The Immunoseasonal Theory of Psychiatric Disorders
Abstract
1. Weather Factors and Psychiatric Disorders
2. Immunology, Microbes, and Psychiatric Disorders
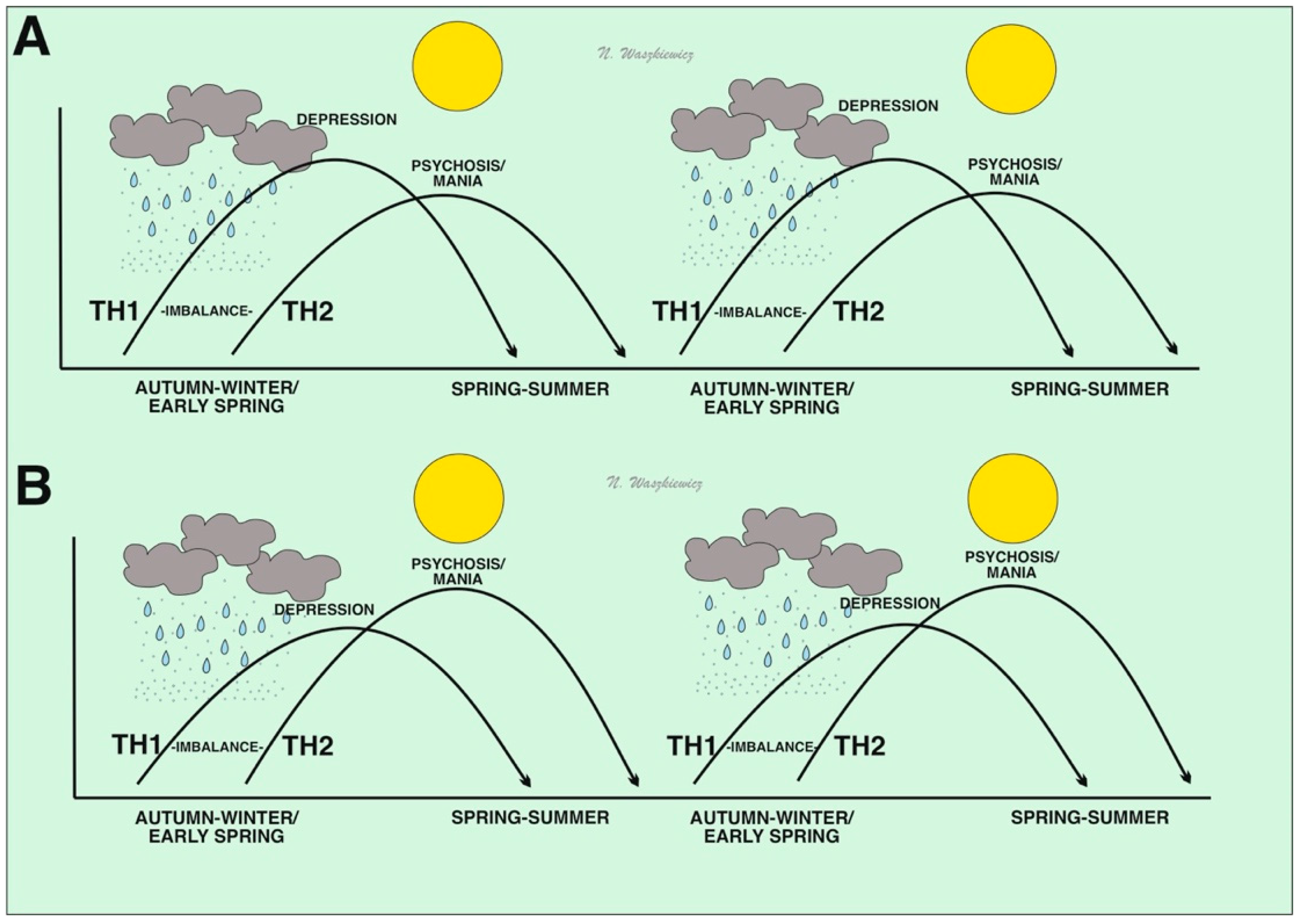
3. Immunoseasonal Theory of Psychiatric/Mental Disorders
4. Stress Axis Pathway
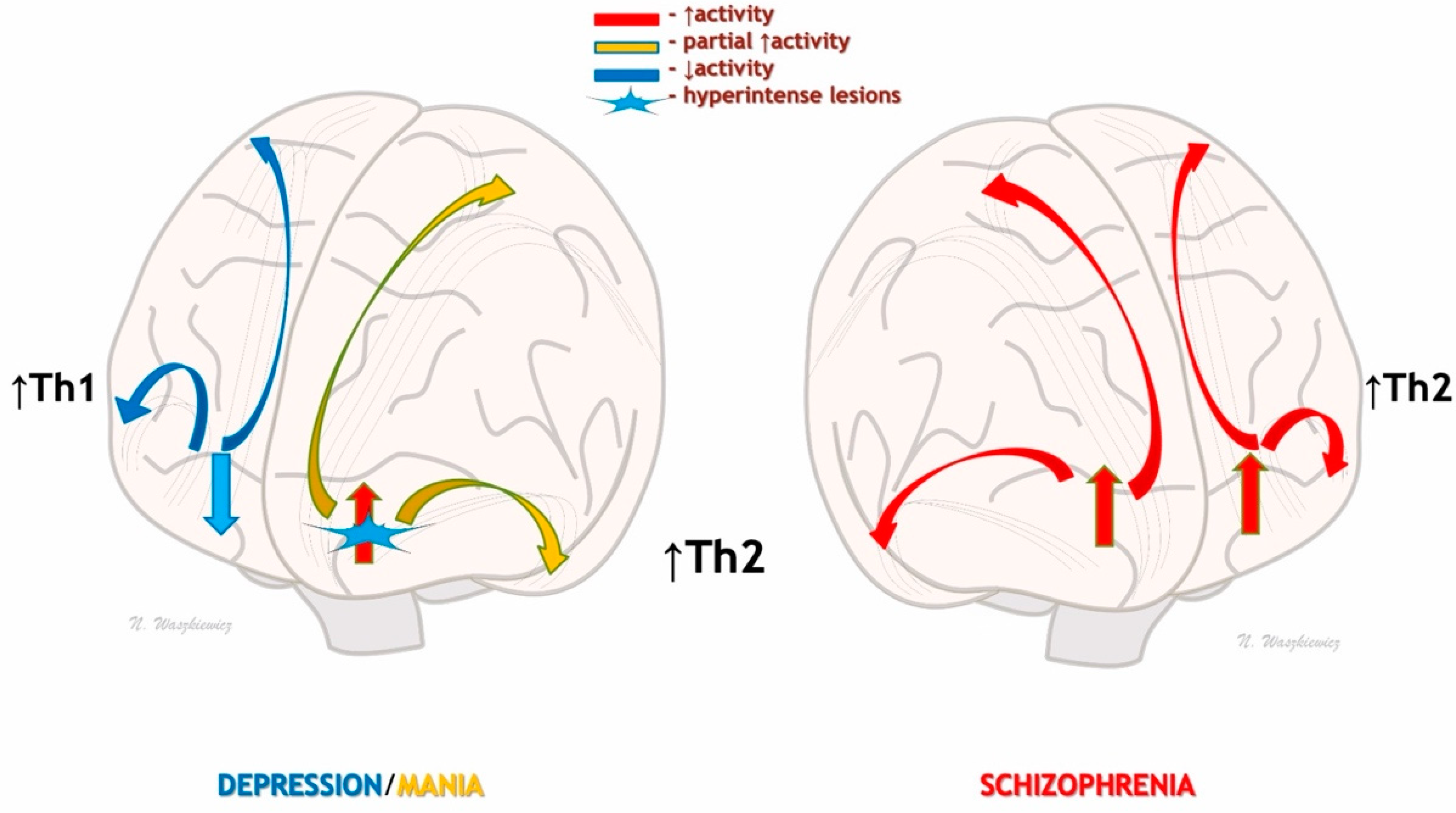
5. Seasonal Immunity and Neurodevelopmental/Neurodegenerative Theories of Mental Disorders
6. Is It a New Psychiatric Episode or a “Flashback” Effect?
7. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majeed, H.; Lee, J. The impact of climate change on youth depression and mental health. Lancet Planet. Health 2017, 1, e94–e95. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, A. An overview of epidemiological studies on seasonal affective disorder. Acta Psychiatr. Scand. 2000, 101, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Bullock, B.; Murray, G.; Meyer, D. Highs and lows, ups and downs: Meteorology and mood in bipolar disorder. PLoS ONE 2017, 12, e0173431. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Mazza, M.; Panaccione, I.; Moccia, L.; Giuseppin, G.; Marano, G.; Grandinetti, P.; Camardese, G.; De Berardis, D.; Pompili, M.; et al. Sensitivity to Climate and Weather Changes in Euthymic Bipolar Subjects: Association With Suicide Attempts. Front. Psychiatry 2020, 11, 95. [Google Scholar] [CrossRef]
- Brandl, E.J.; Lett, T.A.; Bakanidze, G.; Heinz, A.; Bermpohl, F.; Schouler-Ocak, M. Weather conditions influence the number of psychiatric emergency room patients. Int. J. Biometeorol. 2018, 62, 843–850. [Google Scholar] [CrossRef]
- McWilliams, S.; Kinsella, A.; O’Callaghan, E. The effects of daily weather variables on psychosis admissions to psychiatric hospitals. Int. J. Biometeorol. 2013, 57, 497–508. [Google Scholar] [CrossRef]
- Hinterbuchinger, B.; König, D.; Gmeiner, A.; Listabarth, S.; Fellinger, M.; Thenius, C.; Baumgartner, J.S.; Vyssoki, S.; Waldhoer, T.; Vyssoki, B.; et al. Seasonality in schizophrenia-An analysis of a nationwide registry with 110,735 hospital admissions. Eur. Psychiatry 2020, 63, e55. [Google Scholar] [CrossRef]
- Liu, J.; Varghese, B.M.; Hansen, A.; Xiang, J.; Zhang, Y.; Dear, K.; Gourley, M.; Driscoll, T.; Morgan, G.; Capon, A.; et al. Is there an association between hot weather and poor mental health outcomes? A systematic review and meta-analysis. Environ. Int. 2021, 153, 106533. [Google Scholar] [CrossRef]
