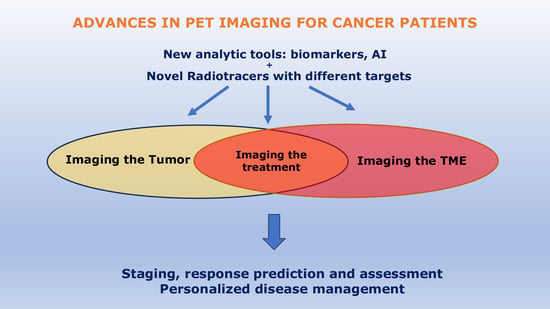
Advances in PET/CT Imaging for Breast Cancer
Abstract
1. Introduction
2. Current Imaging Practices for Breast Cancer Screening, Monitoring, and Potential for Future Avenues
2.1. Imaging Modalities for Breast Cancer Screening
2.2. Imaging Modalities for Breast Cancer Monitoring
3. New Analytic Tools in PET/CT Breast Imaging
3.1. Tumor-Related Biomarkers
3.2. Non-Tumor-Related Biomarkers
3.3. Novel Applications of FDG PET/CT-Tumor-Related Biomarkers
4. Novel Radiotracers for PET/CT Imaging of Breast Cancer
4.1. Promising Tracers for Tumor Imaging
4.1.1. PD-L1
4.1.2. EGFR and Her 2
4.1.3. HDAC
4.1.4. FES
4.1.5. Mucin 1
4.1.6. Tissue Factor
4.1.7. CD146
| Targets | Radiopharmaceuticals | References |
|---|---|---|
| PD-L1 | ||
| [64Cu]-NOTA-PD-L1 | [79] | |
| [89Zr]-atezolizumab | [82] | |
| EGFR | ||
| [89Zr]-cetuximab | [100] | |
| [89Zr]-trastuzumab | [117] | |
| [64Cu]-DOTA-trastuzumab | [122] | |
| HDAC | ||
| [11C]-Martinostat | [127] | |
| Estrogen receptor | ||
| [18F]-FES | [129] | |
| Tissue factor | ||
| [64Cu]-NOTA-ALT-836-Fab | [161] | |
| [18F]-ASIS | [166] | |
| Mucin 1 | ||
| MUC1-FA-[18F] SFB | [140] | |
| CD146 | ||
| [52Mn]-DOTA-YY146 | [170] | |
| [89Zr]-Df-YY146 | [170] | |
| [64Cu]-YY146 | [170] | |
| Trop 2 | ||
| [89Zr]-DFO-AF650 | [172] | |
| Nectin 4 | ||
| [68Ga]-N188 | [173] |
4.2. Additional Tumoral Radiotracers Being Investigated
4.3. Promising Tracers Targeting the Tumoral Microenvironement (TME)
4.3.1. FAPI
4.3.2. Imaging Immune Cells
4.3.3. Imaging the Treatment in Real-Time: Monitoring CAR T Cell Therapy
4.3.4. Imaging Hypoxia and Vasculature
| Target | Radiopharmaceuticals | Reference |
|---|---|---|
| Fibroblast | ||
| FAP | [68Ga]-FAPI-04 | [182] |
| [225Ac]-FAPI-46 | [191] | |
| [177Lu]-FAPI-46 | [191] | |
| T cell | ||
| CD3 | 89Zr-DFO-CD3 | [196] |
| CD8 | 9ZED88082A | [197] |
| [18F]-GEH200521 | [198] | |
| Activated T cell | ||
| Synapse for T cell activation | [64Cu]-synTac | [203] |
| T cell activation in tissue | [89Zr]-deferoxamine-ICOS | [205] |
| Granzyme B | [68Ga]-NOTA-GZP | [204] |
| TAM | ||
| Folate receptor | [18F]-AzaFol | [201] |
| Arginase | [18F]-FMARS | |
| CSF-1R | [11C]-AZ683 | |
| Hypoxia | ||
| pO2 < 10 mmHg | [18F]-HX4 | [211] |
| [18F]-FMISO | ||
| [18F]-FAZA | ||
| Angiogenesis | ||
| Integrin | [18F]-F-Galacto-RGD | [214] |
| VEGF | [89Zr]-Bevacizumab | |
| NGR tripeptide | [64Cu]-labelled NGR |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA: A Cancer. J. Clin. 2017, 67, 7–30. [Google Scholar]
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef] [PubMed]
- United Nations Development Programme. Human Development Data Center. Available online: http://hdr.undp.org/en/data (accessed on 27 January 2021).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. Breast Cancer Statistics. 2021. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer (accessed on 31 August 2021).
