Interaction of Diamine Oxidase with Psychostimulant Drugs for ADHD Management
Abstract
1. Introduction
2. Materials and Methods
3. Results
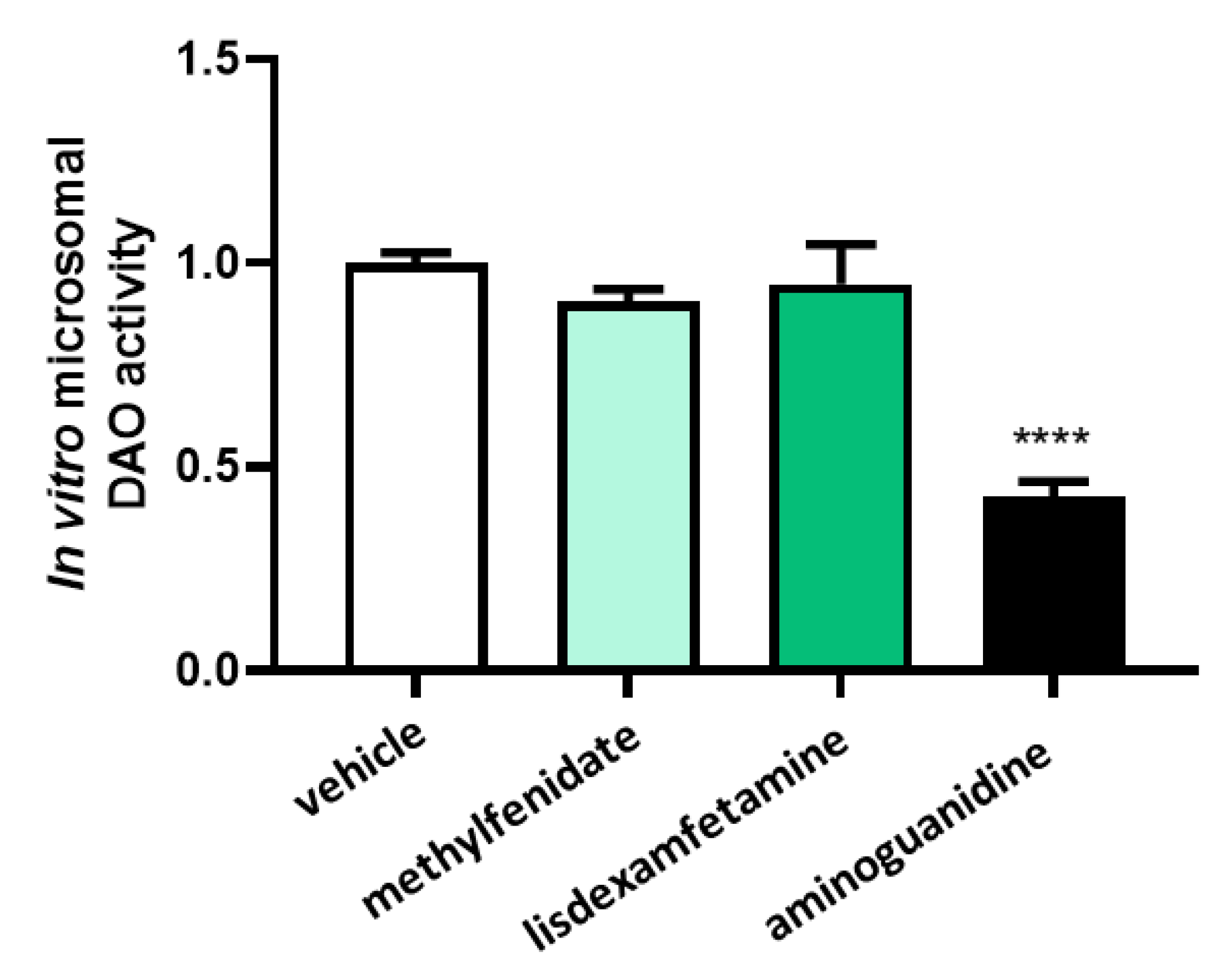
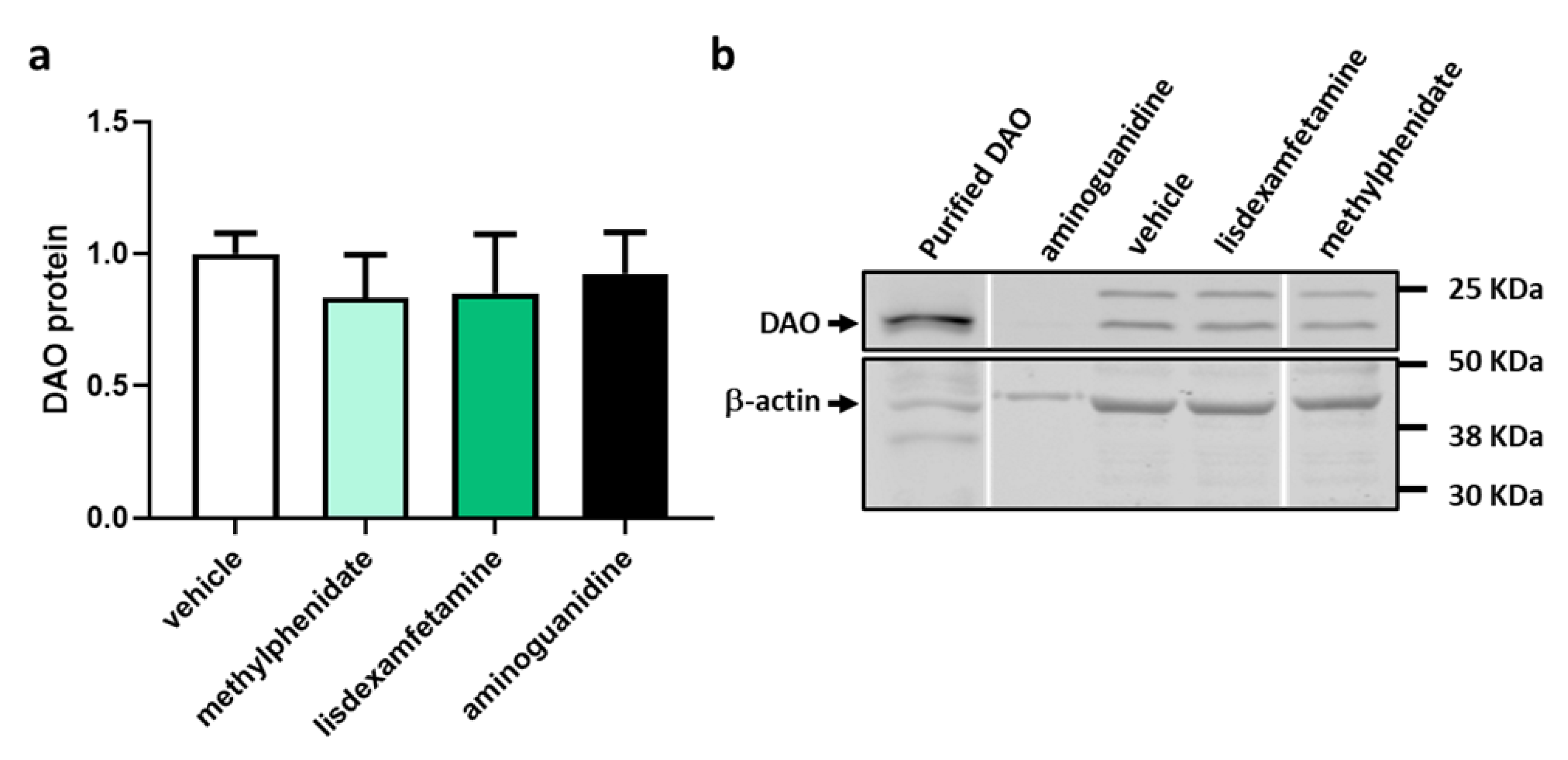
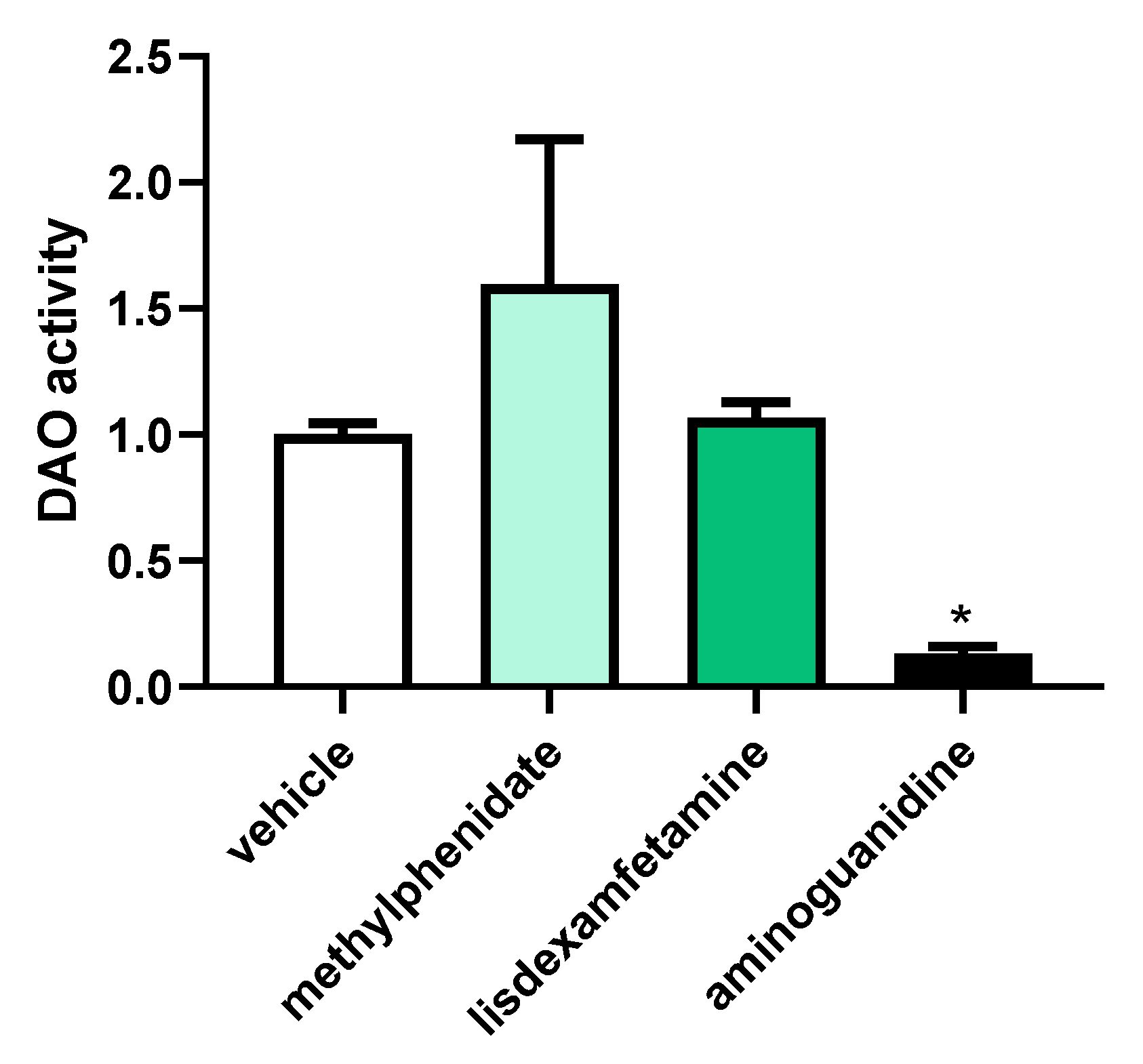
3.1. DAO Activity Was Not Affected by the Presence of Methylphenidate or Lisdexamfetamine
3.2. Effects of Selected Psychostimulant Drugs on Intestinal DAO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.S.; Tannock, R.; Franke, B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Prim. 2015, 1, 15020. [Google Scholar] [CrossRef] [PubMed]
- Majarwitz, D.J.; Perumareddi, P. Attention-Deficit/Hyperactivity Disorder Across the Spectrum: From Childhood to Adulthood. Prim. Care 2023, 50, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Samuel, S.; Patel, D.R. Pharmacologic management of attention deficit hyperactivity disorder in children and adolescents: A review for practitioners. Transl. Pediatr. 2018, 7, 36–47. [Google Scholar] [CrossRef]
- Wolraich, M.L.; Hagan, J.F.; Allan, C.; Chan, E.; Davison, D.; Earls, M.; Evans, S.W.; Flinn, S.K.; Froehlich, T.; Frost, J.; et al. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics 2019, 144, e20192528. [Google Scholar] [CrossRef] [PubMed]
- Branco, A.C.C.C.; Yoshikawa, F.S.Y.; Pietrobon, A.J.; Sato, M.N. Role of Histamine in Modulating the Immune Response and Inflammation. Mediat. Inflamm. 2018, 2018, 9524075. [Google Scholar] [CrossRef] [PubMed]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.D.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef]
- Izquierdo-Casas, J.; Comas-Basté, O.; Latorre-Moratalla, M.L.; Lorente-Gascón, M.; Duelo, A.; Soler-Singla, L.; Vidal-Carou, M.C. Diamine oxidase (DAO) supplement reduces headache in episodic migraine patients with DAO deficiency: A randomized double-blind trial. Clin. Nutr. 2019, 38, 152–158. