Prospects for Precision Medicine in Acute Myocardial Infarction: Patient-Level Insights into Myocardial Injury and Repair
Abstract
Highlights
- Highly controlled experimental studies have illustrated the importance of ischaemia time in relation to myocyte necrosis in acute myocardial infarction. However, in humans, the situation is more complex and the relationship between clinical outcomes and ischaemia time is non-linear over a time window that is relevant to clinical presentation.
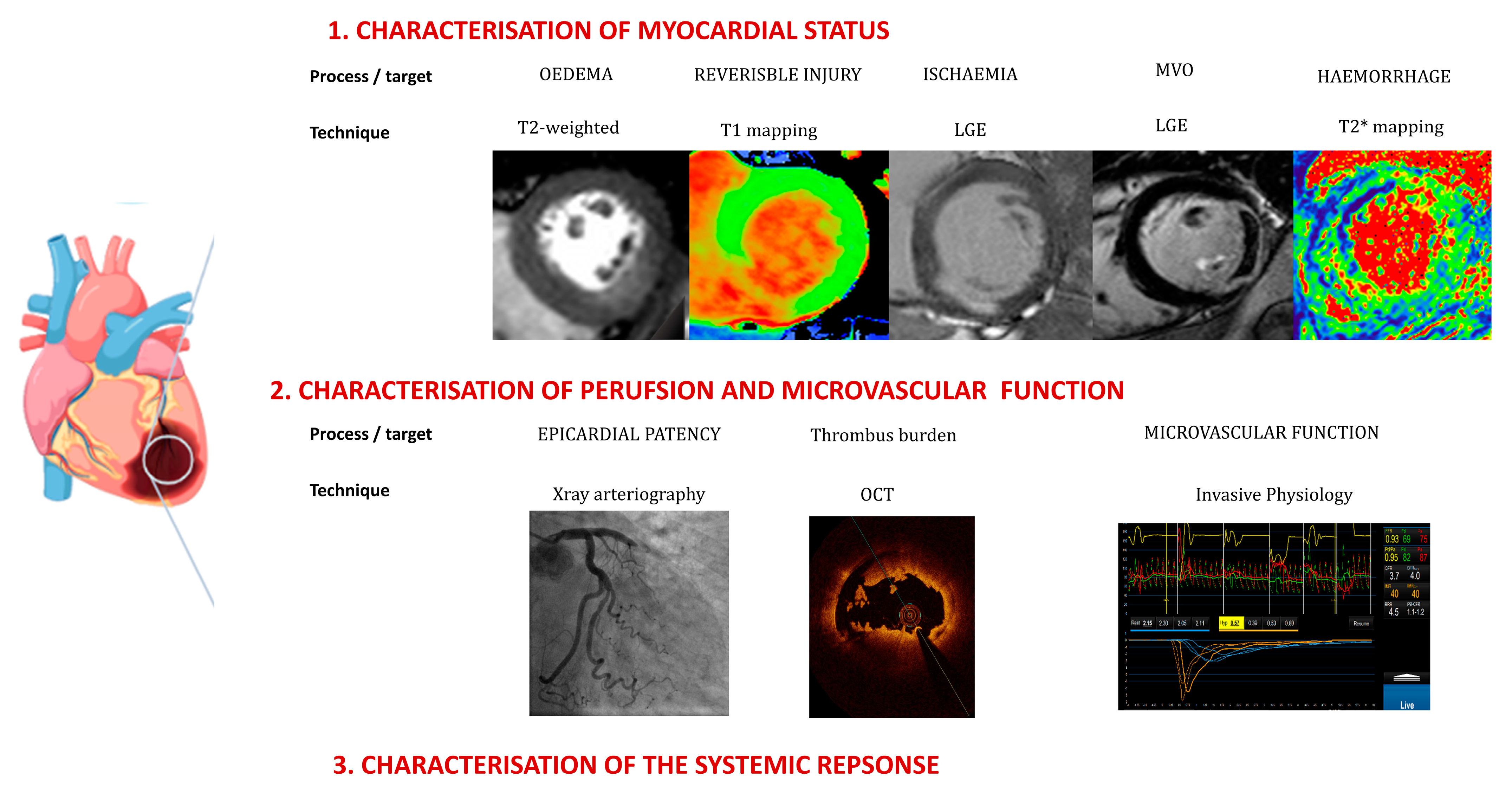
- Emerging techniques can characterise myocardia in individual patients, providing enhanced understanding of the heterogeneity of the response to ischemic injury.
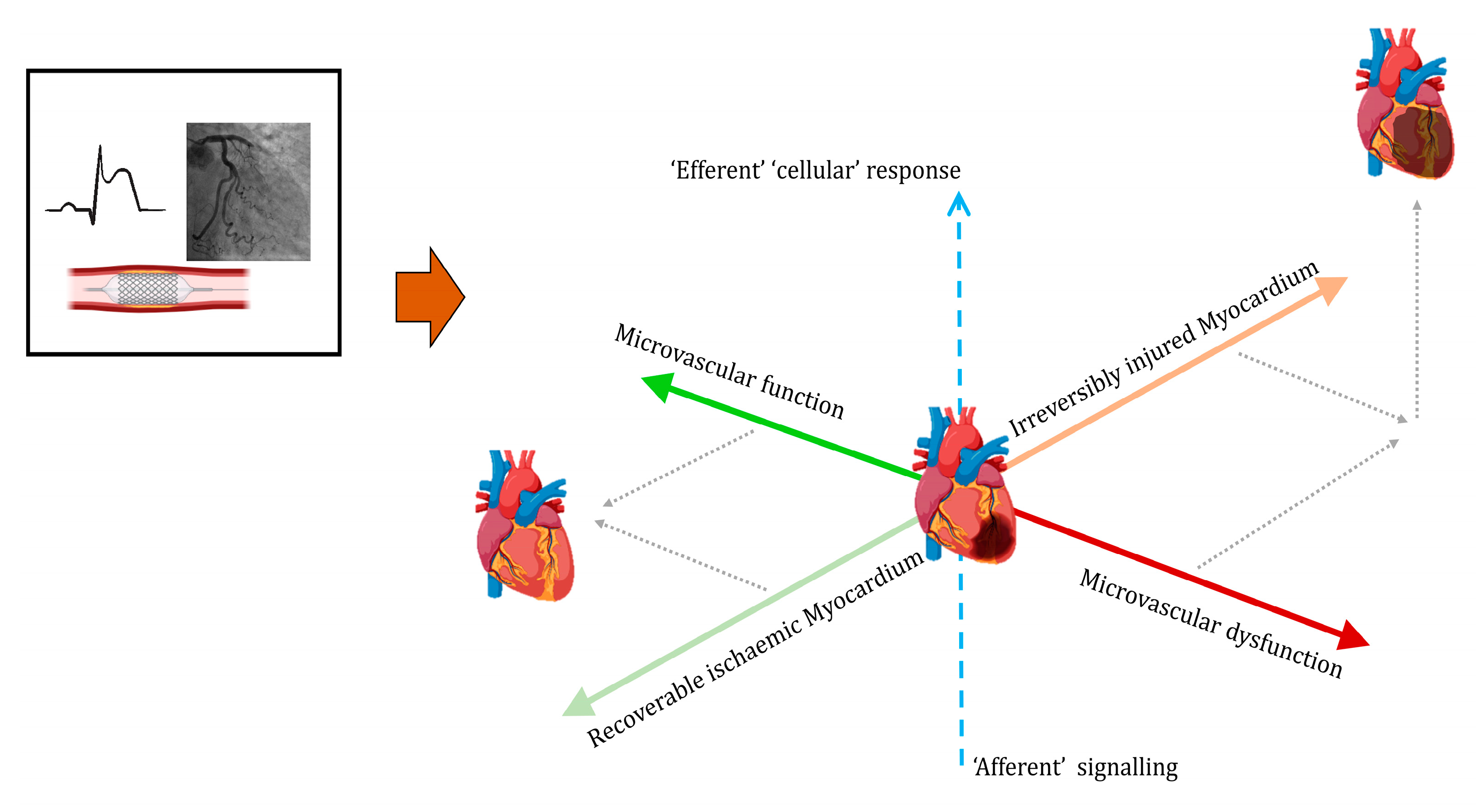
- Assessments of microvascular function, including secondary changes such as intramyocardial haemorrhaging, should inform decision making regarding additional therapies.