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: Collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet 2002, 360, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012, 13, 1141–1151. [Google Scholar] [CrossRef]
- Key, T.; Appleby, P.; Barnes, I.; Reeves, G. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J. Natl. Cancer Inst. 2002, 94, 606–616. [Google Scholar]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.E.P.P.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef]
- Johnson, K.S.; Conant, E.F.; Soo, M.S. Molecular Subtypes of Breast Cancer: A Review for Breast Radiologists. J. Breast Imaging 2020, 3, 12–24. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 1–13. [Google Scholar] [CrossRef]
- Howard, F.M.; Olopade, O.I. Epidemiology of Triple-Negative Breast Cancer: A Review. Cancer J. 2021, 27, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 181–204. [Google Scholar] [CrossRef]
- Ho-Pun-Cheung, A.; Bazin, H.; Boissière-Michot, F.; Mollevi, C.; Simony-Lafontaine, J.; Landas, E.; Bleuse, J.P.; Chardès, T.; Prost, J.F.; Pèlegrin, A.; et al. Quantification of HER1, HER2 and HER3 by time-resolved Förster resonance energy transfer in FFPE triple-negative breast cancer samples. Br. J. Cancer 2020, 122, 397–404. [Google Scholar] [CrossRef]
- Available online: https://acsearch.acr.org/docs/70910/Narrative (accessed on 25 May 2023).
- Lee, C.H.; Dershaw, D.D.; Kopans, D.; Evans, P.; Monsees, B.; Monticciolo, D.; Brenner, R.J.; Bassett, L.; Berg, W.; Feig, S.; et al. Breast Cancer Screening With Imaging: Recommendations from the Society of Breast Imaging and the ACR on the Use of Mammography, Breast MRI, Breast Ultrasound, and other Technologies for the Detection of Clinically Occult Breast Cancer. J. Am. Coll. Radiol. 2010, 7, 18–27. [Google Scholar] [CrossRef]
- Monticciolo, D.L.; Newell, M.S.; Hendrick, R.E.; Helvie, M.A.; Moy, L.; Monsees, B.; Kopans, D.B.; Eby, P.R.; Sickles, E.A. Breast Cancer Screening for Average-Risk Women: Recommendations from the ACR Commission on Breast Imaging. J. Am. Coll. Radiol. 2017, 14, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.S.; Javitt, M.C.; Katzen, J.; Michael, S.; Holland, A.E. Clinical performance metrics of 3D digital breast tomosyn-thesis compared with 2D digital mammography for breast cancer screening in community practice. AJR Am. J. Roentgenol. 2014, 203, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Houssami, N.; Macaskill, P.; Bernardi, D.; Caumo, F.; Pellegrini, M.; Brunelli, S.; Tuttobene, P.; Bricolo, P.; Fantò, C.; Valentini, M.; et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading—Evidence to guide future screening strategies. Eur. J. Cancer 2014, 50, 1799–1807. [Google Scholar] [CrossRef]
- Bernardi, D.; Caumo, F.; Macaskill, P.; Ciatto, S.; Pellegrini, M.; Brunelli, S.; Tuttobene, P.; Bricolo, P.; Fantò, C.; Valentini, M.; et al. Effect of integrating 3D-mammography (digital breast tomosynthesis) with 2D-mammography on radiologists’ true-positive and false-positive detection in a population breast screening trial. Eur. J. Cancer 2014, 50, 1232–1238. [Google Scholar] [CrossRef]
- Caumo, F.; Bernardi, D.; Ciatto, S.; Macaskill, P.; Pellegrini, M.; Brunelli, S.; Tuttobene, P.; Bricolo, P.; Fantò, C.; Valentini, M.; et al. Incremental effect from integrating 3D-mammography (tomosynthesis) with 2D-mammography: Increased breast cancer detection evident for screening centres in a population-based trial. Breast 2013, 23, 76–80. [Google Scholar] [CrossRef]
- Takamoto, Y.; Tsunoda, H.; Kikuchi, M.; Hayashi, N.; Honda, S.; Koyama, T.; Ohde, S.; Yagata, H.; Yoshida, A.; Yamauchi, H. Role of Breast Tomosynthesis in Diagnosis of Breast Cancer for Japanese Women. Asian Pac. J. Cancer Prev. 2013, 14, 3037–3040. [Google Scholar] [CrossRef]