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Enko, D. Histamine Intolerance Originates in the Gut. Nutrients 2021, 13, 1262. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Schenk, M.; Lackner, S.; Enko, D.; Mangge, H.; Forster, F. Diamine oxidase supplementation improves symptoms in patients with histamine intolerance. Food Sci. Biotechnol. 2019, 28, 1779–1784. [Google Scholar] [CrossRef]
- Kedem, S.; Yust-Katz, S.; Carter, D.; Levi, Z.; Kedem, R.; Dickstein, A.; Daher, S.; Katz, L.H. Attention deficit hyperactivity disorder and gastrointestinal morbidity in a large cohort of young adults. World J. Gastroenterol. 2020, 26, 6626–6637. [Google Scholar] [CrossRef] [PubMed]
- Carthy, E.; Ellender, T. Histamine, Neuroinflammation and Neurodevelopment: A Review. Front. Neurosci. 2021, 15, 680214. [Google Scholar] [CrossRef] [PubMed]
- Melamed, I.; Heffron, M. Attention Deficit Disorder and Allergic Rhinitis: Are They Related? J. Immunol. Res. 2016, 2016, 1596828. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Buske-Kirschbaum, A.; Tesch, F.; Trikojat, K.; Stephan, V.; Abraham, S.; Bauer, A.; Nemat, K.; Plessow, F.; Roessner, V. Increased attention-deficit/hyperactivity symptoms in atopic dermatitis are associated with history of antihistamine use. Allergy 2018, 73, 615–626. [Google Scholar] [CrossRef]
- Cacabelos, R.; Torrellas, C.; Fernández-Novoa, L.; López-Muñoz, F. Histamine and Immune Biomarkers in CNS Disorders. Mediat. Inflamm. 2016, 2016, 1924603. [Google Scholar] [CrossRef]
- Chen, Q.; Hartman, C.A.; Haavik, J.; Harro, J.; Klungsøyr, K.; Hegvik, T.-A.; Wanders, R.; Ottosen, C.; Dalsgaard, S.; Faraone, S.V.; et al. Common psychiatric and metabolic comorbidity of adult attention-deficit/hyperactivity disorder: A population-based cross-sectional study. PLoS ONE 2018, 13, e0204516. [Google Scholar] [CrossRef]
- Song, Y.; Lu, M.; Yuan, H.; Chen, T.; Han, X. Mast cell-mediated neuroinflammation may have a role in attention deficit hyperactivity disorder (Review). Exp. Ther. Med. 2020, 20, 714–726. [Google Scholar] [CrossRef]
