Role of Selected Circulating Tumor Biomarkers in Patients with Skeletal Metastatic Pancreatic Neuroendocrine Neoplasms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Circulating Biomarkers Measurement
- -
- For ferritin: FERRITIN ELISA, DiaMetra S.r.l. Headquater, SEGRATE (Mi), Italy (catalog number DKO039); reference ranges were 20–400 ng/mL for men and 6–350 ng/mL for women; intra-assay precision and inter-assay precision were ≤7.5% and ≤6.1%, respectively.
- -
- For CY18: TPS ELISA, iDL Biotech AB, Bromma, Sweden (catalog number 10-212), the measuring range was 10–1200 U/L, the normal range was <80 U/L, and the detection limit was <6 U/L.
- -
- For CA125: CanAg CA125 EIA, Fujirebio Diagnostics AB, Goteburg, Sweden (catalog number 400-10), the measuring range was 1.5–500 U/mL, the reference range was 5–39 U/mL, the detection limit was <1.5 U/mL, and the intra-assay precision and inter-assay precision were 2.9–4.4% and 3.1–4.0%, respectively.
- -
- For AFP: CanAg AFP EIA, Fujirebio Diagnostics AB, Goteburg, Sweden (catalog number 600-10), the measuring range was 0.5–500 µg/L, the reference range was 0.1–10 µg/L, the detection limit was <0.5 µg/L, and the intra-assay precision and inter-assay precision were 1.6–2.0% and 1.4–2.0%, respectively.
- -
- For CEA: CanAg CEA EIA, Fujirebio Diagnostics AB, Goteburg, Sweden (catalog number 401-10), the measuring range was 0.25–75 µg/L. the reference range was 0.5–9.1 µg/L, the detection limit was <0.25 µg/L, and the intra-assay precision and inter-assay precision were 2.1–2.7% and 1.5–2.7%, respectively.
- -
- For CA19-9: CanAg CA19-9 EIA, Fujirebio Diagnostics AB, Goteburg, Sweden (catalog number 120010), the measuring range was 1–240 U/mL, the reference range was 0–25 U/mL, the detection limit of the assay was <1 U/mL, and the intra-assay precision and inter-assay precision were 3.3–4.5% and 6.2–7.0%, respectively;
- -
- For B2M: β2-Microglobulin ELISA, Immunodiagnostic AG, Bensheim, Germany (catalog number K 6210), the reference range was <2.5 mg/L, and the detection limit of the assay was <0.1 mg/L.
2.3. Statistical Analysis
3. Results
3.1. Patients with Pancreatic Neuroendocrine Neoplasms vs. Controls
3.2. Patients with Pancreatic Neuroendocrine Neoplasm, Bone Metastases and Tumor Biomarkers
3.3. Diagnostic Accuracy of Tumor Biomarkers
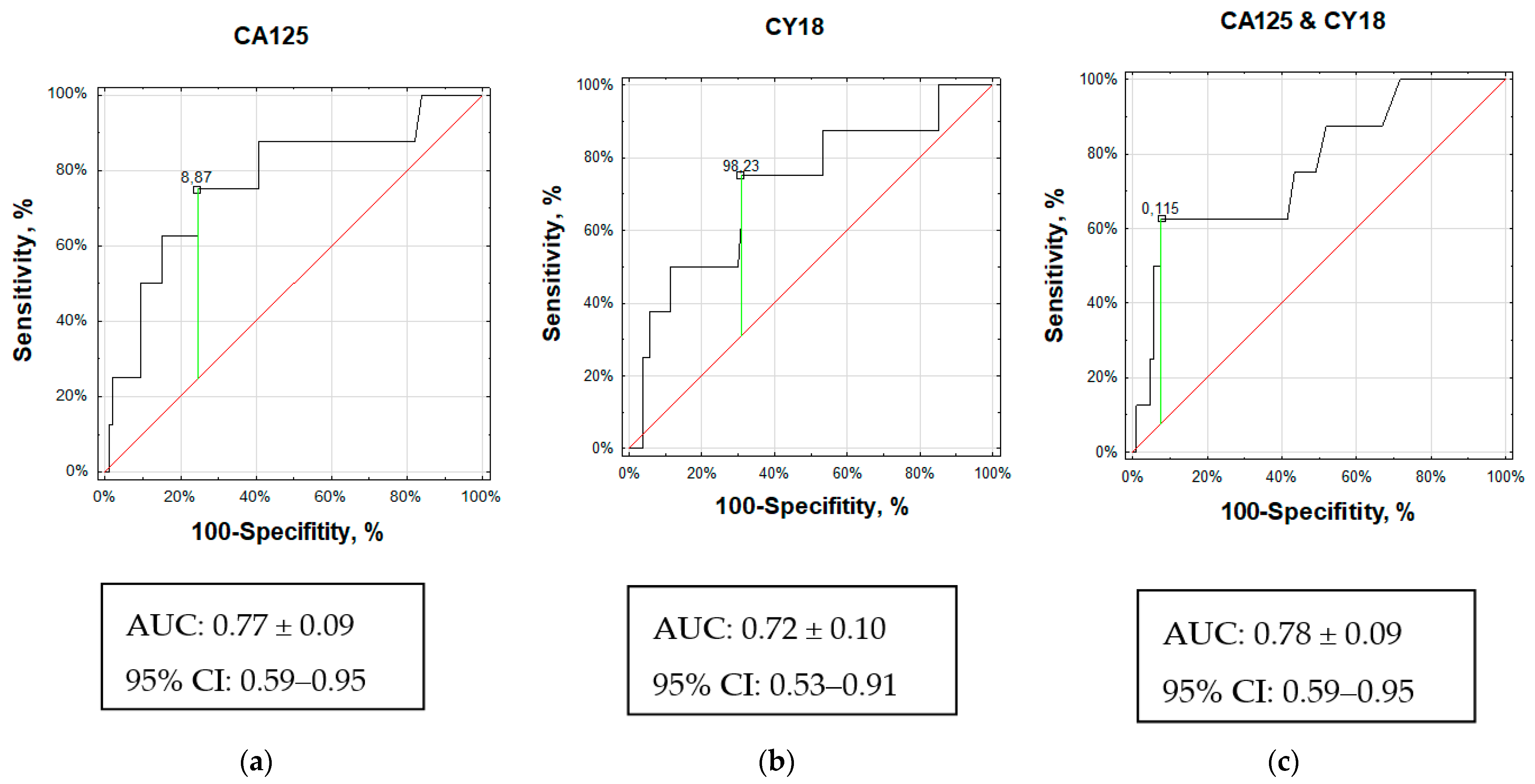
3.3.1. Cancer Antigen 125 (CA125)
3.3.2. Cytokeratin 18 (CY18)
3.3.3. Combination of CY18 and CA125 (multiROC)
3.3.4. Beta-2 Microglobulin (B2M)
3.3.5. Other Tumor Markers
3.3.6. Neuroendocrine Tumor Markers (Chromogranin A, Serotonin, and 5-Hydroxyinoleacetic Acid)
4. Discussion
5. Conclusions
