Improving Pathways to Care for Patients at High Psychosis Risk in Switzerland: PsyYoung Study Protocol
Abstract
1. Introduction
Aims and Hypotheses
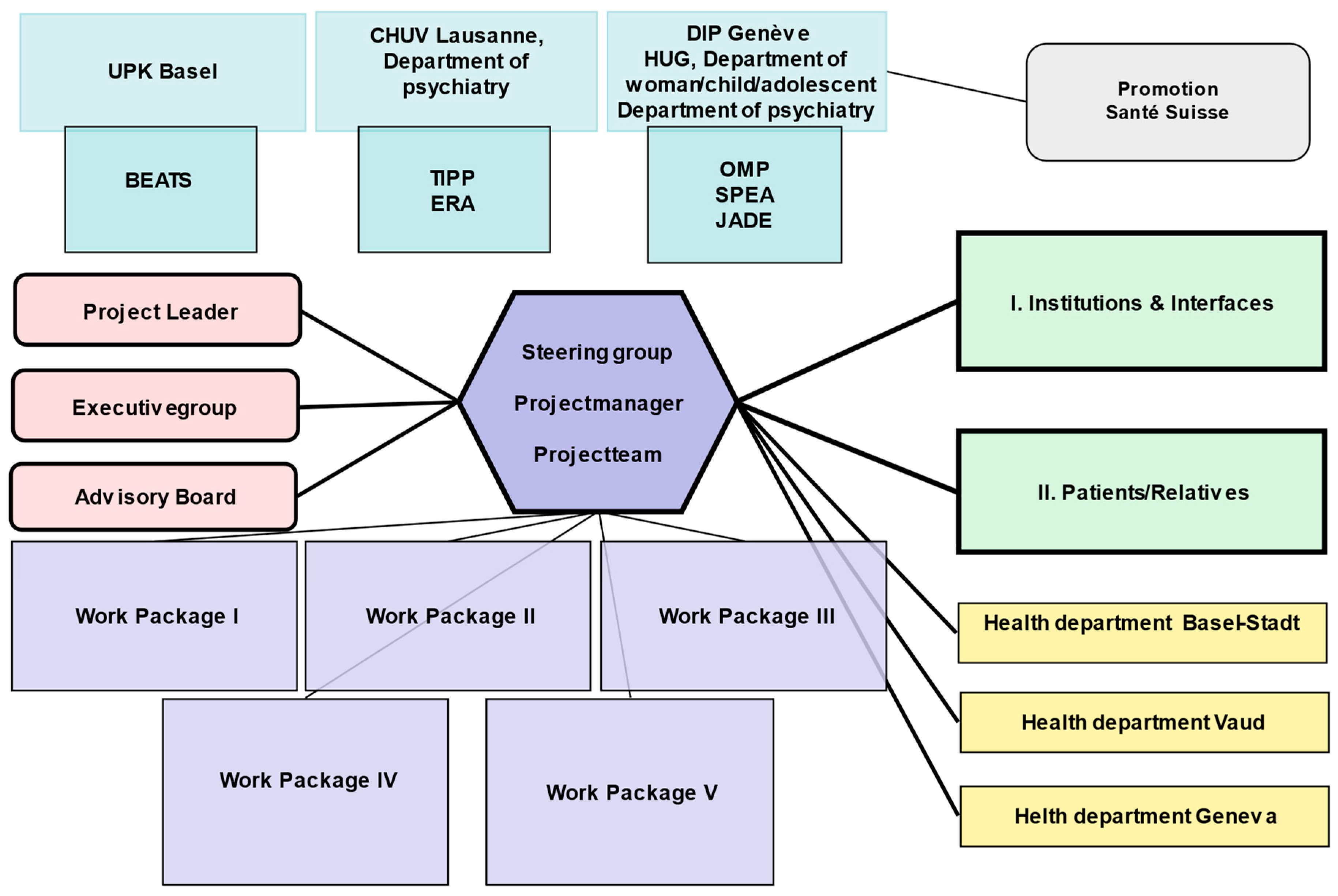
2. Methods
2.1. Intervention: Service Delivery Changes
Standardized Stepped-Care Diagnosis and Assessment Model
2.2. Measures to Increase Awareness, Dissemination and Networking
2.3. Project Population
- (A)
- Patients referred to an early intervention site with a suspected clinical ARMS or FEP (expected n = 625, patients with a FEP are expected to constitute approximately 25% of all referred patients). Inclusion criteria: (i) age 15–35 years; (ii) at least one appointment with a specialized early intervention service in one of the three participating cantons; (iii) reason for referral being a suspected ARMS or a FEP. Participation of a close relative (see B) is not an inclusion criterion for participation of patients in the study.
- (B)
- Close relatives of patients with a suspected ARMS or a FEP (expected n = 625). Inclusion criteria: (i) blood relatives or partners of patients meeting the above inclusion criteria, who (ii) live in a common household with the patient or have a close relationship (defined as contact at least once per week).
- (C)
- Professionals involved in the detection and treatment of mental health disorders in adolescents and young adults (expected n = 550). Inclusion criteria: (i) professionals from one of the following groups: general practitioners/family physicians/pediatricians in the private sector/adult or child and adolescent psychiatrists or psychotherapists in the private or public sector/social workers/psychologists/other therapists in the educational sector (schools and universities, vocational consultation services etc.).
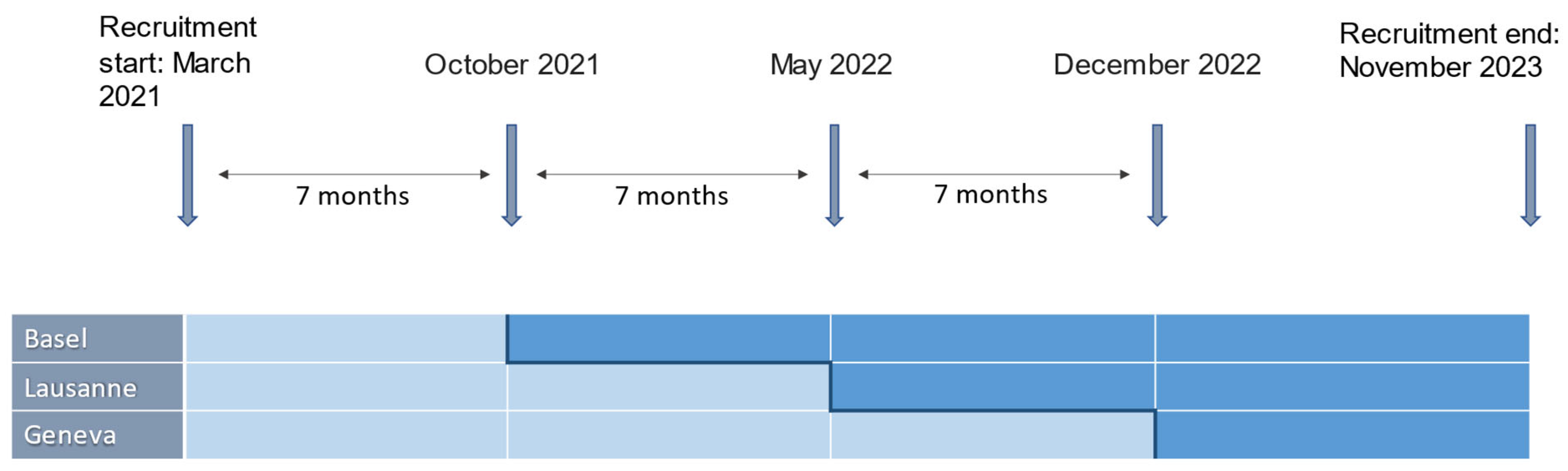
2.4. Study Design
2.5. Setting and Procedure
2.6. Outcomes and Measures
- Percentage of “late” referrals (i.e., referral to the EIS after a first inpatient admission). Percentage of referred patients with a suspected ARMS who are already being treated with antipsychotics at referral (contrary to current guidelines [18], and hence, a measure of insufficient awareness among referring physicians).