- Sujar, A.; Bayona, S.; Delgado-Gómez, D.; Miguélez-Fernández, C.; Ardoy-Cuadros, J.; Peñuelas-Calvo, I.; Baca-García, E.; Blasco-Fontecilla, H. Attention Deficit Hyperactivity Disorder Assessment Based on Patient Behavior Exhibited in a Car Video Game: A Pilot Study. Brain Sci. 2022, 12, 877. [Google Scholar] [CrossRef]
- Blasco-Fontecilla, H.; Wang, P.; Li, C.; Duelo, A.; Ruiz-Casares, E.; Perucho, T. Prevalencia y perfil clínico de la deficiencia de diamino oxidasa (DAO) en pacientes con trastorno por déficit de atención e hiperactividad (TDAH). Rev. Psq. Inf. 2022, 39 (Suppl. 1), 88. [Google Scholar] [CrossRef]
- Pinto, S.; Correia-de-Sá, T.; Sampaio-Maia, B.; Vasconcelos, C.; Moreira, P.; Ferreira-Gomes, J. Eating Patterns and Dietary Interventions in ADHD: A Narrative Review. Nutrients 2022, 14, 4332. [Google Scholar] [CrossRef]
- Agúndez, J.A.G.; Ayuso, P.; Cornejo-García, J.A.; Blanca, M.; Torres, M.J.; Doña, I.; Salas, M.; Blanca-López, N.; Canto, G.; Rondon, C.; et al. The diamine oxidase gene is associated with hypersensitivity response to non-steroidal anti-inflammatory drugs. PLoS ONE 2012, 7, e47571. [Google Scholar] [CrossRef] [PubMed]
- Leitner, R.; Zoernpfenning, E.; Missbichler, A. Evaluation of the inhibitory effect of various drugs/active ingredients on the activity of human diamine oxidase in vitro. Clin. Transl. Allergy 2014, 4, P23. [Google Scholar] [CrossRef]
- Golmirzaei, J.; Mahboobi, H.; Yazdanparast, M.; Mushtaq, G.; Kamal, M.A.; Hamzei, E. Psychopharmacology of Attention-Deficit Hyperactivity Disorder: Effects and Side Effects. Curr. Pharm. Des. 2016, 22, 590–594. [Google Scholar] [CrossRef]