- Early prediction of the nature and extent of myocardial recovery vs. irreversible injury would be useful for prognostic and therapeutic purposes in addition to guiding clinical pathways and safe resource allocation.
- Integrating knowledge of the status of the myocardium and the stages of activation of systemic responses should allow the development and application of therapies based on mechanistic characterisation of upstream signalling pathways and the downstream consequences of effector cells, e.g., of the innate immune system.
Abstract
1. Introduction
2. Current Caveats in the Acute Management of Myocardial Infarction
3. Infarct Distribution
4. Myocardial Characterisation
5. Infarct Complexity and Secondary Processes
6. Infarct Recovery
7. Response to Infarction
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maroko, P.R.; Kjekshus, J.K.; Sobel, B.E.; Watanabe, T.; Covell, J.W.; Ross, J., Jr.; Braunwald, E. Factors influencing infarct size following experimental coronary artery occlusions. Circulation 1971, 43, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Reimer, K.A.; Jennings, R.B. The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab. Investig. 1979, 40, 633–644. [Google Scholar] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Boersma, E.; Maas, A.C.; Deckers, J.W.; Simoons, M.L. Early thrombolytic treatment in acute myocardial infarction: Reappraisal of the golden hour. Lancet 1996, 348, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Alkhalil, M.; Choudhury, R.P. Reperfusion Treatment in Late Presentation Acute Myocardial Infarction. Timing Is Not Everything. Circ. Cardiovasc. Interv. 2018, 11, e007287. [Google Scholar] [CrossRef]
- Betgem, R.P.; de Waard, G.A.; Nijveldt, R.; Beek, A.M.; Escaned, J.; van Royen, N. Intramyocardial haemorrhage after acute myocardial infarction. Nat. Rev. Cardiol. 2015, 12, 156–167. [Google Scholar] [CrossRef]
- McCartney, P.J.; Maznyczka, A.M.; Eteiba, H.; McEntegart, M.; Oldroyd, K.G.; Greenwood, J.P.; Maredia, N.; Schmitt, M.; McCann, G.P.; Fairbairn, T.; et al. Low-Dose Alteplase during Primary Percutaneous Coronary Intervention according to Ischemic Time. J. Am. Coll. Cardiol. 2020, 75, 1406–1421. [Google Scholar] [CrossRef]
- Ward, B.J.; McCarthy, A. Endothelial cell “swelling” in ischaemia and reperfusion. J. Mol. Cell. Cardiol. 1995, 27, 1293–1300. [Google Scholar] [CrossRef]
- Eeckhout, E.; Kern, M.J. The coronary no-reflow phenomenon: A review of mechanisms and therapies. Eur. Heart J. 2001, 22, 729–739. [Google Scholar] [CrossRef]
- Ruparelia, N.; Godec, J.; Lee, R.; Chai, J.T.; Dall’Armellina, E.; McAndrew, D.; Digby, J.E.; Forfar, J.C.; Prendergast, B.D.; Kharbanda, R.K.; et al. Acute myocardial infarction activates distinct inflammation and proliferation pathways in circulating monocytes, prior to recruitment, and identified through conserved transcriptional responses in mice and humans. Eur. Heart J. 2015, 36, 1923–1934. [Google Scholar] [CrossRef]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Swirski, F.K.; Aikawa, E.; Stangenberg, L.; Wurdinger, T.; Figueiredo, J.L.; Libby, P.; Weissleder, R.; Pittet, M.J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007, 204, 3037–3047. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M. Myeloid cell contributions to cardiovascular health and disease. Nat. Med. 2018, 24, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Braithwaite, A.T.; Corr, E.M.; Koelwyn, G.J.; van Solingen, C.; Cochain, C.; Saliba, A.E.; Corbin, A.; Pezzolla, D.; Moller Jorgensen, M.; et al. Rapid neutrophil mobilization by VCAM-1+ endothelial cell-derived extracellular vesicles. Cardiovasc. Res. 2023, 119, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, L.; Corum, J.; Parshina-Kottas, Y.; Roberts, G. Time Is Muscle: Understanding Heart Attacks. 2015. Available online: https://www.nytimes.com/interactive/2015/06/19/health/what-is-a-heart-attack.html (accessed on 6 June 2023).