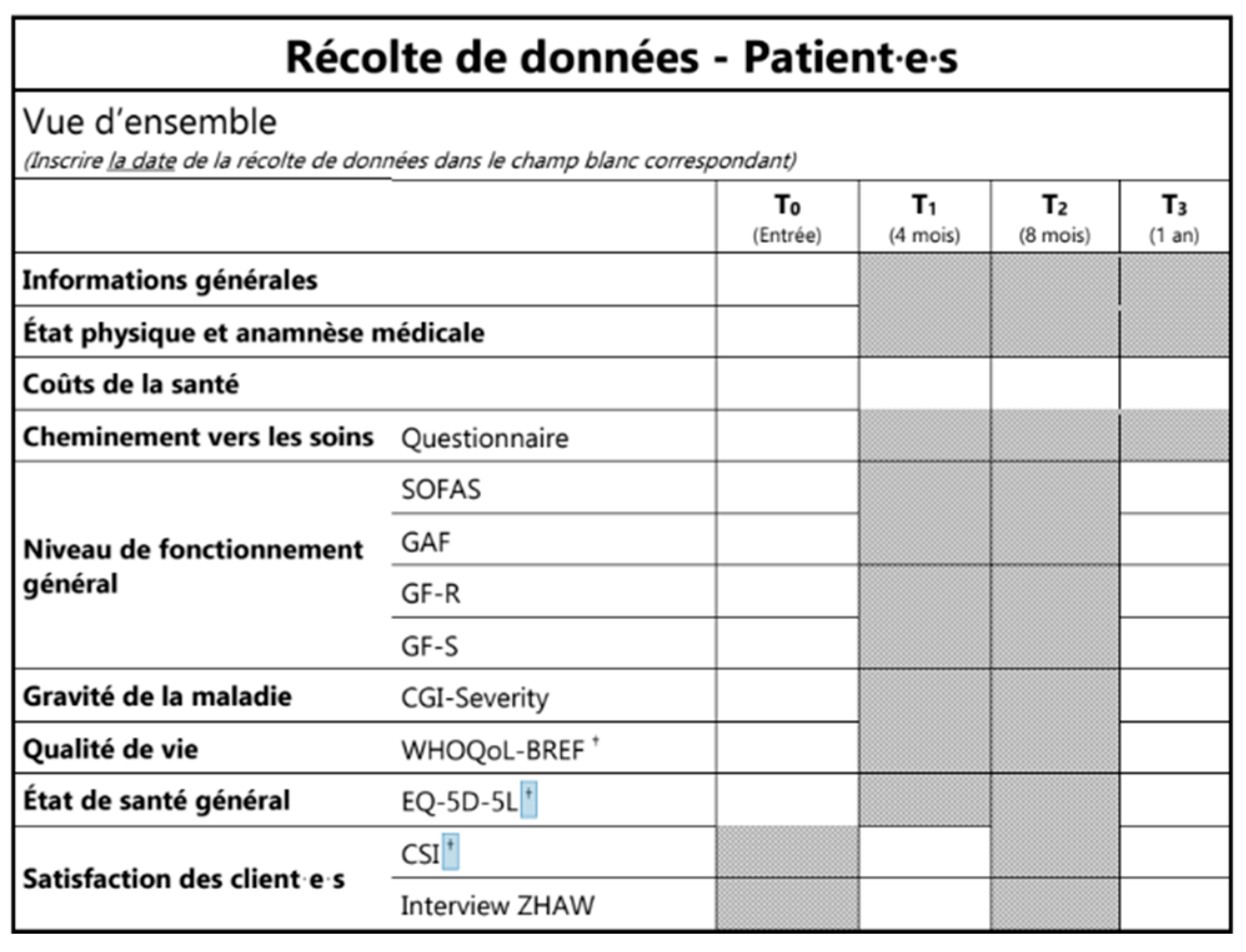
- Pathway to care until the specialized referral of patients including: number of contacts with mental health professionals up until the specialized referral, delay in seeking help, delay in first referral to mental health services, and delay in referral within mental health services (see Appendix B, Table A2 and Figure A1).
- Time from referral to needs-based orientation of referred patients. This is assessed by case managers by means of a concordance index (number of domains covered by the treatment plan divided by the number of domains identified by multiaxial needs assessment at the intake interview), based on all of the available data that is on file 1 year after the intake interview.
- Percentage of patients who receive an individualized treatment plan within a month from first assessment.
- Change in quality of life, functioning, and symptom severity in the first year following referral to the EIS. Quality of life is assessed by means of EuroQol 5 Dimensions (EQ-5D [26,27]) and EQ-5D-Y [28] for adults and adolescents, respectively, as well as the World Health Organization Quality of Life-BREF (WHOQOL-BREF [29,30]); measures of functioning include the functioning assessment scale (SOFAS [31]), global assessment of functioning (GAF [32]), and global functioning role and social scales (GF-R/GF-S [33]); symptom severity is assessed by the clinical global impression scale (CGI [34]).
- Direct healthcare costs and indirect costs for patients in the first year following referral to the EIS. The assessment considers the type and dose of medication, days of absence at work or school due to illness, as well as the number of medical appointments (any specialty; divided by psychiatric, surgical, and non-surgical appointments), emergency room visits, psychotherapy sessions, other therapy sessions, and the number and duration of inpatient admissions (classified as psychiatric and non-psychiatric).
- Duration of untreated psychosis in referred patients with a FEP.
- Satisfaction with the EIS and the treatment plan of referred patients. This is assessed with the CSI [35], as well as qualitative analysis of semi-structured telephone interviews conducted in a subset of patients (n = 300) by the external evaluation institute
- Level of engagement of patients with a ARMS within the EIS (rate of missed appointments, drop-out rate).
- Satisfaction with the EIS of referring institutions and referring health care professionals. Opinions and evaluations from referring professionals are collected by the external evaluation institute through telephone interviews or online surveys, six months after the implementation of the intervention program.
- Satisfaction with the EIS of the relatives of the patients. This is assessed using information collected during focus groups, conducted by the external evaluation institute in a subset of relatives (n = 30).
- Quality of life and burden of care in patients’ relatives. This is assessed by means of self-reported questionnaires regarding quality of life (EQ-5D, see above) as well as the involvement evaluation questionnaire (IEQ) [36], assessing various dimensions of caregiver burden such as tension, supervision, worrying, and urging.