- Işeri, E.; Kiliç, B.G.; Senol, S.; Karabacak, N.I. Effects of methylphenidate on leptin and appetite in children with attention-deficit hyperactivity disorder: An open label trial. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.A.; Weiss, M.; Hlavaty, L. ADHD treatments, sleep, and sleep problems: Complex associations. Neurotherapeutics 2012, 9, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Comas-Basté, O.; Latorre-Moratalla, M.L.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. In vitro determination of diamine oxidase activity in food matrices by an enzymatic assay coupled to UHPLC-FL. Anal. Bioanal. Chem. 2019, 411, 7595–7602. [Google Scholar] [CrossRef]
- Knights, K.M.; Stresser, D.M.; Miners, J.O.; Crespi, C.L. In Vitro Drug Metabolism Using Liver Microsomes. Curr. Protoc. Pharmacol. 2016, 74, 7.8.1–7.8.24. [Google Scholar] [CrossRef]
- Yoshitomo, A.; Asano, S.; Hozuki, S.; Tamemoto, Y.; Shibata, Y.; Hashimoto, N.; Takahashi, K.; Sasaki, Y.; Ozawa, N.; Kageyama, M.; et al. Significance of Basal Membrane Permeability of Epithelial Cells in Predicting Intestinal Drug Absorption. Drug Metab. Dispos. 2023, 51, 318–328. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Ma, S.Z.; Zhang, W.W.; Yao, K.B.; Chen, L.; Zhao, F.; Zhuang, Y.Q. Accumulating pathways of γ-aminobutyric acid during anaerobic and aerobic sequential incubations in fresh tea leaves. Food Chem. 2018, 240, 1081–1086. [Google Scholar] [CrossRef]
- Guo, X.X.; Zeng, Z.; Qian, Y.Z.; Qiu, J.; Wang, K.; Wang, Y.; Ji, B.-P.; Zhou, F. Wheat flour, enriched with γ-oryzanol, phytosterol, and ferulic acid, alleviates lipid and glucose metabolism in high-fat-fructose-fed rats. Nutrients 2019, 11, 1697. [Google Scholar] [CrossRef]
- Chierrito, D.; Villas-Boas, C.B.; Tonin, F.S.; Fernandez-Llimos, F.; Sanches, A.C.C.; de Mello, J.C.P. Using Cell Cultures for the Investigation of Treatments for Attention Deficit Hyperactivity Disorder: A Systematic Review. Curr. Neuropharmacol. 2019, 17, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Pennick, M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr. Dis. Treat. 