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kos-Kudła, B.; Rosiek, V.; Borowska, M.; Bednarczuk, T.; Bolanowski, M.; Chmielik, E.; Ćwikła, J.B.; Foltyn, W.; Gisterek, I.; Handkiewicz-Junak, D.; et al. Pancreatic neuroendocrine neoplasms—Update of the diagnostic and therapeutic guidelines (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2022, 73, 491–548. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O’Dorisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.A.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 863–881. [Google Scholar] [CrossRef]
- Komek, H.; Ansal Balci, T.; Can, C. Efficacy of Galium-68 DOTATATE PET/CT in the Detection of Metastasis Rate of Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors. Asia Ocean J. Nucl. Med. Biol. 2019, 7, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. The epidemiology of metastases in neuroendocrine tumors. Int. J. Cancer 2016, 139, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Hermans, B.C.M.; de Vos-Geelen, J.; Derks, J.L.; Latten, L.; Liem, I.H.; van der Zwan, J.M.; Speel, E.M.; Dercksen, M.W.; Dingemans, A.C. Unique Metastatic Patterns in Neuroendocrine Neoplasms of Different Primary Origin. Neuroendocrinology 2021, 111, 1111–1120. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Pizanias, M.; Uri, I.; Thomas, D.; Page, T.; Kolomodi, D.; Low, C.S.; Adesanya, O.; Tsoli, M.; Gross, D.J.; et al. The prognosis and management of neuroendocrine neoplasms-related metastatic bone disease: Lessons from clinical practice. Endocrine 2019, 64, 690–701. [Google Scholar] [CrossRef]
- Garcia-Torralba, E.; Spada, F.; Lim, K.H.J.; Jacobs, T.; Barriuso, J.; Mansoor, W.; McNamara, M.G.; Hubner, R.A.; Manoharan, P.; Fazio, N.; et al. Knowns and unknowns of bone metastases in patients with neuroendocrine neoplasms: A systematic review and meta-analysis. Cancer Treat. Rev. 2021, 94, 102168. [Google Scholar] [CrossRef]
- Rizzo, F.M.; Vesely, C.; Childs, A.; Marafioti, T.; Khan, M.S.; Mandair, D.; Cives, M.; Ensell, L.; Lowe, H.; Akarca, A.U.; et al. Circulating tumour cells and their association with bone metastases in patients with neuroendocrine tumours. Br. J. Cancer 2019, 120, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Kavecansky, J.; Wei, L.; Caronia, L.; Ramirez, M.T.; Bloomston, M.; Shah, M.H. Bone metastases in well-to-moderately differentiated neuroendocrine tumors: A single institutional review from the Ohio State University Medical Center. Pancreas 2015, 44, 198–203. [Google Scholar] [CrossRef]
- Cives, M.; Quaresmini, D.; Rizzo, F.M.; Felici, C.; D’Oronzo, S.; Simone, V.; Silvestris, F. Osteotropism of neuroendocrine tumors: Role of the CXCL12/CXCR4 pathway in promoting EMT in vitro. Oncotarget 2017, 8, 22534–22549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosiek, V.; Wójcik-Giertuga, M.; Kos-Kudła, B. Serum tumor markers for detection of bone metastases in patients with lung neuroendocrine neoplasms. Cancer Treat Res Commun. 2022, 31, 100533. [Google Scholar] [CrossRef] [PubMed]