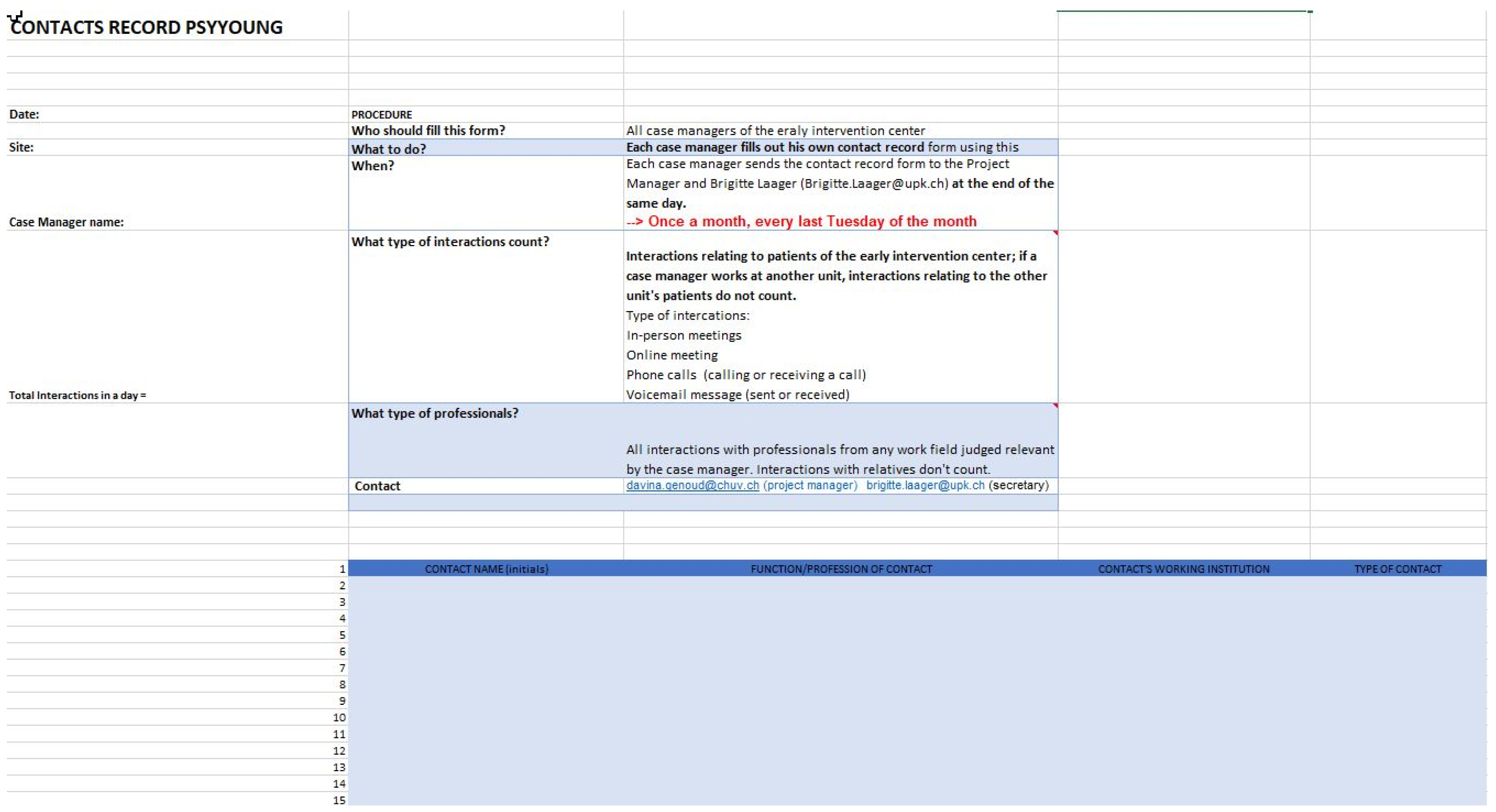
- Number of interactions between specialized early intervention staff and other important players involved in the treatment and education of young people (e.g., general practitioners/pediatricians, psychiatrists and psychologists, social workers, and mental health professionals in schools, workplaces etc.). This is assessed using documentation of all interactions with the EIS clinical staff during one week at quarterly intervals (see Appendix B, Figure A1).
2.7. Statistics and Power Calculation
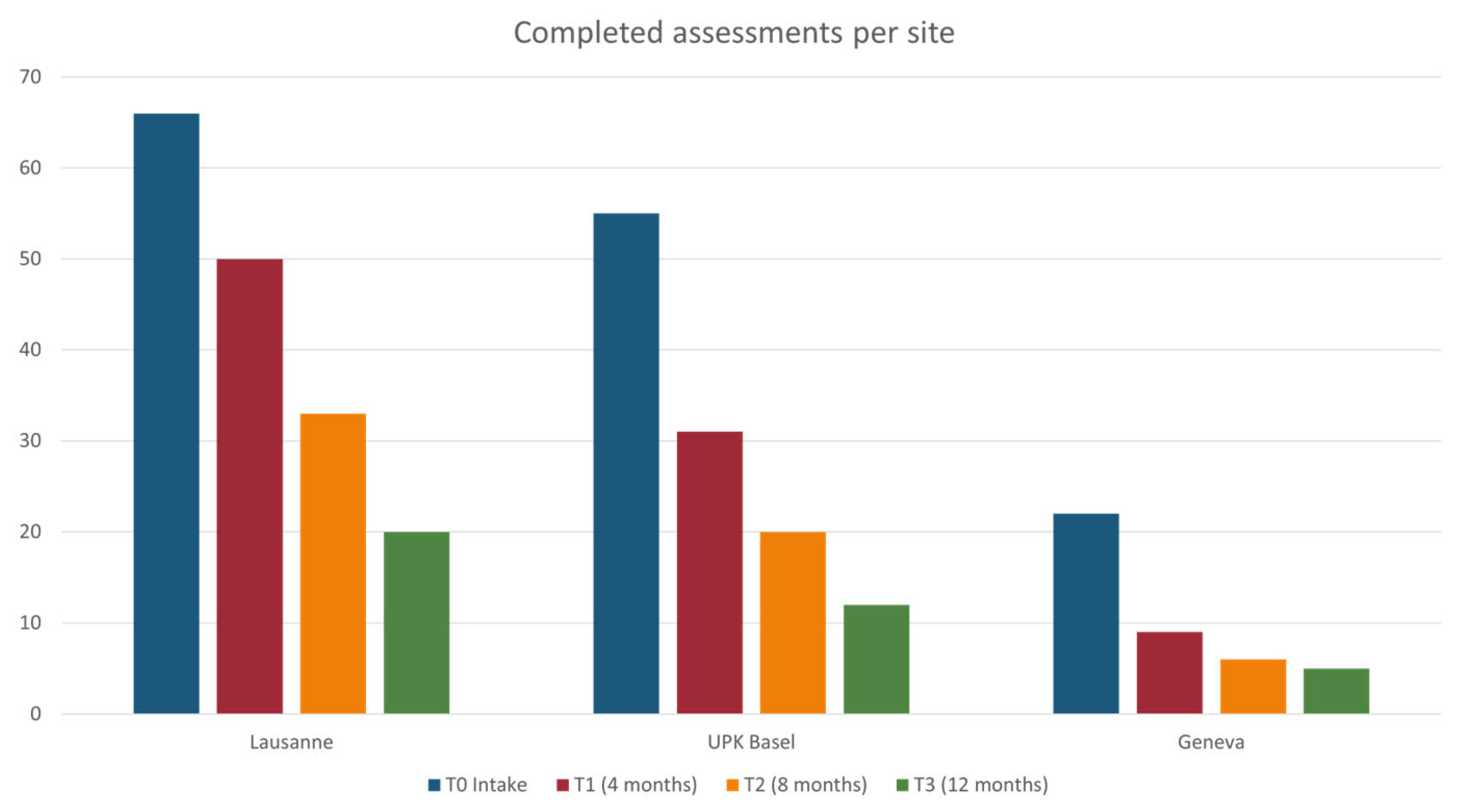
3. Current Implementation Status and Preliminary Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Instrument | Type and Aims |
|---|---|
| Psychiatric Diagnosis (on Structured Level) | |
| International Classification of Diseases (ICD-10/11) or Diagnostic and Statistical Manual of Mental Disorders (DSM-5) | Codified medical classification of diseases [37] Classification manual of diagnosis of mental disorders [32] |
| Mini international neuropsychiatric Interview (M.I.N.I.) | Short, structured diagnostic interview to assess psychiatric diagnosis according to DSM-5 and ICD-10 criteria [38] |
| Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) | Semi-structured interview used to measure current and past symptoms of mood, anxiety, psychotic, and disruptive behavior disorders in children and adolescents [39] |
| The Structured Clinical Interview for DSM-5 Personality Disorders (SCID PD) The Structured Clinical Interview for DSM-5, Clinician Version (SCID CV) | Semi-structured diagnostic interview that guides assessment of the defining DSM-5 alternative model for personality disorders components [40] Structured clinical interview to assess DSM-5 disorders [41] |
| Psychotic Symptoms | |
| Positive and Negative Syndrome Scale (PANSS) | Clinical scale measuring the severity of positive and negative symptoms in patients with schizophrenia [42] |
| The Brief Psychiatric Rating Scale (BPRS) | Brief interview used to measure psychiatric symptoms such as anxiety, depression, and psychoses [43] |
| Scale for the Assessment of Negative Symptoms (SANS) | Rating scale used to measure negative symptoms in schizophrenia [44] |
| Depression/Mania | |
| Montgomery–Asberg Depression Rating Scale (MADRS) | Clinical scale used to assess the severity of depression in patients with mood disorders and to measure the changes brought about by the treatment of depression [45] |
| Beck Depression Inventory (BDI-II) | Self-administered questionnaire providing a quantitative estimation of the intensity of depressive feelings [46] |
| Children’s Depression Rating Scale (CDRS) | Semi-structured interview for the diagnosis of depressive disorders in children and adolescents [47] |
| Children’s Depression Inventory (CDI) | Self-report assessment measuring the cognitive, affective, and behavioral signs of depression [48] |
| Young Mania Rating Scale (YMRS) | Clinical interview scale used to assess the severity of manic states [49] |
| Anxiety Disorders | |
| Beck Anxiety Inventory (BAI) | Self-administered questionnaire assessing anxiety [50] |
| Revised Children’s Manifest Anxiety Scale (RCMAS) or Youth Anxiety Measure for DSM-5 (YAM-5) | Self-administered questionnaire measuring the level and nature of anxiety as experienced by children and adolescents [51] Self-report, or parent-report, assessing the full spectrum of symptoms of anxiety disorders in adolescents and children [52] |
| Social Interaction Anxiety Scale (SIAS) | Self-report scale measuring distress when meeting and talking with others [53] |
| Youth Self-Report for Ages 6-18 (YSR) Child Behavior Checklist for Ages 6-18 (CBCL) | Self-report questionnaire measuring emotional and behavioral problems [54] Parent questionnaire measuring behavioral and emotional problems [55] |
| Adult Self-Report (ASR) | Self-report questionnaire measuring emotional and behavioral problems [56] |
| Adult Behavior Checklist (ABCL) | Proxy Informant Questionnaire assessing psychopathology [57] |
| Liebowitz Social Anxiety Scale (LSAS) | Clinician-administered rating scale measuring the range of social interactions and performance situations that individuals with social phobia may fear and/or avoid [58] |
| Overall Anxiety Severity Impairment Scale (OASIS) | Self-reported questionnaire measuring frequency and severity of anxiety [59] |
| OCD | |
| Yale–Brown Obsessive Compulsive Scale (Y-BOCS) | Semi-structured interview assessing severity and frequency of obsessions and compulsions [60] |
| Substance abuse, cannabis abuse | |
| Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) Alcohol Use Disorders Identification Test (AUDIT) (Alternative to SCID Section) | Self-reported questionnaire for hazardous and harmful alcohol consumption [61] |
| Cannabis Use Disorders Identification Test (CUDIT, alternative to SCID Section) | Self-reported questionnaire for identifying cannabis use disorder in at-risk populations [62] |