2010, 6, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Mettler, L.G.; Brecht, K.; Butterweck, V.; Meyer Zu Schwabedissen, H.E. Impact of the clinically approved Petasites hybridus extract Ze 339 on intestinal mechanisms involved in the handling of histamine. Biomed. Pharmacother. 2022, 148, 112698. [Google Scholar] [CrossRef] [PubMed]
- Rokkas, T.; Vaja, S.; Murphy, G.M.; Dowling, R.H. Aminoguanidine blocks intestinal diamine oxidase (DAO) activity and enhances the intestinal adaptive response to resection in the rat. Digestion 1990, 46, 447–457. [Google Scholar] [CrossRef]
- Yang, R.; Chen, H.; Gu, Z. Factors influencing diamine oxidase activity and γ-aminobutyric acid content of fava bean (Vicia faba L.) during germination. J. Agric. Food Chem. 2011, 59, 11616–11620. [Google Scholar] [CrossRef]
- Jagannath, V.; Marinova, Z.; Monoranu, C.-M.; Walitza, S.; Grünblatt, E. Expression of D-Amino Acid Oxidase (DAO/DAAO) and D-Amino Acid Oxidase Activator (DAOA/G72) during Development and Aging in the Human Post-mortem Brain. Front. Neuroanat. 2017, 11, 31. [Google Scholar] [CrossRef]
- Panina, Y.; Germond, A.; Masui, S.; Watanabe, T.M. Validation of Common Housekeeping Genes as Reference for qPCR Gene Expression Analysis during iPS Reprogramming Process. Sci. Rep. 2018, 8, 8716. [Google Scholar] [CrossRef]
- Quesada-Vázquez, S.; Colom-Pellicer, M.; Navarro-Masip, È.; Aragonès, G.; Del Bas, J.M.; Caimari, A.; Escoté, X. Supplementation with a Specific Combination of Metabolic Cofactors Ameliorates Non-Alcoholic Fatty Liver Disease, Hepatic Fibrosis, and Insulin Resistance in Mice. Nutrients 2021, 13, 3532. [Google Scholar] [CrossRef]
- Beltrán-Ortiz, C.; Peralta, T.; Ramos, V.; Durán, M.; Behrens, C.; Maureira, D.; Guzmán, M.A.; Bastias, C.; Ferrer, P. Standardization of a colorimetric technique for determination of enzymatic activity of diamine oxidase (DAO) and its application in patients with clinical diagnosis of histamine intolerance. World Allergy Organ. J. 2020, 13, 100457. [Google Scholar] [CrossRef]