- McGarrah, R.W.; Crown, S.B.; Zhang, G.F.; Shah, S.H.; Newgard, C.B. Cardiovascular Metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef]
- Surendran, A.; Aliani, M.; Ravandi, A. Metabolomic characterization of myocardial ischemia-reperfusion injury in ST-segment elevation myocardial infarction patients undergoing percutaneous coronary intervention. Sci. Rep. 2019, 9, 11742. [Google Scholar] [CrossRef] [PubMed]
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-coding RNAs in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. Heart J. 2018, 39, 2704–2716. [Google Scholar] [CrossRef]
- Dall’Armellina, E.; Karamitsos, T.D.; Neubauer, S.; Choudhury, R.P. CMR for characterization of the myocardium in acute coronary syndromes. Nat. Rev. Cardiol. 2010, 7, 624–636. [Google Scholar] [CrossRef]
- Maznyczka, A.M.; Oldroyd, K.G.; McCartney, P.; McEntegart, M.; Berry, C. The Potential Use of the Index of Microcirculatory Resistance to Guide Stratification of Patients for Adjunctive Therapy in Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2019, 12, 951–966. [Google Scholar] [CrossRef]
- Ibanez, B.; Aletras, A.H.; Arai, A.E.; Arheden, H.; Bax, J.; Berry, C.; Bucciarelli-Ducci, C.; Croisille, P.; Dall’Armellina, E.; Dharmakumar, R.; et al. Cardiac MRI Endpoints in Myocardial Infarction Experimental and Clinical Trials: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 74, 238–256. [Google Scholar] [CrossRef]
- Thanavaro, S.; Krone, R.J.; Kleiger, R.E.; Province, M.A.; Miller, J.P.; deMello, V.R.; Oliver, G.C. In-hospital prognosis of patients with first nontransmural and transmural infarctions. Circulation 1980, 61, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.J.; Wu, E.; Rafael, A.; Chen, E.L.; Parker, M.A.; Simonetti, O.; Klocke, F.J.; Bonow, R.O.; Judd, R.M. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N. Engl. J. Med. 2000, 343, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Elmariah, S.; Smith, S.C., Jr.; Fuster, V. Late medical versus interventional therapy for stable ST-segment elevation myocardial infarction. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kuwao, S.; Kameya, T.; Kasai, K.; Niitsuya, M.; Nishiyama, Y. Characterization of transmural and subendocardial infarction by typing and grading of ischemic lesions in autopsied human hearts. Acta Pathol. Jpn. 1992, 42, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Montague, T.J.; MacKenzie, B.R.; Henderson, M.A.; Macdonald, R.G.; Forbes, C.J.; Chandler, B.M. Acute non-Q-wave myocardial infarction: A distinct clinical entity of increasing importance. CMAJ 1988, 139, 487–493. [Google Scholar]
- Moon, J.C.; De Arenaza, D.P.; Elkington, A.G.; Taneja, A.K.; John, A.S.; Wang, D.; Janardhanan, R.; Senior, R.; Lahiri, A.; Poole-Wilson, P.A.; et al. The pathologic basis of Q-wave and non-Q-wave myocardial infarction: A cardiovascular magnetic resonance study. J. Am. Coll. Cardiol. 2004, 44, 554–560. [Google Scholar] [CrossRef]
- Taneja, A.K.; Hayat, S.; Swinburn, J.; Senior, R. Usefulness of Q waves on ECG for the prediction of contractile reserve after acute myocardial infarction. Int. J. Cardiol. 2010, 145, 265–266. [Google Scholar] [CrossRef]
- Raitt, M.H.; Maynard, C.; Wagner, G.S.; Cerqueira, M.D.; Selvester, R.H.; Weaver, W.D. Appearance of abnormal Q waves early in the course of acute myocardial infarction: Implications for efficacy of thrombolytic therapy. J. Am. Coll. Cardiol. 1995, 25, 1084–1088. [Google Scholar] [CrossRef]
- Topal, D.G.; Lonborg, J.; Ahtarovski, K.A.; Nepper-Christensen, L.; Helqvist, S.; Holmvang, L.; Pedersen, F.; Clemmensen, P.; Saunamaki, K.; Jorgensen, E.; et al. Association Between Early Q Waves and Reperfusion Success in Patients with ST-Segment-Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention: A Cardiac Magnetic Resonance Imaging Study. Circ. Cardiovasc. Interv. 2017, 10, e004467. [Google Scholar] [CrossRef]
- Delewi, R.; Ijff, G.; van de Hoef, T.P.; Hirsch, A.; Robbers, L.F.; Nijveldt, R.; van der Laan, A.M.; van der Vleuten, P.A.; Lucas, C.; Tijssen, J.G.; et al. Pathological Q waves in myocardial infarction in patients treated by primary PCI. JACC Cardiovasc. Imaging 2013, 6, 324–331. [Google Scholar] [CrossRef]
- Sztajzel, J.; Urban, P. Early and late Q wave regression in the setting of acute myocardial infarction. Heart 2000, 83, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Factor, S.M.; Sonnenblick, E.H.; Kirk, E.S. The histologic border zone of acute myocardial infarction—Islands or peninsulas? Am. J. Pathol. 1978, 92, 111–124. [Google Scholar] [PubMed]
- Mendonca Costa, C.; Plank, G.; Rinaldi, C.A.; Niederer, S.A.; Bishop, M.J. Modeling the Electrophysiological Properties of the Infarct Border Zone. Front. Physiol. 2018, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Jablonowski, R.; Engblom, H.; Kanski, M.; Nordlund, D.; Koul, S.; van der Pals, J.; Englund, E.; Heiberg, E.; Erlinge, D.; Carlsson, M.; et al. Contrast-Enhanced CMR Overestimates Early Myocardial Infarct Size: Mechanistic Insights Using ECV Measurements on Day 1 and Day 7. JACC Cardiovasc. Imaging 2015, 8, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Arai, A.E. Fuzzy or Sharp Borders of Acute Myocardial Ischemia and Infarction? JACC Cardiovasc. Imaging 2015, 8, 1390–1392. [Google Scholar] [CrossRef]
- Alkhalil, M.; Borlotti, A.; De Maria, G.L.; Gaughran, L.; Langrish, J.; Lucking, A.; Ferreira, V.; Kharbanda, R.K.; Banning, A.P.; Channon, K.M.; et al. Dynamic changes in injured myocardium, very early after acute myocardial infarction, quantified using T1 mapping cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2018, 20, 82. [Google Scholar] [CrossRef]