| Traumatic and Relevant Life Experiences | |
| Childhood Trauma Questionnaire (CTQ) | Self-reported questionnaire that aims to detect experiences of childhood abuse and neglect in adults and adolescents [63] |
| Coddington life events scale (CLES) | Self-reported questionnaire measuring the presence and impact of important life events [64] |
| Perceived stress reactivity scale related to life events (PSRS) | Self-reported questionnaire measuring stress reactivity [65] |
| Forms of Bullying Scale (FBS) | Self-reported questionnaire assessing involvement in different forms of both bullying victimization and perpetration [66] |
| Suicide Risk, Ideation | |
| Risk-Urgence-Dangerosité (RUD) | Semi-structural interview evaluating the dimensions of risk, urgency and dangerosity of a suicidal patient [67] |
| Other problems | |
| Birchwood Insight Scale (BIS) | Self-report questionnaire measuring awareness of psychosis [68] |
| Paradox of Self-Stigma Scale (Pass-24) | Self-report questionnaire measuring self-stigma and related constructs (stereotype endorsement, righteous anger, and non-disclosure) [69] |
| Reflective functioning questionnaire (RFQ) | Self-reported measure for mentalizing [70] |
| Parental reflective functioning questionnaire (PRFQ-A, 12–18yrs.) | Self-reported questionnaire that assesses parental reflective functioning or mentalizing, that is, the capacity to treat the infant as a psychological agent [71] |
| Social Cognition Screening Questionnaire (SCSQ) | Vignette-based questionnaire used to screen for neurocognitive deficits and the patient’s needs for social cognitive intervention [72] |
| Multidimensional Scale of Perceived Social Support (MSPSS) | Self-report questionnaire to assess perceived adequacy of social support from family, friends, and significant others [73] |
| Resilience Scale for Adults (RSA) | Self-report questionnaire used to examine intrapersonal and interpersonal protective factors presumed to facilitate adaptation to psychosocial adversities [74] |
| Personality disorders | |
| NEO Five-Factor-Inventory (NEO-FFI) | Self-report questionnaire that provides a measure of the five personality domains [75] |
| Level of Personality Functioning Questionnaire (LoPF-Q 12-18) | Self-report questionnaire measuring alterations in the functional level of personality in four areas [76,77] |
| Autism | |
| Social Communication Questionnaire (SCQ) | Self-report questionnaire to screen and monitor communications skills and social functioning in children who may have autism/autism spectrum disorders [78] |
| TDAH | |
| Conners Adult ADHD Rating Scales (CAARS) Conners Comprehensive Behavior Rating Scales (Conners CBRS) | Self-report, or observer report, to assess, diagnose and monitor treatment of ADHD in adults [79] Self-report questionnaire used to diagnose attention deficit disorder with or without hyperactivity [80] |
| Wender Utah Rating Scale (WURS, adults) | Self-report questionnaire used in the diagnosis of attention deficit hyperactivity disorder based on behavior and feelings experienced during childhood [81] |
| Neurocognition | |
| Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) | Self-report questionnaire measuring cognition in individuals diagnosed with schizophrenia and related disorders [82] |
| Brief Assessment of Cognition in Schizophrenia (BACS) | Test battery assessing aspects of cognition found to be the most impaired in patients with schizophrenia [83] |
| A developmental NEuroPSYchological Assessment (NEPSY) | Self-report questionnaire that is tailored assessment of child and adolescent skills in 6 major neuropsychological areas [84] |
| Somatic Assessment | |
| Recommendations: SGPP Behandlungsempfehlungen Schizophrenie | Recommendations for the treatment of schizophrenia [85] |
| The exams listed are for the exclusion of organic psychosis | For patients with psychosis, our recommendations are as follows; For patients at-risk of psychosis, the exams depend on the specific situation and specific clinical concerns. |
| Complete somatic assessment | |
| Complete blood cell (CBC) count | |
| Electrolyte (incl. Calcium) levels | |
| CRP | |
| Liver and renal function test | |
| TSH | |
| Drug Screening | |
| Vitamin B-12 | |
Appendix B
| Cheminement Vers les Soins | ||||||
|---|---|---|---|---|---|---|
| CONTACTS (du Premier Contact [1] au Dernier [x]; Inscrire Uniquement les Chiffres/Lettres de la Légende) | ||||||
| Date | Contact Initié Par (Légende A–D) | Qui a Été Contacté (Légende E–R) | Bon Contact (Oui/Non) | Raisons de la Prise de Contact (Légende 1–12) | Propositions à L’issu du Contact (Légende 13–18; Entourer les Propositions Suivies) | |
| 1 | __ __/__ __/__ __ __ __ | |||||
| 2 | __ __/__ __/__ __ __ __ | |||||
| 3 | __ __/__ __/__ __ __ __ | |||||
| 4 | __ __/__ __/__ __ __ __ | |||||
| 5 | __ __/__ __/__ __ __ __ | |||||
| 6 | __ __/__ __/__ __ __ __ | |||||
| 7 | __ __/__ __/__ __ __ __ | |||||
| 8 | __ __/__ __/__ __ __ __ | |||||