- Delyukov, A.; Didyk, L. The effects of extra-low-frequency atmospheric pressure oscillations on human mental activity. Int. J. Biometeorol. 1999, 43, 31–37. [Google Scholar] [CrossRef]
- Feng, J.J.; Li, Y.H. Effects of hyperbaric oxygen therapy on depression and anxiety in the patients with incomplete spinal cord injury (a STROBE-compliant article). Medicine 2017, 96, e7334. [Google Scholar] [CrossRef]
- Waszkiewicz, N. Ordering Knowledge in the Markers of Psychiatric/Mental Disorders. J. Clin. Med. 2022, 11, 284. [Google Scholar] [CrossRef]
- Vida, S.; Durocher, M.; Ouarda, T.B.; Gosselin, P. Relationship between ambient temperature and humidity and visits to mental health emergency departments in Québec. Psychiatr. Serv. 2012, 63, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Berry, H.L.; Bennett, C.M. The Importance of Humidity in the Relationship between Heat and Population Mental Health: Evidence from Australia. PLoS ONE 2016, 11, e0164190. [Google Scholar] [CrossRef] [PubMed]
- Florido Ngu, F.; Kelman, I.; Chambers, J.; Ayeb-Karlsson, S. Correlating heatwaves and relative humidity with suicide (fatal intentional self-harm). Sci. Rep. 2021, 11, 22175. [Google Scholar] [CrossRef] [PubMed]
- Waszkiewicz, N.; Chlabicz, M.; Paniczko, M.; Jamiołkowski, J.; Sowa, P.; Szpakowicz, M.; Łapińska, M.; Raczkowsk, A.; Kamiński, K. Humidity as the meteorological factor that influences seasonal depression. Neurosci. Appl. 2022, 1, 100181. [Google Scholar] [CrossRef]
- Fisman, D. Seasonality of viral infections: Mechanisms and unknowns. Clin. Microbiol. Infect. 2012, 18, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Najjar, S.; Pearlman, D.M.; Alper, K.; Najjar, A.; Devinsky, O. Neuroinflammation and psychiatric illness. J. Neuroinflamm. 2013, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Buras, A.; Waszkiewicz, N.; Szulc, A. Depression and inflammation in rheumatic diseases. Postepy Hig. Med. Dosw Online 2016, 70, 162–168. (In Polish) [Google Scholar] [CrossRef]
- Dev, S.I.; Moore, R.C.; Soontornniyomkij, B.; Achim, C.L.; Jeste, D.V.; Eyler, L.T. Peripheral inflammation related to lower fMRI activation during a working memory task and resting functional connectivity among older adults: A preliminary study. Int. J. Geriatr. Psychiatry 2017, 32, 341–349. [Google Scholar] [CrossRef]
- Wang, A.K.; Miller, B.J. Meta-analysis of Cerebrospinal Fluid Cytokine and Tryptophan Catabolite Alterations in Psychiatric Patients: Comparisons Between Schizophrenia, Bipolar Disorder, and Depression. Schizophr. Bull. 2018, 44, 75–83. [Google Scholar] [CrossRef]