- Brem, R.F.; Tabár, L.; Duffy, S.W.; Inciardi, M.F.; Guingrich, J.A.; Hashimoto, B.E.; Lander, M.R.; Lapidus, R.L.; Peterson, M.K.; Rapelyea, J.A.; et al. Assessing Improvement in Detection of Breast Cancer with Three-dimensional Automated Breast US in Women with Dense Breast Tissue: The SomoInsight Study. Radiology 2015, 274, 663–673. [Google Scholar] [CrossRef]
- Chae, E.Y.; Kim, H.H.; Cha, J.H.; Shin, H.J.; Kim, H. Evaluation of Screening Whole-Breast Sonography as a Supplemental Tool in Conjunction with Mammography in Women with Dense Breasts. J. Ultrasound Med. 2013, 32, 1573–1578. [Google Scholar] [CrossRef]
- Giuliano, V.; Giuliano, C. Improved breast cancer detection in asymptomatic women using 3D-automated breast ultrasound in mammographically dense breasts. Clin. Imaging 2013, 37, 480–486. [Google Scholar] [CrossRef]
- Winkler, N.S.; Raza, S.; Mackesy, M.; Birdwell, R.L. Breast Density: Clinical Implications and Assessment Methods. Radiographics 2015, 35, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.J.; Hruska, C.B.; Conners, A.L.; Tortorelli, C.L.; Maxwell, R.W.; Jones, K.N.; Toledano, A.Y.; O’Connor, M.K. JOURNAL CLUB: Molecular Breast Imaging at Reduced Radiation Dose for Supplemental Screening in Mammographically Dense Breasts. Am. J. Roentgenol. 2015, 204, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.J.; Hruska, C.B.; Phillips, S.W.; Whaley, D.H.; O’connor, M.K. Dedicated Dual-Head Gamma Imaging for Breast Cancer Screening in Women with Mammographically Dense Breasts. Radiology 2011, 258, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, A.; Newell, M.S. Alternative screening for women with dense breasts: Breastspecific gamma imaging (molecular breast imaging). AJR Am. J. Roentgenol. 2015, 204, 252–256. [Google Scholar] [CrossRef]
- Hagen, A.I.; Kvistad, K.A.; Maehle, L.; Holmen, M.M.; Aase, H.; Styr, B.; Vabø, A.; Apold, J.; Skaane, P.; Møller, P. Sensitivity of MRI versus conventional screening in the diagnosis of BRCA-associated breast cancer in a national prospective series. Breast 2007, 16, 367–374. [Google Scholar] [CrossRef]
- Kriege, M.; Brekelmans, C.T.M.; Boetes, C.; Muller, S.H.; Zonderland, H.M.; Obdeijn, I.M.; Manoliu, R.A.; Kok, T.; Rutgers, E.J.T.; de Koning, H.J.; et al. Differences between first and subsequent rounds of the MRISC breast cancer screening program for women with a familial or genetic predisposition. Cancer 2006, 106, 2318–2326. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Schrading, S.; Leutner, C.C.; Morakkabati-Spitz, N.; Wardelmann, E.; Fimmers, R.; Kuhn, W.; Schild, H.H. Mammography, Breast Ultrasound, and Magnetic Resonance Imaging for Surveillance of Women at High Familial Risk for Breast Cancer. J. Clin. Oncol. 2005, 23, 8469–8476. [Google Scholar] [CrossRef]
- MARIBS Study Group. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: A prospective multicentre cohort study (MARIBS). Lancet 2005, 365, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Yip, R.; Jirapatnakul, A.; Hu, M.; Chen, X.; Han, D.; Ma, T.; Zhu, Y.; Salvatore, M.M.; Margolies, L.R. I-ELCAP Investigators; et al. Added benefits of early detection of other diseases on low-dose CT screening. Transl. Lung Cancer Res. 2021, 10, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.; Margolies, L.; Bertolini, A.; Singh, A.; Yankelevitz, D.; Henschke, C. The need to be all inclusive: Chest CT scans should include imaged breast parenchyma. Clin. Imaging 2018, 50, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Margolies, L.; Salvatore, M.; Eber, C.; Jacobi, A.; Lee, I.-J.; Liang, M.; Tang, W.; Xu, D.; Zhao, S.; Kale, M.; et al. The general radiologist’s role in breast cancer risk assessment: Breast density measurement on chest CT. Clin. Imaging 2015, 39, 979–982. [Google Scholar] [CrossRef]
