Liver Disease Is a Risk Factor for Recurrent Hyperkalemia: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Objectives
2.2. Statistical Analysis
2.3. Ethical Considerations
3. Results
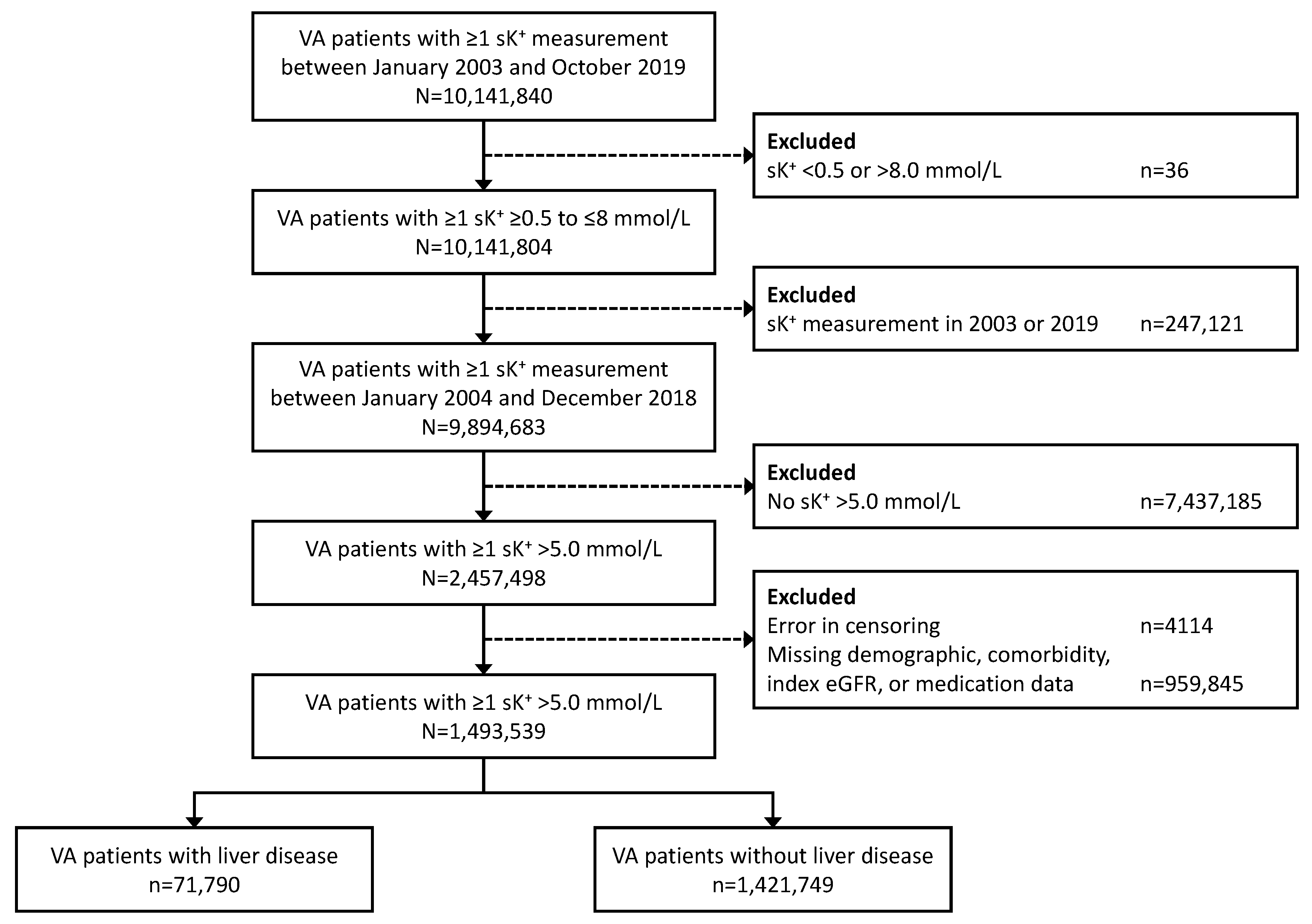
3.1. Patients
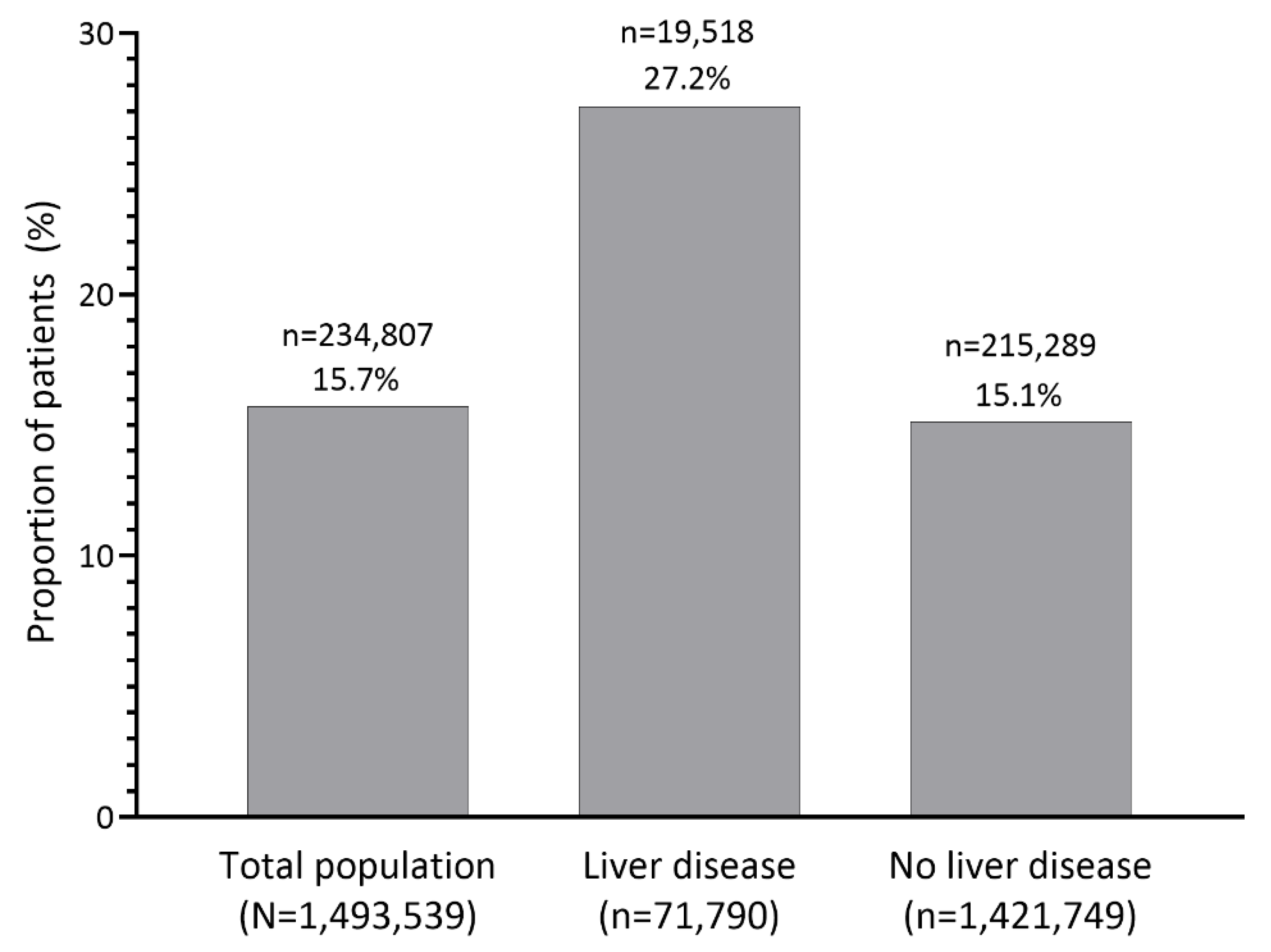
3.2. Hyperkalemia Recurrence
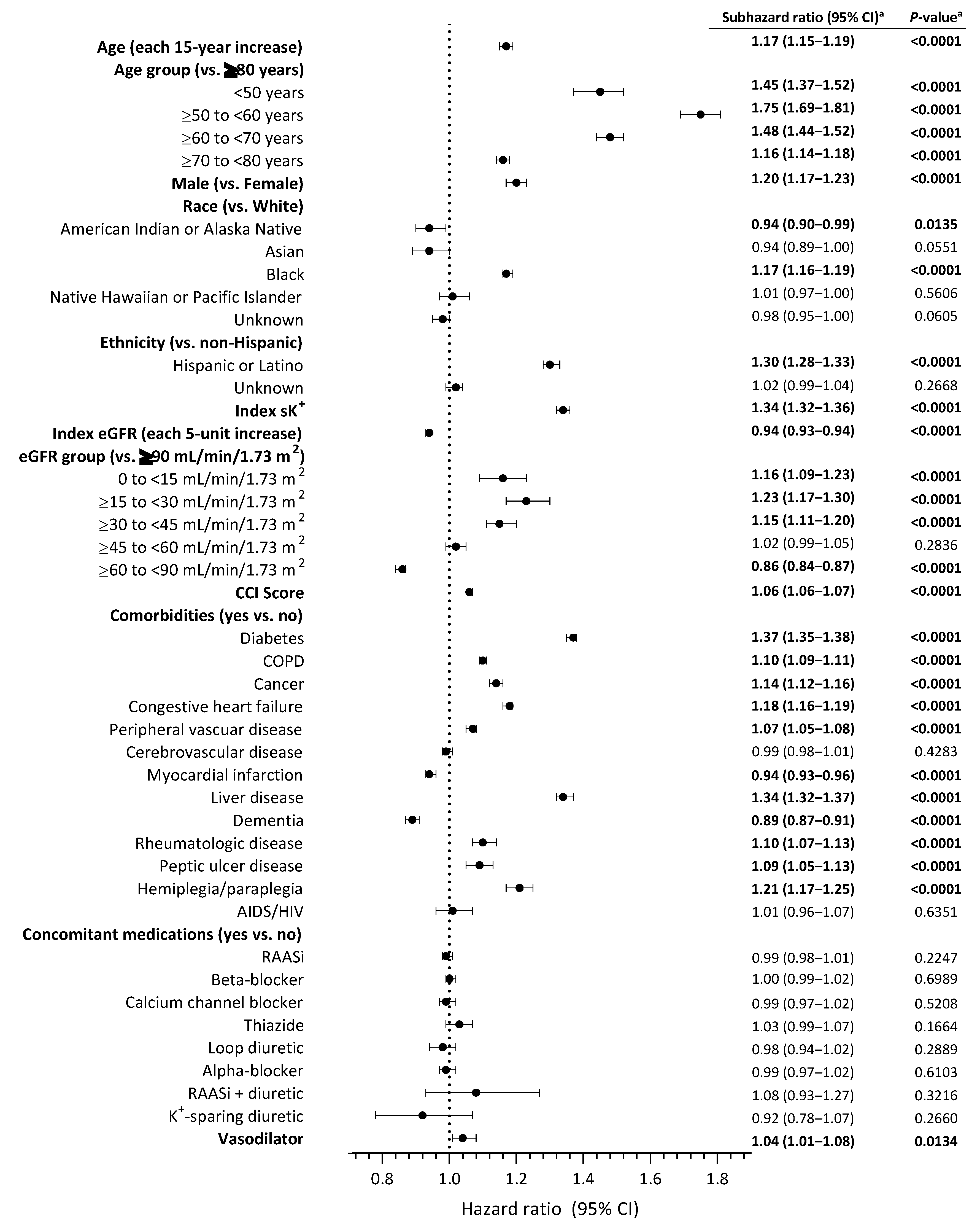
3.3. Characteristics Associated with Hyperkalemia Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paik, J.M.; Golabi, P.; Younossi, Y.; Saleh, N.; Nhyira, A.; Younossi, Z.M. The growing burden of disability related to chronic liver disease in the United States: Data from the Global Burden of Disease Study 2007–2017. Hepatol. Commun. 2021, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Hirode, G.; Saab, S.; Wong, R.J. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw. Open 2020, 3, e201997. [Google Scholar] [CrossRef] [PubMed]
- Maiwall, R.; Kumar, S.; Sharma, M.K.; Wani, Z.; Ozukum, M.; Sarin, S.K. Prevalence and prognostic significance of hyperkalemia in hospitalized patients with cirrhosis. J. Gastroenterol. Hepatol. 2016, 31, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.J.; Wang, K.; Jiang, H.Q.; Han, T. Characteristics, risk factors, and adverse outcomes of hyperkalemia in acute-on-chronic liver failure patients. Biomed. Res. Int. 2019, 2019, 6025726. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.W.; Bailey, M.A. Hyperkalemia: Pathophysiology, risk factors and consequences. Nephrol. Dial. Transpl. 