- Watanabe, E.; Abbasi, S.A.; Heydari, B.; Coelho-Filho, O.R.; Shah, R.; Neilan, T.G.; Murthy, V.L.; Mongeon, F.P.; Barbhaiya, C.; Jerosch-Herold, M.; et al. Infarct tissue heterogeneity by contrast-enhanced magnetic resonance imaging is a novel predictor of mortality in patients with chronic coronary artery disease and left ventricular dysfunction. Circ. Cardiovasc. Imaging 2014, 7, 887–894. [Google Scholar] [CrossRef]
- Roes, S.D.; Borleffs, C.J.; van der Geest, R.J.; Westenberg, J.J.; Marsan, N.A.; Kaandorp, T.A.; Reiber, J.H.; Zeppenfeld, K.; Lamb, H.J.; de Roos, A.; et al. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ. Cardiovasc. Imaging 2009, 2, 183–190. [Google Scholar] [CrossRef]
- Das, A.; Kelly, C.; Teh, I.; Stoeck, C.T.; Kozerke, S.; Chowdhary, A.; Brown, L.A.E.; Saunderson, C.E.D.; Craven, T.P.; Chew, P.G.; et al. Acute Microstructural Changes after ST-Segment Elevation Myocardial Infarction Assessed with Diffusion Tensor Imaging. Radiology 2021, 299, 86–96. [Google Scholar] [CrossRef]
- Reimer, K.A.; Rasmussen, M.M.; Jennings, R.B. Reduction by propranolol of myocardial necrosis following temporary coronary artery occlusion in dogs. Circ. Res. 1973, 33, 353–363. [Google Scholar] [CrossRef]
- Jennings, R.B.; Ganote, C.E. Structural changes in myocardium during acute ischemia. Circ. Res. 1974, 35 (Suppl. S3), 156–172. [Google Scholar] [CrossRef] [PubMed]
- Cleutjens, J.P.; Blankesteijn, W.M.; Daemen, M.J.; Smits, J.F. The infarcted myocardium: Simply dead tissue, or a lively target for therapeutic interventions. Cardiovasc. Res. 1999, 44, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Alkhalil, M.; Borlotti, A.; De Maria, G.L.; Wolfrum, M.; Dawkins, S.; Fahrni, G.; Gaughran, L.; Oxford Acute Myocardial Infarction (OxAMI) Study; Langrish, J.P.; Lucking, A.; et al. Hyper-acute cardiovascular magnetic resonance T1 mapping predicts infarct characteristics in patients with ST elevation myocardial infarction. J. Cardiovasc. Magn. Reson. 2020, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Borlotti, A.; Viliani, D.; Jerosch-Herold, M.; Alkhalil, M.; De Maria, G.L.; Fahrni, G.; Dawkins, S.; Wijesurendra, R.; Francis, J.; et al. CMR Native T1 Mapping Allows Differentiation of Reversible Versus Irreversible Myocardial Damage in ST-Segment-Elevation Myocardial Infarction: An OxAMI Study (Oxford Acute Myocardial Infarction). Circ. Cardiovasc. Imaging 2017, 10, e005986. [Google Scholar] [CrossRef]
- Dall’Armellina, E.; Karia, N.; Lindsay, A.C.; Karamitsos, T.D.; Ferreira, V.; Robson, M.D.; Kellman, P.; Francis, J.M.; Forfar, C.; Prendergast, B.D.; et al. Dynamic changes of edema and late gadolinium enhancement after acute myocardial infarction and their relationship to functional recovery and salvage index. Circ. Cardiovasc. Imaging 2011, 4, 228–236. [Google Scholar] [CrossRef]
- Kim, R.J.; Fieno, D.S.; Parrish, T.B.; Harris, K.; Chen, E.L.; Simonetti, O.; Bundy, J.; Finn, J.P.; Klocke, F.J.; Judd, R.M. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999, 100, 1992–2002. [Google Scholar] [CrossRef]
- Mallory, F.B.; Parker, F. Fixing and staining methods for lead and copper in tissues. Am. J. Pathol. 1939, 15, 517–522.5. [Google Scholar]
- Klein, C.; Schmal, T.R.; Nekolla, S.G.; Schnackenburg, B.; Fleck, E.; Nagel, E. Mechanism of late gadolinium enhancement in patients with acute myocardial infarction. J. Cardiovasc. Magn. Reson. 2007, 9, 653–658. [Google Scholar] [CrossRef]
- Arheden, H.; Saeed, M.; Higgins, C.B.; Gao, D.W.; Ursell, P.C.; Bremerich, J.; Wyttenbach, R.; Dae, M.W.; Wendland, M.F. Reperfused rat myocardium subjected to various durations of ischemia: Estimation of the distribution volume of contrast material with echo-planar MR imaging. Radiology 2000, 215, 520–528. [Google Scholar] [CrossRef]
- Hammer-Hansen, S.; Bandettini, W.P.; Hsu, L.Y.; Leung, S.W.; Shanbhag, S.; Mancini, C.; Greve, A.M.; Kober, L.; Thune, J.J.; Kellman, P.; et al. Mechanisms for overestimating acute myocardial infarct size with gadolinium-enhanced cardiovascular magnetic resonance imaging in humans: A quantitative and kinetic study dagger. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 76–84. [Google Scholar] [CrossRef]
- Oshinski, J.N.; Yang, Z.; Jones, J.R.; Mata, J.F.; French, B.A. Imaging time after Gd-DTPA injection is critical in using delayed enhancement to determine infarct size accurately with magnetic resonance imaging. Circulation 2001, 104, 2838–2842. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Lund, G.; Wendland, M.F.; Bremerich, J.; Weinmann, H.; Higgins, C.B. Magnetic resonance characterization of the peri-infarction zone of reperfused myocardial infarction with necrosis-specific and extracellular nonspecific contrast media. Circulation 2001, 103, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Palomares, J.F.; Ortiz-Perez, J.T.; Lee, D.C.; Bucciarelli-Ducci, C.; Tejedor, P.; Bonow, R.O.; Wu, E. Time elapsed after contrast injection is crucial to determine infarct transmurality and myocardial functional recovery after an acute myocardial infarction. J. Cardiovasc. Magn. Reson. 2015, 17, 43. [Google Scholar] [CrossRef]
- De Maria, G.L.; Alkhalil, M.; Wolfrum, M.; Fahrni, G.; Borlotti, A.; Gaughran, L.; Dawkins, S.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; et al. Index of Microcirculatory Resistance as a Tool to Characterize Microvascular Obstruction and to Predict Infarct Size Regression in Patients With STEMI Undergoing Primary PCI. JACC Cardiovasc. Imaging 2019, 12, 837–848. [Google Scholar] [CrossRef]