- Alkhateeb, A.A.; Connor, J.R. The significance of ferritin in cancer: Anti-oxidation, inflammation and tumorigenesis. Biochim. Biophys. Acta 2013, 1836, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Knovich, M.A.; Coffman, L.G.; Torti, F.M.; Torti, S.V. Serum ferritin: Past, present and future. Biochim. Biophys. Acta 2010, 1800, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Kernan, K.F.; Carcillo, J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017, 29, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.B.; Chen, Y.; Ding, G.R.; Du, H.W.; Fan, H.Y. Keratin 18 induces proliferation, migration, and invasion in gastric cancer via the MAPK signalling pathway. Clin. Exp. Pharmacol. Physiol. 2021, 48, 147–156. [Google Scholar] [CrossRef] [PubMed]
- . Singh Bhangu, J.; Macher-Beer, A.; Schimek, V.; Garmroudi, B.; Tamandl, D.; Unger, L.W.; Bachleitner-Hofmann, T.; Oehler, R. Circulating caspase-cleaved cytokeratin 18 correlates with tumour burden and response to therapy in patients with colorectal cancer liver metastasis. Clin. Chim. Acta 2023, 538, 53–59. [Google Scholar] [CrossRef]
- Eguchi, A.; Iwasa, M.; Tamai, Y.; Yamada, M.; Okuno, K.; Shigefuku, R.; Yoshikawa, K.; Tempaku, M.; Sakaguchi, K.; Tanaka, H.; et al. The prognostic potential of fragmented CK18 serum levels in HCC patients reflecting disease progression and overall hepatocyte damage. Front. Oncol. 2022, 12, 993705. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Teng, T.Z.J.; Shelat, V.G. Carbohydrate antigen 19-9—Tumor marker: Past, present, and future. World J. Gastrointest. Surg. 2020, 12, 468–490. [Google Scholar] [CrossRef]
- Lech, G.; Słotwiński, R.; Słodkowski, M.; Krasnodębski, I.W. Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J. Gastroenterol. 2016, 22, 1745–1755. [Google Scholar] [CrossRef]
- Yamashita, T.; Higashi, M.; Sugiyama, H.; Morozumi, M.; Momose, S.; Tamaru, J.I. Cancer antigen 125 expression enhances the gemcitabine/cisplatin-resistant tumor microenvironment in bladder cancer. Am. J. Pathol. 2022, 193, 350–361. [Google Scholar] [CrossRef]
- Saad, H.M.; Tourky, G.F.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Khattab, A.M.; Elmasry, S.A.; Alsayegh, A.A.; Hakami, Z.H.; Alsulimani, A.; Sabatier, J.M.; et al. The Potential Role of MUC16 (CA125) Biomarker in Lung Cancer: A Magic Biomarker but with Adversity. Diagnostics 2022, 12, 2985. [Google Scholar] [CrossRef]
- Jacobs, I.; Bast, R.C., Jr. The CA 125 tumour-associated antigen: A review of the literature. Hum. Reprod. 1989, 4, 1–12. [Google Scholar] [CrossRef]
- Johnson, P.J. Tumor Markers in Primary malignancies of the liver. In Tumor Markers: Physiology, Pathobiology, Technology and Clinical Applications; Dimandis, E.P., Ed.; AACC Press: Washington, DC, USA, 2002; pp. 269–276. [Google Scholar]