2019, 34, iii2–iii11. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.J.; Pitt, B.; Reaven, N.; Funk, S.; McGaughey, K.; Wilson, D.; Bushinsky, D.A. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am. J. Nephrol. 2017, 46, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Mezzano, G.; Cárdenas, A.; Aguilar, F.; Pavesi, M.; Sole, C.; Napoleone, L.; Graupera, I.; Juanola, A.; Carol, M.; Pose, E.; et al. Hyperkalemia influences the outcome of patients with cirrhosis with acute decompensation (AD) and acute-on-chronic liver failure (ACLF). Dig. Liver Dis. 2021, 53, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Adelborg, K.; Nicolaisen, S.K.; Hasvold, P.; Palaka, E.; Pedersen, L.; Thomsen, R.W. Predictors for repeated hyperkalemia and potassium trajectories in high-risk patients—A population-based cohort study. PLoS ONE 2019, 14, e0218739. [Google Scholar] [CrossRef] [PubMed]
- Sheen, S.S.; Park, R.W.; Yoon, D.; Shin, G.T.; Kim, H.; Park, I.W. The Model for End-stage Liver Disease score is potentially a useful predictor of hyperkalemia occurrence among hospitalized angiotensin receptor blocker users. J. Clin. Pharm. Ther. 2015, 40, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, V.; Kumar, N.; Khan, S.I.; Nawaz, M.U.; Ahmed, H.; Naz, S.; Masood Shah, A.; Jahangir, M. Biochemical risk factors associated with hyperkalemia in cirrhotic patients. Cureus 2021, 13, e18356. [Google Scholar] [CrossRef]