| 9 | __ __/__ __/__ __ __ __ | |||||
| LÉGENDE | ||||||
| Contact Initié Par | Qui a Été Contacté | Raisons de la Prise de Contact | Propositions | |||
|
|
|
| |||
| NOMBRE DE CONTACTS | Durant la phase prodromique: _____ Durant la phase psychotique: _____ Indissociable: _____ total: _____ | |||||
References
- Baer, N.; Altwicker-Hámori, S.; Juvalta, S.; Frick, U.; Rüesch, P. Profile von Jungen IV-Neurentenbeziehenden Mit Psychischen Krankheiten (Profiles of Young Recipients of Disability Pensions Due to Mental Disorders); Bundesamt für Sozialversicherungen: Bern, Switerland, 2016. [Google Scholar]
- Schuler, D.; Tuch, A.; Peter, C. La Santé Psychique en Suisse. Monitorage 2020 (Obsan Rapport 15/2020); Observatoire Suisse de la Santé: Neuchâtel, Switzerland, 2020. [Google Scholar]
- Jääskeläinen, E.; Juola, P.; Hirvonen, N.; McGrath, J.J.; Saha, S.; Isohanni, M.; Veijola, J.; Miettunen, J. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr. Bull. 2013, 39, 1296–1306. [Google Scholar] [CrossRef]
- Howes, O.D.; Whitehurst, T.; Shatalina, E.; Townsend, L.; Onwordi, E.C.; Mak, T.L.A.; Arumuham, A.; O’Brien, O.; Lobo, M.; Vano, L.; et al. The clinical significance of duration of untreated psychosis: An umbrella review and random-effects meta-analysis. World Psychiatry 2021, 20, 75–95. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Borgwardt, S.; Bechdolf, A.; Addington, J.; Riecher-Rössler, A.; Schultze-Lutter, F.; Keshavan, M.; Wood, S.; Ruhrmann, S.; Seidman, L.J. The psychosis high-risk state: A comprehensive state-of-the-art review. JAMA Psychiatry 2013, 70, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Salazar de Pablo, G.; Estradé, A.; Cutroni, M.; Andlauer, O.; Fusar-Poli, P. Establishing a clinical service to prevent psychosis: What, how and when? Systematic review. Transl. Psychiatry 2021, 11, 43. [Google Scholar] [CrossRef]
- Yung, A.R.; Yuen, H.P.; McGorry, P.D.; Phillips, L.J.; Kelly, D.; Dell’Olio, M.; Francey, S.M.; Cosgrave, E.M.; Killackey, E.; Stanford, C.; et al. Mapping the onset of psychosis: The Comprehensive Assessment of At-Risk Mental States. Aust. N. Zeal. J. Psychiatry 2005, 39, 964–971. [Google Scholar] [CrossRef]
- Miller, T.; Rosen, J.; D’andrea, J.; Woods, S.; McGlashan, T. Outcome of Prodromal Syndromes: SIPS Predictive Validity; Schizophrenia Research; Elsevier Science BV: Amsterdam, The Netherlands, 2004; p. 44. [Google Scholar]
- Fusar-Poli, P.; Cappucciati, M.; Rutigliano, G.; Lee, T.; Beverly, Q.; Bonoldi, I.; Lelli, J.; Kaar, S.; Gago, E.; Rocchetti, M. Towards a standard psychometric diagnostic interview for subjects at ultra high risk of psychosis: CAARMS versus SIPS. Psychiatry J. 2016, 2016, 7146341. [Google Scholar] [CrossRef] [PubMed]
- Brady, L.S.; Larrauri, C.A.; AMP SCZ Steering Committee. Accelerating Medicines Partnership® Schizophrenia (AMP® SCZ): Developing tools to enable early intervention in the psychosis high risk state. World Psychiatry 2023, 22, 42–43. [Google Scholar] [CrossRef] [PubMed]
- The National Collaborating Centre for Mental Health; National Institute for Health and Care Excellence. Implementing the Early Intervention in Psychosis Access and Waiting Time Standard: Guidance; NHS: England, UK, 2016. [Google Scholar]
- Baumann, P.S.; Crespi, S.; Marion-Veyron, R.; Solida, A.; Thonney, J.; Favrod, J.; Bonsack, C.; Do, K.Q.; Conus, P. Treatment and early intervention in psychosis program (TIPP-Lausanne): Implementation of an early intervention programme for psychosis in Switzerland. Early Interv. Psychiatry 2013, 7, 322–328. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; McGorry, P.D.; Kane, J.M. Improving outcomes of first-episode psychosis: An overview. World Psychiatry 2017, 16, 251–265. [Google Scholar] [CrossRef]
- Salazar de Pablo, G.; Radua, J.; Pereira, J.; Bonoldi, I.; Arienti, V.; Besana, F.; Soardo, L.; Cabras, A.; Fortea, L.; Catalan, A.; et al. Probability of Transition to Psychosis in Individuals at Clinical High Risk: An Updated Meta-analysis. JAMA Psychiatry 2021, 78, 970–978. [Google Scholar] [CrossRef]
- Salazar de Pablo, G.; Soardo, L.; Cabras, A.; Pereira, J.; Kaur, S.; Besana, F.; Arienti, V.; Coronelli, F.; Shin, J.I.; Solmi, M.; et al. Clinical outcomes in individuals at clinical high risk of psychosis who do not transition to psychosis: A meta-analysis. Epidemiol. Psychiatr. Sci. 2022, 31, e9. [Google Scholar] [CrossRef] [PubMed]
- Salazar de Pablo, G.; Catalan, A.; Vaquerizo Serrano, J.D.; Pedruzo, B.; Alameda, L.; Armendariz, A.; Rodriguez, V.; Arango, C.; Moreno, C.; Downs, J.; et al. Negative symptoms in children and adolescents with early-onset psychosis and at clinical high-risk for psychosis: Systematic review and meta-analysis. Br. J. Psychiatry 2022, 17, 1–13. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Rocchetti, M.; Sardella, A.; Avila, A.; Brandizzi, M.; Caverzasi, E.; Politi, P.; Ruhrmann, S.; McGuire, P. Disorder, not just state of risk: Meta-analysis of functioning and quality of life in people at high risk of psychosis. Br. J. Psychiatry 2015, 207, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.J.; Schultze-Lutter, F.; Schimmelmann, B.G.; Maric, N.P.; Salokangas, R.K.; Riecher-Rössler, A.; van der Gaag, M.; Meneghelli, A.; Nordentoft, M.; Marshall, M.; et al. EPA guidance on the early intervention in clinical high risk states of psychoses. Eur. Psychiatry 2015, 30, 388–404. [Google Scholar] [CrossRef]