- Meuwissen, M.; Chamuleau, S.A.; Siebes, M.; Schotborgh, C.E.; Koch, K.T.; de Winter, R.J.; Bax, M.; de Jong, A.; Spaan, J.A.; Piek, J.J. Role of variability in microvascular resistance on fractional flow reserve and coronary blood flow velocity reserve in intermediate coronary lesions. Circulation 2001, 103, 184–187. [Google Scholar] [CrossRef]
- Fearon, W.F.; Balsam, L.B.; Farouque, H.M.; Caffarelli, A.D.; Robbins, R.C.; Fitzgerald, P.J.; Yock, P.G.; Yeung, A.C. Novel index for invasively assessing the coronary microcirculation. Circulation 2003, 107, 3129–3132. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, P.F.; de Waard, G.A.; Hollander, M.R.; Robbers, L.F.; Danad, I.; Biesbroek, P.S.; Amier, R.P.; Echavarria-Pinto, M.; Quiros, A.; Broyd, C.; et al. Doppler-derived intracoronary physiology indices predict the occurrence of microvascular injury and microvascular perfusion deficits after angiographically successful primary percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2015, 8, e001786. [Google Scholar] [CrossRef] [PubMed]
- McGeoch, R.; Watkins, S.; Berry, C.; Steedman, T.; Davie, A.; Byrne, J.; Hillis, S.; Lindsay, M.; Robb, S.; Dargie, H.; et al. The index of microcirculatory resistance measured acutely predicts the extent and severity of myocardial infarction in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 2010, 3, 715–722. [Google Scholar] [CrossRef]
- Carrick, D.; Haig, C.; Ahmed, N.; Carberry, J.; Yue May, V.T.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Lindsay, M.; Hood, S.; et al. Comparative Prognostic Utility of Indexes of Microvascular Function Alone or in Combination in Patients With an Acute ST-Segment-Elevation Myocardial Infarction. Circulation 2016, 134, 1833–1847. [Google Scholar] [CrossRef]
- de Waard, G.A.; Fahrni, G.; de Wit, D.; Kitabata, H.; Williams, R.; Patel, N.; Teunissen, P.F.; van de Ven, P.M.; Umman, S.; Knaapen, P.; et al. Hyperaemic microvascular resistance predicts clinical outcome and microvascular injury after myocardial infarction. Heart 2018, 104, 127–134. [Google Scholar] [CrossRef]
- Fearon, W.F.; Low, A.F.; Yong, A.S.; McGeoch, R.; Berry, C.; Shah, M.G.; Ho, M.Y.; Kim, H.S.; Loh, J.P.; Oldroyd, K.G. Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation 2013, 127, 2436–2441. [Google Scholar] [CrossRef] [PubMed]
- Aletras, A.H.; Tilak, G.S.; Natanzon, A.; Hsu, L.Y.; Gonzalez, F.M.; Hoyt, R.F., Jr.; Arai, A.E. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: Histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation 2006, 113, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aty, H.; Cocker, M.; Meek, C.; Tyberg, J.V.; Friedrich, M.G. Edema as a very early marker for acute myocardial ischemia: A cardiovascular magnetic resonance study. J. Am. Coll. Cardiol. 2009, 53, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Bulluck, H.; Dharmakumar, R.; Arai, A.E.; Berry, C.; Hausenloy, D.J. Cardiovascular Magnetic Resonance in Acute ST-Segment-Elevation Myocardial Infarction: Recent Advances, Controversies, and Future Directions. Circulation 2018, 137, 1949–1964. [Google Scholar] [CrossRef]
- Perazzolo Marra, M.; Lima, J.A.; Iliceto, S. MRI in acute myocardial infarction. Eur. Heart J. 2011, 32, 284–293. [Google Scholar] [CrossRef]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef]
- Moon, J.C.; Messroghli, D.R.; Kellman, P.; Piechnik, S.K.; Robson, M.D.; Ugander, M.; Gatehouse, P.D.; Arai, A.E.; Friedrich, M.G.; Neubauer, S.; et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J. Cardiovasc. Magn. Reson. 2013, 15, 92. [Google Scholar] [CrossRef]
- Bulluck, H.; Hammond-Haley, M.; Fontana, M.; Knight, D.S.; Sirker, A.; Herrey, A.S.; Manisty, C.; Kellman, P.; Moon, J.C.; Hausenloy, D.J. Quantification of both the area-at-risk and acute myocardial infarct size in ST-segment elevation myocardial infarction using T1-mapping. J. Cardiovasc. Magn. Reson. 2017, 19, 57. [Google Scholar] [CrossRef]
- Kidambi, A.; Motwani, M.; Uddin, A.; Ripley, D.P.; McDiarmid, A.K.; Swoboda, P.P.; Broadbent, D.A.; Musa, T.A.; Erhayiem, B.; Leader, J.; et al. Myocardial Extracellular Volume Estimation by CMR Predicts Functional Recovery Following Acute MI. JACC Cardiovasc. Imaging 2017, 10, 989–999. [Google Scholar] [CrossRef]
- Garg, P.; Broadbent, D.A.; Swoboda, P.P.; Foley, J.R.J.; Fent, G.J.; Musa, T.A.; Ripley, D.P.; Erhayiem, B.; Dobson, L.E.; McDiarmid, A.K.; et al. Acute Infarct Extracellular Volume Mapping to Quantify Myocardial Area at Risk and Chronic Infarct Size on Cardiovascular Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2017, 10, e006182. [Google Scholar] [CrossRef]
- Wamil, M.; Borlotti, A.; Liu, D.; Briosa, E.G.A.; Bracco, A.; Alkhalil, M.; De Maria, G.L.; Piechnik, S.K.; Ferreira, V.M.; Banning, A.P.; et al. Combined T1-mapping and tissue tracking analysis predicts severity of ischemic injury following acute STEMI-an Oxford Acute Myocardial Infarction (OxAMI) study. Int. J. Cardiovasc. Imaging 2019, 35, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Borlotti, A.; Jerosch-Herold, M.; Liu, D.; Viliani, D.; Bracco, A.; Alkhalil, M.; De Maria, G.; Channon, K.; Banning, A.; Choudhury, R.; et al. Acute microvascular impairment post reperfused STEMI is reversible and has additional clinical predictive value—A CMR OxAMI study. JACC Cardiovasc. Imaging 2019, 12, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Bagai, A.; Huang, Z.; Lokhnygina, Y.; Harrington, R.A.; Armstrong, P.W.; Strony, J.; White, H.D.; Leonardi, S.; Held, C.; Van de Werf, F.; et al. Magnitude of troponin elevation and long-term clinical outcomes in acute coronary syndrome patients treated with and without revascularization. Circ. Cardiovasc. Interv. 2015, 8, e002314. [Google Scholar] [CrossRef] [PubMed]