- Biggins, S.W.; Angeli, P.; Garcia-Tsao, G.; Gines, P.; Ling, S.C.; Nadim, M.K.; Wong, F.; Kim, W.R. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1014–1048. [Google Scholar] [CrossRef]
- Burkholder, D.A.; Moran, I.J.; DiBattista, J.V.; Lok, A.S.; Parikh, N.D.; Chen, V.L. Accuracy of International Classification of Diseases-10 Codes for cirrhosis and portal hypertensive complications. Dig. Dis. Sci. 2021, 67, 3623–3631. [Google Scholar] [CrossRef]
| Total Population (n = 1,493,539) | Liver Disease (n = 71,790) | No Liver Disease (n = 1,421,749) | |
|---|---|---|---|
| Age (years), mean ± SD | 61.5 ± 13.0 | 56.4 ± 9.4 | 61.7 ± 13.1 |
| Sex, n (%) | |||
| Male | 1,433,710 (96.0) | 69,796 (97.2) | 1,363,914 (95.9) |
| Female | 59,829 (4.0) | 1994 (2.8) | 57,835 (4.1) |
| Race, n (%) | |||
| White | 1,184,008 (79.3) | 48,647 (67.8) | 1,135,361 (79.9) |
| Black | 213,725 (14.3) | 18,454 (25.7) | 195,271 (13.7) |
| Asian | 7832 (0.5) | 248 (0.3) | 7584 (0.5) |
| American Indian or Alaska Native | 11,741 (0.8) | 726 (1.0) | 11,015 (0.8) |
| Native Hawaiian or other Pacific Islander | 13,747 (0.9) | 638 (0.9) | 13,109 (0.9) |
| Unknown/declined to answer | 62,486 (4.2) | 3077 (4.3) | 59,409 (4.2) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 77,069 (5.2) | 4927 (6.9) | 72,142 (5.1) |
| Not Hispanic or Latino | 1,364,656 (91.4) | 64,410 (89.7) | 1,300,246 (91.5) |