- Andreou, C.; Bailey, B.; Armando, M.; Micali, N.; Huber, C.; Herbrecht, E.; Curtis, L.; Genoud, D.; Borgwardt, S.; Plessen, K.J. PsyYoung: A transcantonal project for facilitating access to care for young people at risk for psychotic disorders. Rev. Med. Suisse 2021, 17, 1597–1601. [Google Scholar] [PubMed]
- Ising, H.K.; Veling, W.; Loewy, R.L.; Rietveld, M.W.; Rietdijk, J.; Dragt, S.; Klaassen, R.M.C.; Nieman, D.H.; Wunderink, L.; Linszen, D.H.; et al. The Validity of the 16-Item Version of the Prodromal Questionnaire (PQ-16) to Screen for Ultra High Risk of Developing Psychosis in the General Help-Seeking Population. Schizophr. Bull. 2012, 38, 1288–1296. [Google Scholar] [CrossRef]
- Lejuste, F.; Pedron, L.; Bonnard, E.; Urban, M.; Morvan, Y.; Urben, S.; Gaillard, R.; Conus, P.; Krebs, M.O. Validation d’une version française du 16-items Prodromal Questionnaire (fPQ16) chez des adolescents et jeunes adultes consultant en psychiatrie. L’Encéphale 2021, 47, 547–553. [Google Scholar] [CrossRef]
- Miller, T.J.; McGlashan, T.H.; Rosen, J.L.; Somjee, L.; Markovich, P.J.; Stein, K.; Woods, S.W. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: Preliminary evidence of interrater reliability and predictive validity. Am. J. Psychiatry 2002, 159, 863–865. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Rutigliano, G.; Stahl, D.; Schmidt, A.; Ramella-Cravaro, V.; Hitesh, S.; McGuire, P. Deconstructing Pretest Risk Enrichment to Optimize Prediction of Psychosis in Individuals at Clinical High Risk. JAMA Psychiatry 2016, 73, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Conus, P. Premier traitement pour une psychose: Quels défis et quels enjeux? L’Inf. Psychiatr. 2018, 94, 301–306. [Google Scholar]
- Hemming, K.; Haines, T.P.; Chilton, P.J.; Girling, A.J.; Lilford, R.J. The stepped wedge cluster randomised trial: Rationale, design, analysis, and reporting. BMJ 2015, 350, h391. [Google Scholar] [CrossRef]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Andrade, L.F.; Ludwig, K.; Goni, J.M.R.; Oppe, M.; de Pouvourville, G. A French Value Set for the EQ-5D-5L. Pharmacoeconomics 2020, 38, 413–425. [Google Scholar] [CrossRef]
- Van Reenen, A.; de Jong, A.M.; den Toonder, J.M.; Prins, M.W. Integrated lab-on-chip biosensing systems based on magnetic particle actuation–a comprehensive review. Lab. A Chip 2014, 14, 1966–1986. [Google Scholar] [CrossRef]
- Baumann, C.; Erpelding, M.L.; Régat, S.; Collin, J.F.; Briançon, S. The WHOQOL-BREF questionnaire: French adult population norms for the physical health, psychological health and social relationship dimensions. Rev. Epidemiol. Sante Publique 2010, 58, 33–39. [Google Scholar] [CrossRef]
- Skevington, S.M.; Lotfy, M.; O’Connell, K.A. The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A Report from the WHOQOL Group. Qual. Life Res. 2004, 13, 299–310. [Google Scholar] [CrossRef]
- Morosini, P.L.; Magliano, L.; Brambilla, L.; Ugolini, S.; Pioli, R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social funtioning. Acta Psychiatr. Scand. 2000, 101, 323–329. [Google Scholar] [CrossRef]
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; APA: Washington, DC, USA, 2013. [Google Scholar]
- Lucarini, V.; Kazes, M.; Krebs, E.; Morin, V.; Godignon, M.; De Gasquet, M.; Ton, T.; Féron, M.; Tanguy, G.; Lévi, A.; et al. Validation of the French version of the Global Functioning: Social and Global Functioning: Role Scales in adolescents and young adults seeking help in early intervention clinics. Early Interv. Psychiatry 2023. [Google Scholar] [CrossRef]
- Guy, W. ECDEU Assessment Manual for Psychopharmacology; US Department of Health, Education, and Welfare, Public Health Service: Bethesda, MD, USA, 1976.
- McMurtry, S.L.; Hudson, W.W. The Client Satisfaction Inventory: Results of an Initial Validation Study. Res. Soc. Work Pract. 2000, 10, 644–663. [Google Scholar] [CrossRef]
- van Wijngaarden, B.; Schene, A.H.; Koeter, M.; Vázquez-Barquero, J.L.; Knudsen, H.C.; Lasalvia, A.; McCrone, P. Caregiving in schizophrenia: Development, internal consistency and reliability of the Involvement Evaluation Questionnaire—European Version: EPSILON Study 4. Br. J. Psychiatry 2000, 177, s21–s27. [Google Scholar] [CrossRef]
- World Health Organization. WHO International Statistical Classification of Diseases and Related Health Problems, 11th ed.; World Health Organization: Geneva, Switzerland, 2019. Available online: https://www.who.int/standards/classifications/classification-of-diseases (accessed on 1 May 2023).