- Stenman, U.H.; Alfthan, H. Markers for Testicular Cancer. In Tumor Markers: Physiology, Pathobiology, Technology and Clinical Applications; Dimandis, E.P., Ed.; AACC Press: Washington, DC, USA, 2002; pp. 351–359. [Google Scholar]
- Katzmann, J.A.; Greipp, P.R.; O’Fallon, W.M.; Kyle, R.A. Serum beta 2-microglobulin. Mayo. Clin. Proc. 1986, 61, 752–753. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, B.; Wei, J. Beta2-microglobulin(B2M) in cancer immunotherapies: Biological function, resistance and remedy. Cancer Lett. 2021, 517, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Linder, S. Cytokeratin markers come of age. Tumour. Biol. 2007, 28, 189–195. [Google Scholar] [CrossRef]
- Menz, A.; Weitbrecht, T.; Gorbokon, N.; Büscheck, F.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Hinsch, A.; Höflmayer, D.; Weidemann, S.; et al. Diagnostic and prognostic impact of cytokeratin 18 expression in human tumors: A tissue microarray study on 11,952 tumors. Mol. Med. 2021, 27, 16. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.R.; Cui, Y.; Fang, J.Y. Biological functions of cytokeratin 18 in cancer. Mol. Cancer Res. 2012, 10, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yang, L.; Lin, Y.; Chang, X.; Wu, H.; Chen, Y. Prognostic value of non-invasive serum Cytokeratin 18 detection in gastrointestinal cancer: A meta-analysis. J. Cancer 2019, 10, 4814–4823. [Google Scholar] [CrossRef]
- Urano-Takaoka, M.; Sumida, H.; Miyagawa, T.; Awaji, K.; Nagai, K.; Omatsu, J.; Miyake, T.; Sato, S. Serum Cytokeratin 18 as a Metastatic and Therapeutic Marker for Extramammary Paget’s Disease. Acta Derm. Venereol. 2022, 102, adv00636. [Google Scholar] [CrossRef]
- Tas, F.; Karabulut, S.; Yildiz, I.; Duranyildiz, D. Clinical significance of serum M30 and M65 levels in patients with breast cancer. Biomed. Pharmacother. 2014, 68, 1135–1140. [Google Scholar] [CrossRef]
- Shi, R.; Wang, C.; Fu, N.; Liu, L.; Zhu, D.; Wei, Z.; Zhang, H.; Xing, J.; Wang, Y. Downregulation of cytokeratin 18 enhances BCRP-mediated multidrug resistance through induction of epithelial-mesenchymal transition and predicts poor prognosis in breast cancer. Oncol. Rep. 2019, 41, 3015–3026. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, M.; Zeng, Y.; Li, Y.; Zhang, C.; Getzenberg, R.H.; Song, Y. Downregulation of cytokeratin 18 is associated with paclitaxel-resistance and tumor aggressiveness in prostate cancer. Int. J. Oncol. 2016, 48, 1730–1736. [Google Scholar] [CrossRef] [Green Version]
- Moss, E.L.; Hollingworth, J.; Reynolds, T.M. The role of CA 125 in clinical practice. J. Clin. Pathol. 2005, 58, 308–312. [Google Scholar] [CrossRef] [Green Version]