| Unknown/declined to answer | 51,814 (3.5) | 2453 (3.4) | 49,361 (3.5) |
| Index eGFR (mL/min/1.73 m2), mean ± SD | 67.5 ± 25.1 | 67.7 ± 30.7 | 67.5 ± 24.8 |
| CCI score, median (IQR) | 1 (0, 3) | 4 (3, 6) | 1 (0, 2) |
| Comorbidities, n (%) | |||
| Diabetes | 494,831 (33.1) | 27,718 (38.6) | 467,113 (32.9) |
| COPD | 253,274 (17.0) | 20,321 (28.3) | 232,953 (16.4) |
| Cancer | 196,206 (13.1) | 15,805 (22.0) | 180,401 (12.7) |
| Congestive heart failure | 174,229 (11.7) | 13,597 (18.9) | 160,632 (11.3) |
| Peripheral vascular disease | 126,609 (8.5) | 7403 (10.3) | 119,206 (8.4) |
| Cerebrovascular disease | 96,643 (6.5) | 5474 (7.6) | 91,169 (6.4) |
| Myocardial infarction | 77,030 (5.2) | 5639 (7.9) | 71,391 (5.0) |
| Dementia | 51,398 (3.4) | 2803 (3.9) | 48,595 (3.4) |
| Rheumatologic disease | 22,575 (1.5) | 1237 (1.7) | 21,338 (1.5) |
| Peptic ulcer disease | 17,180 (1.2) | 2771 (3.9) | 14,409 (1.0) |
| Hemiplegia/paraplegia | 17,040 (1.1) | 1453 (2.0) | 15,587 (1.1) |
| AIDS/HIV | 8720 (0.6) | 2010 (2.8) | 6710 (0.5) |
| Concomitant medication, n (%) | |||
| RAASi | 719,243 (48.2) | 34,612 (48.2) | 684,631 (48.2) |
| Beta-blocker | 576,679 (38.6) | 27,778 (38.7) | 548,901 (38.6) |
| Calcium channel blocker | 325,981 (21.8) | 15,880 (22.1) | 310,101 (21.8) |
| Thiazides | 280,203 (18.8) | 13,644 (19.0) | 266,559 (18.7) |
| Loop diuretic | 258,552 (17.3) | 12,525 (17.5) | 246,027 (17.3) |
| Alpha-blocker | 163,954 (11.0) | 7969 (11.1) | 155,985 (11.0) |