- Lorkiewicz, P.; Waszkiewicz, N. Is SARS-CoV-2 a Risk Factor of Bipolar Disorder?—A Narrative Review. J. Clin. Med. 2022, 11, 6060. [Google Scholar] [CrossRef] [PubMed]
- Dogaru, I.A.; Puiu, M.G.; Manea, M.; Dionisie, V. Current Perspectives on Pharmacological and Non-Pharmacological Interventions for the Inflammatory Mechanism of Unipolar Depression. Brain Sci. 2022, 12, 1403. [Google Scholar] [CrossRef] [PubMed]
- Suneson, K.; Lindahl, J.; Chamli Hårsmar, S.; Söderberg, G.; Lindqvist, D. Inflammatory Depression-Mechanisms and Non-Pharmacological Interventions. Int. J. Mol. Sci. 2021, 22, 1640. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, I.G.; Machado-Vieira, R.; Soares, J.C.; Teixeira, A.L. The immunology of bipolar disorder. Neuroimmunomodulation 2014, 21, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Więdłocha, M.; Marcinowicz, P.; Krupa, R.; Janoska-Jaździk, M.; Janus, M.; Dębowska, W.; Mosiołek, A.; Waszkiewicz, N.; Szulc, A. Effect of antidepressant treatment on peripheral inflammation markers—A meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80 Pt C, 217–226. [Google Scholar] [CrossRef]
- Dionisie, V.; Filip, G.A.; Manea, M.C.; Movileanu, R.C.; Moisa, E.; Manea, M.; Riga, S.; Ciobanu, A.M. Neutrophil-to-Lymphocyte Ratio, a Novel Inflammatory Marker, as a Predictor of Bipolar Type in Depressed Patients: A Quest for Biological Markers. J. Clin. Med. 2021, 10, 1924. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S. Bipolar Disorder and Immune Dysfunction: Epidemiological Findings, Proposed Pathophysiology and Clinical Implications. Brain Sci. 2017, 7, 144. [Google Scholar] [CrossRef]
- Wollenhaupt-Aguiar, B.; Librenza-Garcia, D.; Bristot, G.; Przybylski, L.; Stertz, L.; Kubiachi Burque, R.; Ceresér, K.M.; Spanemberg, L.; Caldieraro, M.A.; Frey, B.N.; et al. Differential biomarker signatures in unipolar and bipolar depression: A machine learning approach. Aust. N. Z. J. Psychiatry 2020, 54, 393–401. [Google Scholar] [CrossRef]
- Dawidowski, B.; Górniak, A.; Podwalski, P.; Lebiecka, Z.; Misiak, B.; Samochowiec, J. The Role of Cytokines in the Pathogenesis of Schizophrenia. J. Clin. Med. 2021, 10, 3849. [Google Scholar] [CrossRef]
- Barron, H.; Hafizi, S.; Andreazza, A.C.; Mizrahi, R. Neuroinflammation and Oxidative Stress in Psychosis and Psychosis Risk. Int. J. Mol. Sci. 2017, 18, 651. [Google Scholar] [CrossRef]