- Xu, G.; Strathearn, L.; Liu, B.; Yang, B.; Bao, W. Twenty-Year Trends in Diagnosed Attention-Deficit/Hyperactivity Disorder Among US Children and Adolescents, 1997-2016. JAMA Netw. Open 2018, 1, e181471. [Google Scholar] [CrossRef]
- Chung, W.; Jiang, S.-F.; Paksarian, D.; Nikolaidis, A.; Castellanos, F.X.; Merikangas, K.R.; Milham, M.P. Trends in the Prevalence and Incidence of Attention-Deficit/Hyperactivity Disorder Among Adults and Children of Different Racial and Ethnic Groups. JAMA Netw. Open 2019, 2, e1914344. [Google Scholar] [CrossRef]
- Pinzer, T.C.; Tietz, E.; Waldmann, E.; Schink, M.; Neurath, M.F.; Zopf, Y. Circadian profiling reveals higher histamine plasma levels and lower diamine oxidase serum activities in 24% of patients with suspected histamine intolerance compared to food allergy and controls. Allergy 2018, 73, 949–957. [Google Scholar] [CrossRef]
- Yacoub, M.-R.; Ramirez, G.A.; Berti, A.; Mercurio, G.; Breda, D.; Saporiti, N.; Burastero, S.; Dagna, L.; Colombo, G. Diamine Oxidase Supplementation in Chronic Spontaneous Urticaria: A Randomized, Double-Blind Placebo-Controlled Study. Int. Arch. Allergy Immunol. 2018, 176, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Lunsford-Avery, J.R.; Kollins, S.H. Editorial Perspective: Delayed circadian rhythm phase: A cause of late-onset attention-deficit/hyperactivity disorder among adolescents? J. Child Psychol. Psychiatry 2018, 59, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Bondopadhyay, U.; Diaz-Orueta, U.; Coogan, A.N. The Role of the Circadian System in Attention Deficit Hyperactivity Disorder. Adv. Exp. Med. Biol. 2021, 1344, 113–127. [Google Scholar] [CrossRef]