- Salvatore, M.; Margolies, L.; Kale, M.; Wisnivesky, J.; Kotkin, S.; Henschke, C.I.; Yankelevitz, D.F. Breast Density: Comparison of Chest CT with Mammography. Radiology 2013, 270, 67–73. [Google Scholar] [CrossRef]
- Desperito, E.; Schwartz, L.; Capaccione, K.M.; Collins, B.T.; Jamabawalikar, S.; Peng, B.; Patrizio, R.; Salvatore, M.M. Chest CT for Breast Cancer Diagnosis. Life 2022, 12, 1699. [Google Scholar] [CrossRef]
- Margolies, L.R.; Salvatore, M.; Yip, R.; Tam, K.; Bertolini, A.; Henschke, C.; Yankelevitz, D. The chest radiologist’s role in invasive breast cancer detection. Clin. Imaging 2017, 50, 13–19. [Google Scholar] [CrossRef]
- de Mooij, C.M.; Ploumen, R.A.W.; Nelemans, P.J.; Mottaghy, F.M.; Smidt, M.L.; van Nijnatten, T.J.A. The in-fluence of receptor expression and clinical subtypes on baseline [18F]FDG uptake in breast cancer: Systematic review and meta-analysis. EJNMMI Res. 2023, 13, 5. [Google Scholar] [CrossRef]
- O’Neill, H.; Malik, V.; Johnston, C.; Reynolds, J.V.; O’sullivan, J. Can the Efficacy of [18F]FDG-PET/CT in Clinical Oncology Be Enhanced by Screening Biomolecular Profiles? Pharmaceuticals 2019, 12, 16. [Google Scholar] [CrossRef]
- Podoloff, D.A.; Advani, R.H.; Allred, C.; Benson, A.B., 3rd; Brown, E.; Burstein, H.J.; Carlson, R.W.; Coleman, R.E.; Czuczman, M.S.; Delbeke, D.; et al. NCCN task force report: Positron emission tomography (PET)/computed tomography (CT) scanning in cancer. J. Natl. Compr. Cancer Netw. 2007, 5 (Suppl. 1), S-1. [Google Scholar] [CrossRef]
- Rosen, E.L.; Eubank, W.B.; Mankoff, D.A. FDG PET, PET/CT, and breast cancer imaging. Radiographics 2007, 27 (Suppl. 1), S215–S229. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Chauhan, A.; Zhuang, H.; Chandra, P.; Schnall, M.; Alavi, A. Clinicopathologic factors associated with false negative FDG–PET in primary breast cancer. Breast Cancer Res. Treat. 2006, 98, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Vercellino, L.; de Jong, D.; Dercle, L.; Hosten, B.; Braumuller, B.; Das, J.P.; Deng, A.; Moya-Plana, A.; A’keen, C.; Yeh, R.; et al. Translating Molecules into Imaging—The Development of New PET Tracers for Patients with Melanoma. Diagnostics 2022, 12, 1116. [Google Scholar] [CrossRef] [PubMed]
- Krarup, M.M.; Fischer, B.M.; Christensen, T.N. New PET Tracers: Current Knowledge and Perspectives in Lung Cancer. Semin. Nucl. Med. 2022, 52, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Aukema, T.S.; Rutgers, E.J.; Vogel, W.V.; Teertstra, H.J.; Oldenburg, H.S.; Vrancken Peeters, M.T.; Wesseling, J.; Russell, N.S.; Valdés Olmos, R.A. The role of FDG PET/CT in patients with locoregional breast cancer recurrence: A comparison to conventional imaging techniques. Eur. J. Surg. Oncol. 2010, 36, 387–392. [Google Scholar] [CrossRef]
- Murakami, R.; Kumita, S.-I.; Yoshida, T.; Ishihara, K.; Kiriyama, T.; Hakozaki, K.; Yanagihara, K.; Iida, S.; Tsuchiya, S.-I. FDG-PET/CT in the diagnosis of recurrent breast cancer. Acta Radiol. 2012, 53, 12–16. [Google Scholar] [CrossRef]
- Avril, S.; Muzic, R.F., Jr.; Plecha, D.; Traughber, B.J.; Vinayak, S.; Avril, N. ¹⁸F-FDG PET/CT for Monitoring of Treatment Response in Breast Cancer. J. Nucl. Med. 2016, 57 (Suppl. 1), 34S–39S. [Google Scholar] [CrossRef]
- Alberini, J.-L.; Lerebours, F.; Wartski, M.; Fourme, E.; Le Stanc, E.; Gontier, E.; Madar, O.; Cherel, P.; Pecking, A.P. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) imaging in the staging and prognosis of inflammatory breast cancer. Cancer 2009, 115, 5038–5047. [Google Scholar] [CrossRef]
- Koo, H.R.; Park, J.S.; Kang, K.W.; Han, W.; Park, I.A.; Moon, W.K. Correlation between 18F-FDG uptake on PET/CT and prognostic factors in triple-negative breast cancer. Eur. Radiol. 2015, 25, 3314–3321. [Google Scholar] [CrossRef]