- Tortorella, A. We Should Improve Personalization of Management in Patients with a Diagnosis of Schizophrenia. J. Clin. Med. 2021, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Müller, N. Inflammation in Schizophrenia: Pathogenetic Aspects and Therapeutic Considerations. Schizophr. Bull. 2018, 44, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Kuhlman, K.R. Psychoneuroimmunology: An Introduction to Immune-to-Brain Communication and Its Implications for Clinical Psychology. Annu. Rev. Clin. Psychol. 2023, 19, 331–359. [Google Scholar] [CrossRef]
- Müller, N. Infectious diseases and mental health. In Comorbidity of Mental and Physical Disorders; Sartorius, N., Holt, R.I.G., Maj, M., Eds.; Karger: Basel, Switzerland, 2015; Volume 179, pp. 99–113. [Google Scholar] [CrossRef]
- Audi, A.; AlIbrahim, M.; Kaddoura, M.; Hijazi, G.; Yassine, H.M.; Zaraket, H. Sea-sonality of Respiratory Viral Infections: Will COVID-19 Follow Suit? Front. Public Health 2020, 8, 567184. [Google Scholar] [CrossRef] [PubMed]
- Upthegrove, R.; Marwaha, S.; Birchwood, M. Depression and Schizophrenia: Cause, Consequence, or Trans-diagnostic Issue? Schizophr. Bull. 2017, 43, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Budu-Aggrey, A.; Joyce, S.; Davies, N.M.; Paternoster, L.; Munafò, M.R.; Brown, S.J.; Evans, J.; Sallis, H.M. Investigating the causal relationship between allergic disease and mental health. Clin. Exp. Allergy 2021, 51, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Brüne, M. Schizophrenia as parasitic behavior manipulation: Can we put together the pieces of an evolutionary puzzle? World Psychiatry 2020, 19, 119–120. [Google Scholar] [CrossRef]
- Yang, J.; Sundrud, M.S.; Skepner, J.; Yamagata, T. Targeting Th17 cells in autoimmune diseases. Trends Pharmacol. Sci. 2014, 35, 493–500. [Google Scholar] [CrossRef]
- Łoś, K.; Kulikowska, J.; Waszkiewicz, N. The Impact of the COVID-19 Virus Pandemic on the Incidence of First Psychotic Spectrum Disorders. Int. J. Environ. Res. Public Health 2022, 19, 3781. [Google Scholar] [CrossRef]
- Galińska-Skok, B.; Waszkiewicz, N. Markers of Schizophrenia-A Critical Narrative Update. J. Clin. Med. 2022, 11, 3964. [Google Scholar] [CrossRef]
- de Baumont, A.; Maschietto, M.; Lima, L.; Carraro, D.M.; Olivieri, E.H.; Fiorini, A.; Barreta, L.A.; Palha, J.A.; Belmonte-de-Abreu, P.; Moreira Filho, C.A.; et al. Innate immune response is differentially dysregulated between bipolar disease and schizophrenia. Schizophr. Res. 2015, 161, 215–221. [Google Scholar] [CrossRef]