- Bast, R.C., Jr.; Xu, F.J.; Yu, Y.H.; Barnhill, S.; Zhang, Z.; Mills, G.B. CA 125: The past and the future. Int. J. Biol. Markers 1998, 13, 179–187. [Google Scholar] [CrossRef]
- Falcão, F.; de Oliveira, F.R.A.; da Silva, M.C.F.C.; Sobral Filho, D.C. Carbohydrate antigen 125: A promising tool for risk stratification in heart diseases. Biomark. Med. 2018, 12, 367–381. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Xiubin, Z.; Wei, H.; Chenghao, G. Cancer antigen-125 and ICAM-1 are together responsible for ascites in liver cirrhosis. Clin. Lab. 2014, 60, 653–658. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, X.; Liu, X.; Zhu, S. Prognostic Value of Elevated Pre-treatment Serum CA-125 in Epithelial Ovarian Cancer: A Meta-Analysis. Front. Oncol. 2022, 12, 868061. [Google Scholar] [CrossRef]
- Wohlmuth, C.; Djedovic, V.; Kjaer, S.K.; Jensen, A.; Glasspool, R.; Roxburgh, P.; DeFazio, A.; Johnatty, S.E.; Webb, P.M.; Modugno, F.; et al. CA-125 Levels Are Predictive of Survival in Low-Grade Serous Ovarian Cancer-A Multicenter Analysis. Cancers 2022, 14, 1954. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.N.; Koh, E.Y.; Jang, J.Y.; Kim, C.W. Multiple biomarkers are more accurate than a combination of carbohydrate antigen 125 and human epididymis protein 4 for ovarian cancer screening. Obstet. Gynecol. Sci. 2022, 65, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Fu, J.; Zhang, L. Serum CA125 levels are decreased in rectal cancer but increased in fibrosis-associated diseases and in most types of cancers. Prog. Mol. Biol. Transl. Sci. 2019, 162, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, M.; Wang, X.G. The Implication and Significance of Beta 2 Microglobulin: A Conservative Multifunctional Regulator. Chin. Med. J. 2016, 129, 448–455. [Google Scholar] [CrossRef]
- Althubiti, M.; Elzubier, M.; Alotaibi, G.S.; Althubaiti, M.A.; Alsadi, H.H.; Alhazmi, Z.A.; Alghamdi, F.; El-Readi, M.Z.; Almaimani, R.; Babakr, A. Beta 2 microglobulin correlates with oxidative stress in elderly. Exp. Gerontol. 2021, 150, 111359. [Google Scholar] [CrossRef] [PubMed]
- Prizment, A.E.; Linabery, A.M.; Lutsey, P.L.; Selvin, E.; Nelson, H.H.; Folsom, A.R.; Church, T.R.; Drake, C.G.; Platz, E.A.; Joshu, C. Circulating Beta-2 Microglobulin and Risk of Cancer: The Atherosclerosis Risk in Communities Study (ARIC). Cancer Epidemiol. Biomarkers. Prev. 2016, 25, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.X.; Wang, L.; Ji, P.Y.; Zhao, G.G.; Zhong, G.P.; Wang, Z.P. Correlation of serum β2-microglobulin levels with prostate-specific antigen, Gleason score, clinical stage, tumor metastasis and therapy efficacy in prostate cancer. Arch. Med. Res. 2013, 44, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Mink, S.R.; Hodge, A.; Agus, D.B.; Jain, A.; Gross, M.E. Beta-2-microglobulin expression correlates with high-grade prostate cancer and specific defects in androgen signaling. Prostate 2010, 70, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, P.; Migliori, M.; Simoni, P.; Casadei, R.; De Iasio, R.; Corinaldesi, R.; Gullo, L. Diagnostic value of plasma chromogranin A in neuroendocrine tumours. Eur. J. Gastroenterol. Hepatol. 2001, 13, 55–58. [Google Scholar] [CrossRef]