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Balker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. S20), 22–33. [Google Scholar] [PubMed]
- Chambers, W.J.; Puig-Antich, J.; Hirsch, M.; Paez, P.; Ambrosini, P.J.; Tabrizi, M.A.; Davies, M. The Assessment of Affective Disorders in Children and Adolescents by Semistructured Interview: Test-Retest Reliability of the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present Episode Version. Arch. Gen. Psychiatry 1985, 42, 696–702. [Google Scholar] [CrossRef]
- First, M.B.; Gibbon, M.; Spitzer, R.L.; Williams, J.B.W.; Benjamin, L.S. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II); American Psychiatric Press: Washington, DC, USA, 1997. [Google Scholar]
- First, M.B.; Williams, J.B.W.; Karg, R.S.; Spitzer, R.L. Structured Clinical Interview for DSM-5–Clinician Version (SCID-5 for DSM-5, Clinician Version; SCID-5-RV); American Psychiatric Association: Arlington, TX, USA, 2015; Available online: https://www.appi.org/products/structured-clinical-interview-for-dsm-5-scid-5 (accessed on 1 May 2023).
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Lukoff, D.; Liberman, R.P.; Nuechterlein, K.H. Symptom Monitoring in the Rehabilitation of Schizophrenic Patients. Schizophr. Bull. 1986, 12, 578–603. [Google Scholar] [CrossRef]
- Andreasen, N.C. The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and Theoretical Foundations. Br. J. Psychiatry 1989, 155, 49–52. [Google Scholar] [CrossRef]
- Montgomery, S.A.; Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Poznanski, E.O.; Grossman, J.A.; Buchsbaum, Y.; Banegas, M.; Freeman, L.; Gibbons, R. Children’s Depression Rating Scale-Revised (CDRS-R); APA PsycTests: Washington, DC, USA, 1984; Available online: https://psycnet.apa.org/doiLanding?doi=10.1037%2Ft55280-000 (accessed on 1 May 2023).
- Kovacs, M. Children’s Depression Inventory (CDI); APA PsycTests: Washington, DC, USA, 1978; Available online: https://psycnet.apa.org/doiLanding?doi=10.1037%2Ft00788-000 (accessed on 1 May 2023).
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A Rating Scale for Mania: Reliability, Validity and Sensitivity. Br. J. Psychiatry 1978, 133, 429–435. [Google Scholar] [CrossRef]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Gerard, A.B.R.; Reynolds, C.R. Characteristics and applications of the Revised Children’s Manifest Anxiety Scale (RCMAS). In The Use of Psychological Testing for Treatment Planning and Outcomes Assessment, 2nd ed.; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 1999; pp. 323–340. Available online: https://www.taylorfrancis.com/chapters/edit/10.4324/9781410610621-3/characteristics-applications-revised-children-manifest-anxiety-scale-rcmas-anthony-gerard-cecil-reynolds (accessed on 1 May 2023).
- Muris, P.; Simon, E.; Lijphart, H.; Bos, A.; Hale, W.; Schmeitz, K.; Albano, A.M.; Bar-Haim, Y.; Beesdo-Baum, K.; Beidel, D.; et al. The Youth Anxiety Measure for DSM-5 (YAM-5): Development and First Psychometric Evidence of a New Scale for Assessing Anxiety Disorders Symptoms of Children and Adolescents. Child Psychiatry Hum. Dev. 2017, 48, 1–17. [Google Scholar] [CrossRef]
- Mattick, R.P.; Clarke, J.C. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behav. Res. Ther. 1998, 36, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA School-Age Forms & Profiles; University of Vermont, Research Center for Children, Youth, & Families: Burlington, VT, USA, 2001; Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=1855203 (accessed on 1 May 2023).
- Achenbach, T.M. Manual for the Child Behaviour Check-List/4-18 and 1991 Profile. 1991. Available online: https://books.google.ro/books/about/Manual_for_the_Child_Behavior_Checklist.html?id=I5btOwAACAAJ&redir_esc=y (accessed on 1 May 2023).
- Achenbach, T.M.; Rescorla, L. Manual for the ASEBA Adult Forms & Profiles; University of Vermont, Research Center for Children, Youth: Burlington, VT, USA, 2003; Available online: https://www.scirp.org/(S(351jmbntvnsjt1aadkozje))/reference/ReferencesPapers.aspx?ReferenceID=2239454 (accessed on 1 May 2023).
- Achenbach, T.M. Manual for the Young Adult Self-Report and Young Adult Behavior Checklist; University of Vermont, Department of Psychiatry: Burlington, VT, USA, 1997; Available online: https://scirp.org/reference/referencespapers.aspx?referenceid=721748 (accessed on 1 May 2023).
- Heimberg, R.G.; Horner, K.J.; Juster, H.R.; Safren, S.A.; Brown, E.J.; Schneier, F.R.; Liebowitz, M.R. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychol. Med. 1999, 29, 199–212. [Google Scholar] [CrossRef]
- Campbell-Sills, L.; Norman, S.B.; Craske, M.G.; Sullivan, G.; Lang, A.J.; Chavira, D.A.; Bystritsky, A.; Sherbourne, C.; Roy-Byrne, P.; Stein, M.B. Validation of a brief measure of anxiety-related severity and impairment: The Overall Anxiety Severity and Impairment Scale (OASIS). J. Affect. Disord. 2009, 112, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.K.; Price, L.H.; Rasmussen, S.A.; Mazure, C.; Fleischmann, R.L.; Hill, C.L.; Heninger, G.R.; Charney, D.S. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch. Gen. Psychiatry 1989, 46, 1006–1011. [Google Scholar] [CrossRef]
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; De La Fuente, J.R.; Grant, M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef]
- Adamson, S.J.; Sellman, J.D. A prototype screening instrument for cannabis use disorder: The Cannabis Use Disorders Identification Test (CUDIT) in an alcohol-dependent clinical sample. Drug Alcohol Rev. 2003, 22, 309–315. [Google Scholar] [CrossRef]
- Pennebaker, J.W.; Susman, J.R. Disclosure of traumas and psychosomatic processes. Soc. Sci. Med. 1988, 26, 327–332. [Google Scholar] [CrossRef]
- Coddington, R.D.; Sitarenios, G.; Barry, S.; Morrison, J. Coddington Life Events Scales (CLES): Technical Manual; Multi-Health Systems, Inc.: North Tonawanda, NY, USA, 1999; Available online: https://search.tcsedsystem.edu/discovery/fulldisplay?context=L&vid=01TCSEDSYSTEM_INST:TCSPP&search_scope=TCSPP_and_CI&tab=Everything_tcspp&docid=alma991000207689706756 (accessed on 1 May 2023).