| RAASi + diuretic | 96,985 (6.5) | 4679 (6.5) | 92,306 (6.5) |
| K+-sparing diuretic | 96,011 (6.4) | 4647 (6.5) | 91,364 (6.4) |
| Vasodilator | 49,515 (3.3) | 2416 (3.4) | 47,099 (3.3) |
| Laboratory Measurement a | Total Population (n = 1,493,539) | Liver Disease (n = 71,790) | No Liver Disease (n = 1,421,749) |
|---|---|---|---|
| Serum albumin (g/dL) | 4.0 ± 4.1 | 3.4 ± 1.2 | 4.0 ± 4.2 |
| UACR (mg/g) | 111.0 ± 716.5 | 258.6 ± 2588.6 | 107.1 ± 590.3 |
| Serum ALP (U/L) | 86.0 ± 67.0 | 129.8 ± 127.2 | 83.5 ± 60.9 |
| Serum bicarbonate (mmol/L) | 26.8 ± 4.7 | 24.9 ± 5.5 | 26.9 ± 4.6 |
| BUN (mg/dL) | 22.9 ± 15.3 | 27.0 ± 21.1 | 22.7 ± 15.0 |
| Serum calcium (mg/dL) | 9.4 ± 0.7 | 9.0 ± 0.9 | 9.4 ± 0.7 |
| Serum ferritin (ng/mL) | 318.7 ± 1283.8 | 587.4 ± 2988.8 | 286.1 ± 867.9 |
| Blood glucose (mg/dL) | 129.3 ± 76.4 | 140.3 ± 98.2 | 128.8 ± 75.1 |
| Blood glucose POC (mg/dL) | 170.1 ± 116.4 | 168.3 ± 113.9 | 170.4 ± 116.7 |
| Hemoglobin (g/dL) | 13.6 ± 2.2 | 12.4 ± 2.5 | 13.7 ± 2.1 |
| HbA1c (%) | 6.8 ± 2.5 | 6.8 ± 2.3 | 6.8 ± 2.5 |
| Serum sodium (mmol/L) | 138.7 ± 5.3 | 136.1 ± 6.8 | 138.8 ± 5.2 |
| Serum phosphorus (mg/dL) | 3.8 ± 1.3 | 4.0 ± 1.6 | 3.8 ± 1.3 |
| Serum K+ (mmol/L) | 5.3 ± 0.3 | 5.4 ± 0.4 | 5.3 ± 0.3 |
| WBC count (× 109/L) | 8.6 ± 131.1 | 10.2 ± 112.9 | 8.5 ± 132.1 |
| Lipid panel (mg/dL) | |||
| Total cholesterol | 173.4 ± 44.8 | 158.5 ± 52.2 | 173.8 ± 44.6 |
| HDL-cholesterol | 45.3 ± 15.4 | 42.4 ± 18.5 | 45.4 ± 15.3 |