- Paik, W.H.; Ryu, J.K.; Song, B.J.; Kim, J.; Park, J.K.; Kim, Y.T.; Yoon, Y.B. Clinical usefulness of plasma chromogranin a in pancreatic neuroendocrine neoplasm. J. Korean. Med. Sci. 2013, 28, 750–754. [Google Scholar] [CrossRef] [Green Version]
- Szarvas, T.; Csizmarik, A.; Fazekas, T.; Hüttl, A.; Nyirády, P.; Hadaschik, B.; Grünwald, V.; Püllen, L.; Jurányi, Z.; Kocsis, Z.; et al. Comprehensive analysis of serum chromogranin A and neuron-specific enolase levels in localized and castration-resistant prostate cancer. BJU Int. 2021, 127, 44–55. [Google Scholar] [CrossRef]
- Sarrouilhe, D.; Clarhaut, J.; Defamie, N.; Mesnil, M. Serotonin and Cancer: What Is the Link? Curr. Mol. Med. 2015, 15, 62–77. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017, 20, 48. [Google Scholar] [CrossRef] [Green Version]
- Massironi, S.; Rossi, R.E.; Casazza, G.; Conte, D.; Ciafardini, C.; Galeazzi, M.; Peracchi, M. Chromogranin A in diagnosing and monitoring patients with gastroenteropancreatic neuroendocrine neoplasms: A large series from a single institution. Neuroendocrinology 2014, 100, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.E.; Ciafardini, C.; Sciola, V.; Conte, D.; Massironi, S. Chromogranin A in the Follow-up of Gastroenteropancreatic Neuroendocrine Neoplasms: Is It Really Game over? A Systematic Review and Meta-analysis. Pancreas 2018, 47, 1249–1255. [Google Scholar] [CrossRef] [PubMed]

| Variable | Category | PanNEN Patients | Controls |
|---|---|---|---|
| Number | No. | 115 | 40 |
| Age (years) | Mean (range) | 53 (19–79) | 50 (25–78) |
| Gender | Males | 49 (43%) | 9 (23%) |
| Females | 66 (57%) | 31 (77%) | |
| BMI (kg/m2) | <30 | 101 (88%) | N/A |
| >30 | 14 (12%) | ||
| Grade | NET G1 | 52 (45%) | N/A |
| NET G2 | 45 (39%) | ||
| NET G3 | 3 (3%) | ||
| NEC | 5 (4%) | ||
| Clinical stage | I | 31 (27%) | N/A |
| II | 26 (23%) | ||
| III | 14 (12%) | ||
| IV | 44 (38%) | ||
| Bone metastases | Yes | 8 (7%) | N/A |
| No | 107 (93%) |
| ID | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 |
|---|---|---|---|---|---|---|---|---|
| Sex | Female | Male | Female | Male | Male | Female | Female | Female |
| Age (year) | 64 | 25 | 70 | 33 | 74 | 54 | 42 | 60 |
| BMI (kg/m2) | 23.23 | 21.48 | 20.78 | 19.32 | 29.54 | 17.31 | 19.71 | 27.34 |
| Functional status | NF-PNEN | NF-PNEN | NF-PNEN | NF-PNEN | NF-PNEN | F-PNEN | NF-PNEN | NF-PNEN |