- Younger, J.F.; Plein, S.; Barth, J.; Ridgway, J.P.; Ball, S.G.; Greenwood, J.P. Troponin-I concentration 72 h after myocardial infarction correlates with infarct size and presence of microvascular obstruction. Heart 2007, 93, 1547–1551. [Google Scholar] [CrossRef]
- Henriques, J.P.; Zijlstra, F.; van ‘t Hof, A.W.; de Boer, M.J.; Dambrink, J.H.; Gosselink, M.; Hoorntje, J.C.; Suryapranata, H. Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation 2003, 107, 2115–2119. [Google Scholar] [CrossRef]
- de Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef]
- Ibanez, B.; Heusch, G.; Ovize, M.; Van de Werf, F. Evolving therapies for myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1454–1471. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Burke, A.; Farb, A.; Kaye, D.; Lesser, J.R.; Henry, T.D.; Virmani, R. Microemboli and microvascular obstruction in acute coronary thrombosis and sudden coronary death: Relation to epicardial plaque histopathology. J. Am. Coll. Cardiol. 2009, 54, 2167–2173. [Google Scholar] [CrossRef]
- Robbers, L.F.; Eerenberg, E.S.; Teunissen, P.F.; Jansen, M.F.; Hollander, M.R.; Horrevoets, A.J.; Knaapen, P.; Nijveldt, R.; Heymans, M.W.; Levi, M.M.; et al. Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur. Heart J. 2013, 34, 2346–2353. [Google Scholar] [CrossRef]
- Sezer, M.; Oflaz, H.; Goren, T.; Okcular, I.; Umman, B.; Nisanci, Y.; Bilge, A.K.; Sanli, Y.; Meric, M.; Umman, S. Intracoronary streptokinase after primary percutaneous coronary intervention. N. Engl. J. Med. 2007, 356, 1823–1834. [Google Scholar] [CrossRef]
- McCartney, P.J.; Eteiba, H.; Maznyczka, A.M.; McEntegart, M.; Greenwood, J.P.; Muir, D.F.; Chowdhary, S.; Gershlick, A.H.; Appleby, C.; Cotton, J.M.; et al. Effect of Low-Dose Intracoronary Alteplase During Primary Percutaneous Coronary Intervention on Microvascular Obstruction in Patients With Acute Myocardial Infarction: A Randomized Clinical Trial. JAMA 2019, 321, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Carrick, D.; Haig, C.; Ahmed, N.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Hood, S.; Watkins, S.; Lindsay, M.M.; Davie, A.; et al. Myocardial Hemorrhage After Acute Reperfused ST-Segment-Elevation Myocardial Infarction: Relation to Microvascular Obstruction and Prognostic Significance. Circ. Cardiovasc. Imaging 2016, 9, e004148. [Google Scholar] [CrossRef] [PubMed]
- Reinstadler, S.J.; Stiermaier, T.; Reindl, M.; Feistritzer, H.J.; Fuernau, G.; Eitel, C.; Desch, S.; Klug, G.; Thiele, H.; Metzler, B.; et al. Intramyocardial haemorrhage and prognosis after ST-elevation myocardial infarction. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 138–146. [Google Scholar] [CrossRef] [PubMed]
- De Maria, G.L.; Alkhalil, M.; Wolfrum, M.; Fahrni, G.; Borlotti, A.; Gaughran, L.; Dawkins, S.; Langrish, J.; Lucking, A.; Choudhury, R.; et al. The ATI score (Age-Thrombotic burden-Index of microcirculatory resistance) determined during primary percutaneous coronary intervention predicts final infarct size in patients with ST elevation myocardial infarction: A cardiac magnetic resonance validation study. EuroIntervention 2017, 13, 935–943. [Google Scholar] [CrossRef] [PubMed]
- De Maria, G.L.; Fahrni, G.; Alkhalil, M.; Cuculi, F.; Dawkins, S.; Wolfrum, M.; Choudhury, R.P.; Forfar, J.C.; Prendergast, B.D.; Yetgin, T.; et al. A tool for predicting the outcome of reperfusion in ST-elevation myocardial infarction using age, thrombotic burden and index of microcirculatory resistance (ATI score). EuroIntervention 2016, 12, 1223–1230. [Google Scholar] [CrossRef]
- De Maria, G.L.; Alkhalil, M.; Borlotti, A.; Wolfrum, M.; Gaughran, L.; Dall’Armellina, E.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.K.; et al. Index of microcirculatory resistance-guided therapy with pressure-controlled intermittent coronary sinus occlusion improves coronary microvascular function and reduces infarct size in patients with ST-elevation myocardial infarction: The Oxford Acute Myocardial Infarction—Pressure-controlled Intermittent Coronary Sinus Occlusion study (OxAMI-PICSO study). EuroIntervention 2018, 14, 352–359. [Google Scholar] [CrossRef]
- Buja, L.M. Myocardial ischemia and reperfusion injury. Cardiovasc. Pathol. 2005, 14, 170–175. [Google Scholar] [CrossRef]
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory processes in cardiovascular disease: A route to targeted therapies. Nat. Rev. Cardiol. 2017, 14, 133–144. [Google Scholar] [CrossRef]
- Larose, E.; Tizon-Marcos, H.; Rodes-Cabau, J.; Rinfret, S.; Dery, J.P.; Nguyen, C.M.; Gleeton, O.; Boudreault, J.R.; Roy, L.; Noel, B.; et al. Improving myocardial salvage in late presentation acute ST-elevation myocardial infarction with proximal embolic protection. Catheter. Cardiovasc. Interv. 2010, 76, 461–470. [Google Scholar] [CrossRef]
- Eitel, I.; Stiermaier, T.; Rommel, K.P.; Fuernau, G.; Sandri, M.; Mangner, N.; Linke, A.; Erbs, S.; Lurz, P.; Boudriot, E.; et al. Cardioprotection by combined intrahospital remote ischaemic perconditioning and postconditioning in ST-elevation myocardial infarction: The randomized LIPSIA CONDITIONING trial. Eur. Heart J. 2015, 36, 3049–3057. [Google Scholar] [CrossRef]
