential pulmonary vein ablation. Pace Clin. Electrophysiol. 2005, 28, 366–371. [Google Scholar] [CrossRef]
- Arya, A.; Hindricks, G.; Sommer, P.; Huo, Y.; Bollmann, A.; Gaspar, T.; Bode, K.; Husser, D.; Kottkamp, H.; Piorkowski, C. Long-term results and the predictors of outcome of catheter ablation of atrial fibrillation using steerable sheath catheter navigation after single procedure in 674 patients. Europace 2009, 12, 173–180. [Google Scholar] [CrossRef]
- Hof, I.; Chilukuri, K.; Arbab-Zadeh, A.; Scherr, D.; Dalal, D.; Nazarian, S.; Henrikson, C.; Spragg, D.; Berger, R.; Marine, J.; et al. Does Left Atrial Volume and Pulmonary Venous Anatomy Predict the Outcome of Catheter Ablation of Atrial Fibrillation? J. Cardiovasc. Electrophysiol. 2009, 20, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Njoku, A.; Kannabhiran, M.; Arora, R.; Reddy, P.; Gopinathannair, R.; Lakkireddy, D.; Dominic, P. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: A meta-analysis. Europace 2018, 20, 33–42. [Google Scholar] [CrossRef] [PubMed]
- A Clarnette, J.; Brooks, A.G.; Mahajan, R.; Elliott, A.D.; Twomey, D.J.; Pathak, R.K.; Kumar, S.; A Munawar, D.; Young, G.D.; Kalman, J.M.; et al. Outcomes of persistent and long-standing persistent atrial fibrillation ablation: A systematic review and meta-analysis. Europace 2017, 20, f366–f376. [Google Scholar] [CrossRef]
- Hussein, A.; Das, M.; Riva, S.; Morgan, M.; Ronayne, C.; Sahni, A.; Shaw, M.; Todd, D.; Hall, M.; Modi, S.; et al. Use of Ablation Index-Guided Ablation Results in High Rates of Durable Pulmonary Vein Isolation and Freedom From Arrhythmia in Persistent Atrial Fibrillation Patients. Circ. Arrhythmia Electrophysiol. 2018, 11, e006576. [Google Scholar] [CrossRef] [PubMed]
- Solimene, F.; Schillaci, V.; Shopova, G.; Urraro, F.; Arestia, A.; Iuliano, A.; Maresca, F.; Agresta, A.; La Rocca, V.; De Simone, A.; et al. Safety and efficacy of atrial fibrillation ablation guided by Ablation Index module. J. Interv. Card. Electrophysiol. 2018, 54, 9–15. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Takahashi, Y.; Yamamoto, T.; Amemiya, M.; Sekigawa, M.; Shirai, Y.; Tao, S.; Hayashi, T.; Yagishita, A.; Takigawa, M.; et al. Clinical outcome of pulmonary vein isolation alone ablation strategy using VISITAG SURPOINT in nonparoxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2020, 31, 2592–2599. [Google Scholar] [CrossRef]
- Stabile, G.; Lepillier, A.; De Ruvo, E.; Scaglione, M.; Anselmino, M.; Sebag, F.; Pecora, D.; Gallagher, M.; Rillo, M.; Viola, G.; et al. Reproducibility of pulmonary vein isolation guided by the ablation index: 1-year outcome of the AIR registry. J. Cardiovasc. Electrophysiol. 2020, 31, 1694–1701. [Google Scholar] [CrossRef]
- Derval, N.; Duchateau, J.; Denis, A.; Ramirez, F.D.; Mahida, S.; André, C.; Krisai, P.; Nakatani, Y.; Kitamura, T.; Takigawa, M.; et al. Marshall bundle elimination, Pulmonary vein isolation, and Line completion for ANatomical ablation of persistent atrial fibrillation (Marshall-PLAN): Prospective, single-center study. Heart Rhythm. 2020, 18, 529–537. [Google Scholar] [CrossRef]
- Duytschaever, M.; Demolder, A.; Phlips, T.; Sarkozy, A.; El Haddad, M.; Taghji, P.; Knecht, S.; Tavernier, R.; Vandekerckhove, Y.; De Potter, T. PulmOnary vein isolation With vs. without continued antiarrhythmic Drug trEatment in subjects with Recurrent Atrial Fibrillation (POWDER AF): Results from a multicentre randomized trial. Eur. Heart J. 2017, 39, 1429–1437. [Google Scholar] [CrossRef]
- Duytschaever, M. Pulmonary Vein Iisolation with Versus without Continued Antiarrhythmic Drugs in Persistent Aatrial Fibrillation (POWDER-AF2). ClinicalTrials.gov Identifier: NCT03437356. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03437356 (accessed on 1 February 2023).