| Ki-67 (%) of primary | 1 | 10 | 10 | 3 | 3 | 2 | 50 | 60 |
| Grade | NET G1 | NET G2 | NET G2 | NET G2 | NET G1 | NET G1 | NEC | NEC |
| No. of BM lesion | single | multiple | multiple | multiple | single | multiple | single | single |
| Localisation of BMets | right pubic bone | vertebrae rib sternum | vertebrae humerus | vertebrae | right rib | vertebrae sacrum | right hip bone | right shoulder blade |
| Method used for detection of BMets | 68Ga PET/CT | CT | 68Ga PET/CT | CT | 68Ga PET/CT | CT | FDG PET/CT | 68Ga PET/CT |
| Time point of BMets occurrence after initial diagnosis (months) | 8 | 41 | 16 | 29 | 5 | 3 | 7 | 1 |
| Pancreatic primary | body | body | tail | tail | body | tail | head | head |
| Tumor size (mm) | 11 | 16 | 43 | 35 | 10 | 83 | 84 | 36 |
| Previous type of treatment | surgery | SSA PRRT everolimus CHTH | SSA | CHTH | N/A | N/A | Surgery CHTH RTH | CHTH |
| Variable | Metastatic PanNEN Patients (n = 8) Median [IR] | Non-Metastatic PanNEN Patients (n = 107) Median [IR] | p Value |
|---|---|---|---|
| Age (years) | 57 [38–67] | 55 [42–65] | NS |
| BMI (kg/m2) | 21 [20–25] | 25 [23–28] | NS |
| CY18 (U/L) | 144 [79–288] | 62 [36–120] | 0.04 |
| CA125 (U/mL) | 13 [8–50] | 6 [3–9] | 0.01 |
| Ferritin (ng/mL) | 129 [47–194] | 73 [28–135] | NS |
| CA19-9 (U/mL) | 15 [4–19] | 9 [5–16] | NS |
| AFP (µg/L) | 3 [2–12] | 3 [2–5] | NS |
| CEA (µg/L) | 2 [1–5] | 1 [1–2] | NS |
| B2M (mg/L) | 1 [1–2] | 1 [1–2] | NS |
| CgA (µg/L) | 84 [44–678] | 45 [27–98] | NS |
| serotonin (ng/mL) | 200 [169–318] | 245 [146–372] | NS |
| 5-HIAA (mg/24 h) | 3 [3–3] | 3 [2–5] | NS |
| Ki-67 (%) | 7 [2–35] | 3 [1–5] | NS |
| Tumor size (mm) | 43 [11–83] | 27 [16–47] | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosiek, V.; Janas, K.; Witkowska, M.; Kos-Kudła, B. Role of Selected Circulating Tumor Biomarkers in Patients with Skeletal Metastatic Pancreatic Neuroendocrine Neoplasms. J. Clin. Med. 2023, 12, 4687. https://doi.org/10.3390/jcm12144687
Rosiek V, Janas K, Witkowska M, Kos-Kudła B. Role of Selected Circulating Tumor Biomarkers in Patients with Skeletal Metastatic Pancreatic Neuroendocrine Neoplasms. Journal of Clinical Medicine. 2023; 12(14):4687. https://doi.org/10.3390/jcm12144687
Chicago/Turabian StyleRosiek, Violetta, Ksenia Janas, Magdalena Witkowska, and Beata Kos-Kudła. 2023. "Role of Selected Circulating Tumor Biomarkers in Patients with Skeletal Metastatic Pancreatic Neuroendocrine Neoplasms" Journal of Clinical Medicine 12, no. 14: 4687. https://doi.org/10.3390/jcm12144687
APA StyleRosiek, V., Janas, K., Witkowska, M., & Kos-Kudła, B. (2023). Role of Selected Circulating Tumor Biomarkers in Patients with Skeletal Metastatic Pancreatic Neuroendocrine Neoplasms. Journal of Clinical Medicine, 12(14), 4687. https://doi.org/10.3390/jcm12144687