- Advokat, C.; Scheithauer, M. Attention-deficit hyperactivity disorder (ADHD) stimulant medications as cognitive enhancers. Front. Neurosci. 2013, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Storebø, O.J.; Pedersen, N.; Ramstad, E.; Kielsholm, M.L.; Nielsen, S.S.; Krogh, H.B.; Moreira-Maia, C.R.; Magnusson, F.L.; Holmskov, M.; Gerner, T.; et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents—Assessment of adverse events in non-randomised studies. Cochrane Database Syst. Rev. 2018, 5, CD012069. [Google Scholar] [CrossRef]
- Jaeschke, R.R.; Sujkowska, E.; Sowa-Kućma, M. Methylphenidate for attention-deficit/hyperactivity disorder in adults: A narrative review. Psychopharmacology 2021, 238, 2667–2691. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobajas, Y.; Alemany-Fornés, M.; Samarra, I.; Romero-Giménez, J.; Tintoré, M.; del Pino, A.; Canela, N.; del Bas, J.M.; Ortega-Olivé, N.; de Lecea, C.; et al. Interaction of Diamine Oxidase with Psychostimulant Drugs for ADHD Management. J. Clin. Med. 2023, 12, 4666. https://doi.org/10.3390/jcm12144666
Tobajas Y, Alemany-Fornés M, Samarra I, Romero-Giménez J, Tintoré M, del Pino A, Canela N, del Bas JM, Ortega-Olivé N, de Lecea C, et al. Interaction of Diamine Oxidase with Psychostimulant Drugs for ADHD Management. Journal of Clinical Medicine. 2023; 12(14):4666. https://doi.org/10.3390/jcm12144666
Chicago/Turabian StyleTobajas, Yaiza, Marc Alemany-Fornés, Iris Samarra, Jordi Romero-Giménez, Maria Tintoré, Antoni del Pino, Núria Canela, Josep M. del Bas, Nàdia Ortega-Olivé, Carlos de Lecea, and et al. 2023. "Interaction of Diamine Oxidase with Psychostimulant Drugs for ADHD Management" Journal of Clinical Medicine 12, no. 14: 4666. https://doi.org/10.3390/jcm12144666
APA StyleTobajas, Y., Alemany-Fornés, M., Samarra, I., Romero-Giménez, J., Tintoré, M., del Pino, A., Canela, N., del Bas, J. M., Ortega-Olivé, N., de Lecea, C., & Escoté, X. (2023). Interaction of Diamine Oxidase with Psychostimulant Drugs for ADHD Management. Journal of Clinical Medicine, 12(14), 4666. https://doi.org/10.3390/jcm12144666