- Schlotz, W.; Yim, I.S.; Zoccola, P.M.; Jansen, L.; Schulz, P. The perceived stress reactivity scale: Measurement invariance, stability, and validity in three countries. Psychol. Assess. 2011, 23, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Shaw, T.; Cross, D.; Zubrick, S.R. Testing for Response Shift Bias in Evaluations of School Antibullying Programs. Eval. Rev. 2015, 39, 527–554. [Google Scholar] [CrossRef]
- Rudd, D.M.; Rajab, H.M. Use of the Modified Scale for Suicidal Ideation with suicide ideators and attempters. J. Clin. Psychol. 1995, 51, 632–635. [Google Scholar] [CrossRef]
- Birchwood, M.; Smith, J.; Drury, V.; Healy, J.; Macmillan, F.; Slade, M. A self-report Insight Scale for psychosis: Reliability, validity and sensitivity to change. Acta Psychiatr. Scand. 1994, 89, 62–67. [Google Scholar] [CrossRef]
- Golay, P.; Moga, M.; Devas, C.; Staecheli, M.; Poisat, Y.; Israël, M.; Suter, C.; Silva, B.; Morandi, S.; Ferrari, P.; et al. Measuring the paradox of self-stigma: Psychometric properties of a brief scale. Ann. Gen. Psychiatry 2021, 20, 5. [Google Scholar] [CrossRef]
- Fonagy, P.; Luyten, P.; Moulton-Perkins, A.; Lee, Y.-W.; Warren, F.; Howard, S.; Ghinai, R.; Fearon, P.; Lowyck, B. Development and Validation of a Self-Report Measure of Mentalizing: The Reflective Functioning Questionnaire. PLoS ONE 2016, 11, e0158678. [Google Scholar] [CrossRef] [PubMed]
- Luyten, P.; Mayes, L.C.; Nijssens, L.; Fonagy, P. The parental reflective functioning questionnaire: Development and preliminary validation. PLoS ONE 2017, 12, e0176218. [Google Scholar] [CrossRef]
- Roberts, D.L.; Fiszdon, J.; Tek, C. Ecological Validity of the Social Cognition Screening Questionnaire (Scsq), Schizophrenia Bulletin; Oxford University Press: Oxford, UK, 2011; p. 280. [Google Scholar]
- Zimet, G.D.; Dahlem, N.W.; Zimet, S.G.; Farley, G.K. The Multidimensional Scale of Perceived Social Support. J. Pers. Assess. 1988, 52, 30–41. [Google Scholar] [CrossRef]
- Friborg, O.; Hjemdal, O.; Rosenvinge, J.H.; Martinussen, M. A new rating scale for adult resilience: What are the central protective resources behind healthy adjustment? Int. J. Methods Psychiatr. Res. 2003, 12, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Widiger, T.A.; Costa, P.T., Jr. Personality Disorders and the Five-Factor Model of Personality: Rationale for the Third Edition; American Psychological Association: Washington, DC, USA, 2013. [Google Scholar]
- Goth, K.; Schmeck, K. AIDA—Assessment of Identity Development in Adolescence; Academic-Tests: Offenbach, Germany, 2018; Available online: https://academic-tests.com/aida/ (accessed on 1 May 2023).
- Goth, K.; Birkhölzer, M.; Schmeck, K. LoPF Q 12-18–Levels of Personality Functioning Questionnaire; Academic-Tests: Offenbach, Germany, 2018; Available online: https://academic-tests.com/lopf-q/ (accessed on 1 May 2023).
- Rutter, M.; Bailey, A.; Lord, C. The Social Communication Questionnaire: Manual; Western Psychological Services: Los Angeles, CA, USA, 2003. [Google Scholar]
- Conners, C.K.; Erhardt, D.; Sparrow, E.P. Conners’ Adult ADHD Rating Scales (CAARS): Technical Manual; Multi-Health Systems Inc. (MHS): North Tonawanda, NY, USA, 1999. [Google Scholar]
- Conners, C.K. Conners Comprehensive Behavior Rating Scales (Conners CBRS); Multi-Health Systems Toronto: Toronto, ON, Canada, 2010. [Google Scholar]
- Ward, M.F.; Wender, P.H.; Reimherr, F.W. The Wender Utah Rating Scale: An aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am. J. Psychiatry 1993, 150, 885–890, Erratum in Am. J. Psychiatry 1993, 150, 1280. [Google Scholar] [CrossRef]
- Green, M.F.; Nuechterlein, K.H.; Gold, J.M.; Barch, D.M.; Cohen, J.; Essock, S.; Fenton, W.S.; Frese, F.; Goldberg, T.E.; Heaton, R.K.; et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: The NIMH-MATRICS conference to select cognitive domains and test criteria. Biol. Psychiatry 2004, 56, 301–307. [Google Scholar] [CrossRef]
- Keefe, R.S.; E Goldberg, T.; Harvey, P.D.; Gold, J.M.; Poe, M.P.; Coughenour, L. The Brief Assessment of Cognition in Schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2003, 68, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Korkman, M.; Kirk, U.; Kemp, S. NEPSY-II: Manuel D’administration; ECPA: Kuala Lumpur, Malaysia, 2012. [Google Scholar]
- Kaiser, S.; Berger, G.; Conus, P.; Kawohl, W.; Müller, T.J.; Schimmelmann, B.G.; Traber, R.; Trächsel, N.; Vauth, R.; Seifritz, E. Recommandations thérapeutiques de la SSPP pour le traitement de la schizophrénie. Swiss Medical Forum 2018, 18, 532–539. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conchon, C.; Sprüngli-Toffel, E.; Alameda, L.; Edan, A.; Bailey, B.; Solida, A.; Plessen, K.J.; Conus, P.; Kapsaridi, A.; Genoud, D.; et al. Improving Pathways to Care for Patients at High Psychosis Risk in Switzerland: PsyYoung Study Protocol. J. Clin. Med. 2023, 12, 4642. https://doi.org/10.3390/jcm12144642
Conchon C, Sprüngli-Toffel E, Alameda L, Edan A, Bailey B, Solida A, Plessen KJ, Conus P, Kapsaridi A, Genoud D, et al. Improving Pathways to Care for Patients at High Psychosis Risk in Switzerland: PsyYoung Study Protocol. Journal of Clinical Medicine. 2023; 12(14):4642. https://doi.org/10.3390/jcm12144642
Chicago/Turabian StyleConchon, Caroline, Elodie Sprüngli-Toffel, Luis Alameda, Anne Edan, Barbara Bailey, Alessandra Solida, Kerstin Jessica Plessen, Philippe Conus, Afroditi Kapsaridi, Davina Genoud, and et al. 2023. "Improving Pathways to Care for Patients at High Psychosis Risk in Switzerland: PsyYoung Study Protocol" Journal of Clinical Medicine 12, no. 14: 4642. https://doi.org/10.3390/jcm12144642
APA StyleConchon, C., Sprüngli-Toffel, E., Alameda, L., Edan, A., Bailey, B., Solida, A., Plessen, K. J., Conus, P., Kapsaridi, A., Genoud, D., Crameri, A., Jouabli, S., Caron, C., Grob, C., Gros, J., Senn, S., Curtis, L., Liso Navarro, A., Barbe, R., ... Andreou, C. (2023). Improving Pathways to Care for Patients at High Psychosis Risk in Switzerland: PsyYoung Study Protocol. Journal of Clinical Medicine, 12(14), 4642. https://doi.org/10.3390/jcm12144642