- Beyer, J.L.; Young, R.; Kuchibhatla, M.; Krishnan, K.R. Hyperintense MRI lesions in bipolar disorder: A meta-analysis and review. Int. Rev. Psychiatry 2009, 21, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Kochunov, P.; Hong, L.E. Neurodevelopmental and neurodegenerative models of schizophrenia: White matter at the center stage. Schizophr. Bull. 2014, 40, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, F. Psychiatric disorders: Neurodevelopmental disorders, neurodegenerative disorders, or both? Dialogues Clin. Neurosci. 2018, 20, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Gałecki, P.; Talarowska, M. Neurodevelopmental theory of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 267–272. [Google Scholar] [CrossRef]
- Waszkiewicz, N. Mentally Sick or Not-(Bio)Markers of Psychiatric Disorders Needed. J. Clin. Med. 2020, 9, 2375. [Google Scholar] [CrossRef]
- Nestsiarovich, A.; Obyedkov, V.; Kandratsenka, H.; Siniauskaya, M.; Goloenko, I.; Waszkiewicz, N. Disorganization at the stage of schizophrenia clinical outcome: Clinical-biological study. Eur. Psychiatry 2017, 42, 44–48. [Google Scholar] [CrossRef]
- Martinotti, G.; Santacroce, R.; Pettorruso, M.; Montemitro, C.; Spano, M.C.; Lorusso, M.; di Giannantonio, M.; Lerner, A.G. Hallucinogen Persisting Perception Disorder: Etiology, Clinical Features, and Therapeutic Perspectives. Brain Sci. 2018, 8, 47. [Google Scholar] [CrossRef]
- Patil, A.; Goldust, M.; Wollina, U. Herpes zoster: A Review of Clinical Manifestations and Management. Viruses 2022, 14, 192. [Google Scholar] [CrossRef]
- Dalmau, J.; Graus, F. Antibody-Mediated Encephalitis. N. Engl. J. Med. 2018, 378, 840–851. [Google Scholar] [CrossRef]
- Waszkiewicz, N. Immunosezonowa teoria zaburzeń psychicznych. Psychiatra 2022, 39, 10–13. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waszkiewicz, N. The Immunoseasonal Theory of Psychiatric Disorders. J. Clin. Med. 2023, 12, 4615. https://doi.org/10.3390/jcm12144615
Waszkiewicz N. The Immunoseasonal Theory of Psychiatric Disorders. Journal of Clinical Medicine. 2023; 12(14):4615. https://doi.org/10.3390/jcm12144615
Chicago/Turabian StyleWaszkiewicz, Napoleon. 2023. "The Immunoseasonal Theory of Psychiatric Disorders" Journal of Clinical Medicine 12, no. 14: 4615. https://doi.org/10.3390/jcm12144615
APA StyleWaszkiewicz, N. (2023). The Immunoseasonal Theory of Psychiatric Disorders. Journal of Clinical Medicine, 12(14), 4615. https://doi.org/10.3390/jcm12144615