- Wyse, D.G.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; E Dalquist, J.; et al. A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar] [CrossRef]
- Vorperian, V.R.; Havighurst, T.C.; Miller, S.; January, C.T. Adverse Effects of Low Dose Amiodarone: A Meta-Analysis. J. Am. Coll. Cardiol. 1997, 30, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Boveda, S.; Metzner, A.; Nguyen, D.Q.; Chun, K.J.; Goehl, K.; Noelker, G.; Deharo, J.-C.; Andrikopoulos, G.; Dahme, T.; Lellouche, N.; et al. Single-Procedure Outcomes and Quality-of-Life Improvement 12 Months Post-Cryoballoon Ablation in Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2018, 4, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Neuzil, P.; Luik, A.; Osca Asensi, J.; Schrickel, J.W.; Deneke, T.; Bordignon, S.; Petru, J.; Merkel, M.; Sediva, L.; et al. Laser Balloon or Wide-Area Circumferential Irrigated Radiofrequency Ablation for Persistent Atrial Fibrillation: A Multicenter Prospective Randomized Study. Circ. Arrhythm. Electrophysiol. 2017, 10, e005767. [Google Scholar] [CrossRef] [PubMed]
- Ciconte, G.; Ottaviano, L.; de Asmundis, C.; Baltogiannis, G.; Conte, G.; Sieira, J.; Di Giovanni, G.; Saitoh, Y.; Irfan, G.; Mugnai, G.; et al. Pulmonary vein isolation as index procedure for persistent atrial fibrillation: One-year clinical outcome after ablation using the second-generation cryoballoon. Heart Rhythm. 2015, 12, 60–66. [Google Scholar] [CrossRef]
- Su, W.W.; Reddy, V.Y.; Bhasin, K.; Champagne, J.; Sangrigoli, R.M.; Braegelmann, K.M.; Kueffer, F.J.; Novak, P.; Gupta, S.K.; Yamane, T.; et al. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: Results from the multicenter STOP Persistent AF trial. Heart Rhythm. 2020, 17, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Andreassi, M.G.; Piccaluga, E.; Guagliumi, G.; Del Greco, M.; Gaita, F.; Picano, E. Occupational Health Risks in Cardiac Catheterization Laboratory Workers. Circ. Cardiovasc. Interv. 2016, 9, e003273. [Google Scholar] [CrossRef]
- Tu, C.Y.; Lin, C.J.; Yang, B.H.; Wu, J.; Wu, T.H. Cardiac catheterization real-time dynamic radiation dose measurement to estimate lifetime attributable risk of cancer. PLoS ONE 2020, 15, e0234461. [Google Scholar] [CrossRef] [PubMed]
- Bassiouny, M.; Saliba, W.; Hussein, A.; Rickard, J.; Diab, M.; Aman, W.; Dresing, T.; Callahan, T.; Bhargava, M.; Martin, D.O.; et al. Randomized Study of Persistent Atrial Fibrillation Ablation: Ablate in Sinus Rhythm Versus Ablate Complex-Fractionated Atrial Electrograms in Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2016, 9, e003596. [Google Scholar] [CrossRef] [PubMed]
- Charitos, E.I.; Stierle, U.; Ziegler, P.D.; Baldewig, M.; Robinson, D.R.; Sievers, H.-H.; Hanke, T. A Comprehensive Evaluation of Rhythm Monitoring Strategies for the Detection of Atrial Fibrillation Recurrence: Insights from 647 continuously monitored patients and implications for monitoring after therapeutic interventions. Circulation 2012, 126, 806–814. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).