- Carlsson, M.; Ubachs, J.F.; Hedstrom, E.; Heiberg, E.; Jovinge, S.; Arheden, H. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance: Quantitative assessment during follow-up and validation with single-photon emission computed tomography. JACC Cardiovasc. Imaging 2009, 2, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Jimenez, R.; Sanchez-Gonzalez, J.; Aguero, J.; Garcia-Prieto, J.; Lopez-Martin, G.J.; Garcia-Ruiz, J.M.; Molina-Iracheta, A.; Rossello, X.; Fernandez-Friera, L.; Pizarro, G.; et al. Myocardial edema after ischemia/reperfusion is not stable and follows a bimodal pattern: Imaging and histological tissue characterization. J. Am. Coll. Cardiol. 2015, 65, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Jimenez, R.; Barreiro-Perez, M.; Martin-Garcia, A.; Sanchez-Gonzalez, J.; Aguero, J.; Galan-Arriola, C.; Garcia-Prieto, J.; Diaz-Pelaez, E.; Vara, P.; Martinez, I.; et al. Dynamic Edematous Response of the Human Heart to Myocardial Infarction: Implications for Assessing Myocardial Area at Risk and Salvage. Circulation 2017, 136, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Carberry, J.; Carrick, D.; Haig, C.; Ahmed, N.; Mordi, I.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Hood, S.; Watkins, S.; et al. Persistence of Infarct Zone T2 Hyperintensity at 6 Months After Acute ST-Segment-Elevation Myocardial Infarction: Incidence, Pathophysiology, and Prognostic Implications. Circ. Cardiovasc. Imaging 2017, 10, e006586. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.C.; Nielsen, G.; Groenning, B.A.; Fritz-Hansen, T.; Sondergaard, L.; Jensen, G.B.; Larsson, H.B. Sustained postinfarction myocardial oedema in humans visualised by magnetic resonance imaging. Heart 2001, 85, 639–642. [Google Scholar] [CrossRef]
- Orn, S.; Manhenke, C.; Greve, O.J.; Larsen, A.I.; Bonarjee, V.V.; Edvardsen, T.; Dickstein, K. Microvascular obstruction is a major determinant of infarct healing and subsequent left ventricular remodelling following primary percutaneous coronary intervention. Eur. Heart J. 2009, 30, 1978–1985. [Google Scholar] [CrossRef]
- Bekkers, S.C.; Backes, W.H.; Kim, R.J.; Snoep, G.; Gorgels, A.P.; Passos, V.L.; Waltenberger, J.; Crijns, H.J.; Schalla, S. Detection and characteristics of microvascular obstruction in reperfused acute myocardial infarction using an optimized protocol for contrast-enhanced cardiovascular magnetic resonance imaging. Eur. Radiol. 2009, 19, 2904–2912. [Google Scholar] [CrossRef]
- Kali, A.; Kumar, A.; Cokic, I.; Tang, R.L.; Tsaftaris, S.A.; Friedrich, M.G.; Dharmakumar, R. Chronic manifestation of postreperfusion intramyocardial hemorrhage as regional iron deposition: A cardiovascular magnetic resonance study with ex vivo validation. Circ. Cardiovasc. Imaging 2013, 6, 218–228. [Google Scholar] [CrossRef]
- Kali, A.; Cokic, I.; Tang, R.; Dohnalkova, A.; Kovarik, L.; Yang, H.J.; Kumar, A.; Prato, F.S.; Wood, J.C.; Underhill, D.; et al. Persistent Microvascular Obstruction After Myocardial Infarction Culminates in the Confluence of Ferric Iron Oxide Crystals, Proinflammatory Burden, and Adverse Remodeling. Circ. Cardiovasc. Imaging 2016, 9, e004996. [Google Scholar] [CrossRef]
- Carberry, J.; Carrick, D.; Haig, C.; Ahmed, N.; Mordi, I.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Hood, S.; Watkins, S.; et al. Persistent Iron Within the Infarct Core After ST-Segment Elevation Myocardial Infarction: Implications for Left Ventricular Remodeling and Health Outcomes. JACC Cardiovasc. Imaging 2018, 11, 1248–1256. [Google Scholar] [CrossRef]
- De Maria, G.L.; Cuculi, F.; Patel, N.; Dawkins, S.; Fahrni, G.; Kassimis, G.; Choudhury, R.P.; Forfar, J.C.; Prendergast, B.D.; Channon, K.M.; et al. How does coronary stent implantation impact on the status of the microcirculation during primary percutaneous coronary intervention in patients with ST-elevation myocardial infarction? Eur. Heart J. 2015, 36, 3165–3177. [Google Scholar] [CrossRef] [PubMed]
- De Maria, G.L.; Patel, N.; Wolfrum, M.; Fahrni, G.; Kassimis, G.; Porto, I.; Dawkins, S.; Choudhury, R.P.; Forfar, J.C.; Prendergast, B.D.; et al. The influence of coronary plaque morphology assessed by optical coherence tomography on final microvascular function after stenting in patients with ST-elevation myocardial infarction. Coron. Artery Dis. 2017, 28, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Cuculi, F.; De Maria, G.L.; Meier, P.; Dall’Armellina, E.; de Caterina, A.R.; Channon, K.M.; Prendergast, B.D.; Choudhury, R.P.; Forfar, J.C.; Kharbanda, R.K.; et al. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2014, 64, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Toldo, S.; Abbate, A. The NLRP3 inflammasome in acute myocardial infarction. Nat. Rev. Cardiol. 2018, 15, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Latet, S.C.; Hoymans, V.Y.; Van Herck, P.L.; Vrints, C.J. The cellular immune system in the post-myocardial infarction repair process. Int. J. Cardiol. 2015, 179, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Evrard, M.; Kwok, I.W.H.; Chong, S.Z.; Teng, K.W.W.; Becht, E.; Chen, J.; Sieow, J.L.; Penny, H.L.; Ching, G.C.; Devi, S.; et al. Developmental Analysis of Bone Marrow Neutrophils Reveals Populations Specialized in Expansion, Trafficking, and Effector Functions. Immunity 2018, 48, 364–379.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Padgett, L.; Dinh, H.Q.; Marcovecchio, P.; Blatchley, A.; Wu, R.; Ehinger, E.; Kim, C.; Mikulski, Z.; Seumois, G.; et al. Identification of an Early Unipotent Neutrophil Progenitor with Pro-tumoral Activity in Mouse and Human Bone Marrow. Cell Rep. 2018, 24, 2329–2341 e2328. [Google Scholar] [CrossRef]