| LDL-cholesterol | 99.4 ± 38.1 | 89.4 ± 41.9 | 99.6 ± 37.9 |
| Triglycerides | 150.3 ± 160.0 | 143.7 ± 197.8 | 150.5 ± 158.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahdoot, R.S.; Hsiung, J.-T.; Agiro, A.; Brahmbhatt, Y.G.; Cooper, K.; Fawaz, S.; Westfall, L.; Kalantar-Zadeh, K.; Streja, E. Liver Disease Is a Risk Factor for Recurrent Hyperkalemia: A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 4562. https://doi.org/10.3390/jcm12144562
Ahdoot RS, Hsiung J-T, Agiro A, Brahmbhatt YG, Cooper K, Fawaz S, Westfall L, Kalantar-Zadeh K, Streja E. Liver Disease Is a Risk Factor for Recurrent Hyperkalemia: A Retrospective Cohort Study. Journal of Clinical Medicine. 2023; 12(14):4562. https://doi.org/10.3390/jcm12144562
Chicago/Turabian StyleAhdoot, Rebecca S., Jui-Ting Hsiung, Abiy Agiro, Yasmin G. Brahmbhatt, Kerry Cooper, Souhiela Fawaz, Laura Westfall, Kamyar Kalantar-Zadeh, and Elani Streja. 2023. "Liver Disease Is a Risk Factor for Recurrent Hyperkalemia: A Retrospective Cohort Study" Journal of Clinical Medicine 12, no. 14: 4562. https://doi.org/10.3390/jcm12144562
APA StyleAhdoot, R. S., Hsiung, J.-T., Agiro, A., Brahmbhatt, Y. G., Cooper, K., Fawaz, S., Westfall, L., Kalantar-Zadeh, K., & Streja, E. (2023). Liver Disease Is a Risk Factor for Recurrent Hyperkalemia: A Retrospective Cohort Study. Journal of Clinical Medicine, 12(14), 4562. https://doi.org/10.3390/jcm12144562