- Sreejit, G.; Abdel-Latif, A.; Athmanathan, B.; Annabathula, R.; Dhyani, A.; Noothi, S.K.; Quaife-Ryan, G.A.; Al-Sharea, A.; Pernes, G.; Dragoljevic, D.; et al. Neutrophil-Derived S100A8/A9 Amplify Granulopoiesis After Myocardial Infarction. Circulation 2020, 141, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- Horckmans, M.; Bianchini, M.; Santovito, D.; Megens, R.T.A.; Springael, J.Y.; Negri, I.; Vacca, M.; Di Eusanio, M.; Moschetta, A.; Weber, C.; et al. Pericardial Adipose Tissue Regulates Granulopoiesis, Fibrosis, and Cardiac Function After Myocardial Infarction. Circulation 2018, 137, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Luboshits, G.; Planer, D.; Keren, G.; George, J. Altered status of CD4+CD25+ regulatory T cells in patients with acute coronary syndromes. Eur. Heart J. 2006, 27, 2530–2537. [Google Scholar] [CrossRef] [PubMed]
- Boag, S.E.; Das, R.; Shmeleva, E.V.; Bagnall, A.; Egred, M.; Howard, N.; Bennaceur, K.; Zaman, A.; Keavney, B.; Spyridopoulos, I. T lymphocytes and fractalkine contribute to myocardial ischemia/reperfusion injury in patients. J. Clin. Investig. 2015, 125, 3063–3076. [Google Scholar] [CrossRef] [PubMed]
- Blancke, F.; Claeys, M.J.; Jorens, P.; Vermeiren, G.; Bosmans, J.; Wuyts, F.L.; Vrints, C.J. Systemic inflammation and reperfusion injury in patients with acute myocardial infarction. Mediat. Inflamm. 2005, 2005, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, N.; Digby, J.E.; Jefferson, A.; Medway, D.J.; Neubauer, S.; Lygate, C.A.; Choudhury, R.P. Myocardial infarction causes inflammation and leukocyte recruitment at remote sites in the myocardium and in the renal glomerulus. Inflamm. Res. 2013, 62, 515–525. [Google Scholar] [CrossRef]
- Park, K.C.; Gaze, D.C.; Collinson, P.O.; Marber, M.S. Cardiac troponins: From myocardial infarction to chronic disease. Cardiovasc. Res. 2017, 113, 1708–1718. [Google Scholar] [CrossRef]
- Schernthaner, C.; Lichtenauer, M.; Wernly, B.; Paar, V.; Pistulli, R.; Rohm, I.; Jung, C.; Figulla, H.R.; Yilmaz, A.; Cadamuro, J.; et al. Multibiomarker analysis in patients with acute myocardial infarction. Eur. J. Clin. Investig. 2017, 47, 638–648. [Google Scholar] [CrossRef]
- Xue, S.; Zhu, W.; Liu, D.; Su, Z.; Zhang, L.; Chang, Q.; Li, P. Circulating miR-26a-1, miR-146a and miR-199a-1 are potential candidate biomarkers for acute myocardial infarction. Mol. Med. 2019, 25, 18. [Google Scholar] [CrossRef]
- Gidlof, O.; Evander, M.; Rezeli, M.; Marko-Varga, G.; Laurell, T.; Erlinge, D. Proteomic profiling of extracellular vesicles reveals additional diagnostic biomarkers for myocardial infarction compared to plasma alone. Sci. Rep. 2019, 9, 8991. [Google Scholar] [CrossRef]
- Burrello, J.; Biemmi, V.; Dei Cas, M.; Amongero, M.; Bolis, S.; Lazzarini, E.; Bollini, S.; Vassalli, G.; Paroni, R.; Barile, L. Sphingolipid composition of circulating extracellular vesicles after myocardial ischemia. Sci. Rep. 2020, 10, 16182. [Google Scholar] [CrossRef]
- Mujalli, A.; Banaganapalli, B.; Alrayes, N.M.; Shaik, N.A.; Elango, R.; Al-Aama, J.Y. Myocardial infarction biomarker discovery with integrated gene expression, pathways and biological networks analysis. Genomics 2020, 112, 5072–5085. [Google Scholar] [CrossRef]
- Abbate, A.; Kontos, M.C.; Abouzaki, N.A.; Melchior, R.D.; Thomas, C.; Van Tassell, B.W.; Oddi, C.; Carbone, S.; Trankle, C.R.; Roberts, C.S.; et al. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am. J. Cardiol. 2015, 115, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Padfield, G.J.; Din, J.N.; Koushiappi, E.; Mills, N.L.; Robinson, S.D.; Cruden Nle, M.; Lucking, A.J.; Chia, S.; Harding, S.A.; Newby, D.E. Cardiovascular effects of tumour necrosis factor alpha antagonism in patients with acute myocardial infarction: A first in human study. Heart 2013, 99, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Q.; Kong, X.S.; Shen, X.B.; Huang, M.Z.; Zheng, J.P.; Sun, J.; Xu, S.H. Identification of Differentially Expressed Genes and Signaling Pathways in Acute Myocardial Infarction Based on Integrated Bioinformatics Analysis. Cardiovasc. Ther. 2019, 2019, 8490707. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhalil, M.; De Maria, G.L.; Akbar, N.; Ruparelia, N.; Choudhury, R.P. Prospects for Precision Medicine in Acute Myocardial Infarction: Patient-Level Insights into Myocardial Injury and Repair. J. Clin. Med. 2023, 12, 4668. https://doi.org/10.3390/jcm12144668
Alkhalil M, De Maria GL, Akbar N, Ruparelia N, Choudhury RP. Prospects for Precision Medicine in Acute Myocardial Infarction: Patient-Level Insights into Myocardial Injury and Repair. Journal of Clinical Medicine. 2023; 12(14):4668. https://doi.org/10.3390/jcm12144668
Chicago/Turabian StyleAlkhalil, Mohammad, Giovanni Luigi De Maria, Naveed Akbar, Neil Ruparelia, and Robin P. Choudhury. 2023. "Prospects for Precision Medicine in Acute Myocardial Infarction: Patient-Level Insights into Myocardial Injury and Repair" Journal of Clinical Medicine 12, no. 14: 4668. https://doi.org/10.3390/jcm12144668
APA StyleAlkhalil, M., De Maria, G. L., Akbar, N., Ruparelia, N., & Choudhury, R. P. (2023). Prospects for Precision Medicine in Acute Myocardial Infarction: Patient-Level Insights into Myocardial Injury and Repair. Journal of Clinical Medicine, 12(14), 4668. https://doi.org/10.3390/jcm12144668