- Lemarignier, C.; Martineau, A.; Teixeira, L.; Vercellino, L.; Espié, M.; Merlet, P.; Groheux, D. Correlation between tumour characteristics, SUV measurements, metabolic tumour volume, TLG and textural features assessed with 18F-FDG PET in a large cohort of oestrogen receptor-positive breast cancer patients. Eur. J. Nucl. Med. 2017, 44, 1145–1154. [Google Scholar] [CrossRef]
- Groheux, D.; Giacchetti, S.; Moretti, J.L.; Porcher, R.; Espié, M.; Lehmann-Che, J.; de Roquancourt, A.; Hamy, A.S.; Cuvier, C.; Vercellino, L.; et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.G.; Ulaner, G.A.; Eaton, A.; Fazio, M.; Jhaveri, K.; Patil, S.; Evangelista, L.; Park, J.Y.; Serna-Tamayo, C.; Howard, J.; et al. Standardized uptake value by positron emission tomog-raphy/computed tomography as a prognostic variable in metastatic breast cancer. Cancer 2012, 118, 5454–5462. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Eaton, A.; Morris, P.G.; Lilienstein, J.; Jhaveri, K.; Patil, S.; Fazio, M.; Larson, S.; Hudis, C.A.; Jochelson, M.S. Prognostic value of quantitative fluorodeoxyglucose measurements in newly diagnosed metastatic breast cancer. Cancer Med. 2013, 2, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, C.; Devillers, A.; Campone, M.; Campion, L.; Ferrer, L.; Sagan, C.; Ricaud, M.; Bridji, B.; Kraeber-Bodéré, F. FDG PET evaluation of early axillary lymph node response to neoadjuvant chemotherapy in stage II and III breast cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Schwarz-Dose, J.; Untch, M.; Tiling, R.; Sassen, S.; Mahner, S.; Kahlert, S.; Harbeck, N.; Lebeau, A.; Brenner, W.; Schwaiger, M.; et al. Monitoring Primary Systemic Therapy of Large and Locally Advanced Breast Cancer by Using Sequential Positron Emission Tomography Imaging With [18F]Fluorodeoxyglucose. J. Clin. Oncol. 2009, 27, 535–541. [Google Scholar] [CrossRef]
- Humbert, O.; Riedinger, J.-M.; Charon-Barra, C.; Berriolo-Riedinger, A.; Desmoulins, I.; Lorgis, V.; Kanoun, S.; Coutant, C.; Fumoleau, P.; Cochet, A.; et al. Identification of Biomarkers Including 18FDG-PET/CT for Early Prediction of Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Clin. Cancer Res. 2015, 21, 5460–5468. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.Y.; Han, S.W.; Lee, J.E.; Lee, H.J.; Heo, N.H.; Lee, S.M. [18F]FDG uptake of bone marrow on PET/CT for predicting distant recurrence in breast cancer patients after surgical resection. EJNMMI Res. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Seban, R.D.; Rouzier, R.; Latouche, A.; Deleval, N.; Guinebretiere, J.M.; Buvat, I.; Bidard, F.C.; Champion, L. Total metabolic tumor volume and spleen metabolism on baseline [18F]-FDG PET/CT as independent prognostic biomarkers of re-currence in resected breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3560–3570. [Google Scholar] [CrossRef]
- Şahin, E.; Elboğa, U. Relationship between reticuloendothelial systems’ FDG uptake level and clinicopathological features in patient with invasive ductal breast cancer. La Radiol. Med. 2017, 122, 785–792. [Google Scholar] [CrossRef]
- Bang, J.-I.; Yoon, H.-J.; Kim, B.S. Clinical utility of FDG uptake within reticuloendothelial system on F-18 FDG PET/CT for prediction of tumor recurrence in breast cancer. PLoS ONE 2018, 13, e0208861. [Google Scholar] [CrossRef]
- Cha, Y.J.; Koo, J.S. Role of Tumor-Associated Myeloid Cells in Breast Cancer. Cells 2020, 9, 1785. [Google Scholar] [CrossRef]
- Hirakata, T.; Fujii, T.; Kurozumi, S.; Katayama, A.; Honda, C.; Yanai, K.; Tokuda, S.; Nakazawa, Y.; Obayashi, S.; Yajima, R.; et al. FDG uptake reflects breast cancer immunological features: The PD-L1 expression and degree of TILs in primary breast cancer. Breast Cancer Res. Treat. 2020, 181, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Can, C.; Komek, H. Metabolic and volume-based parameters of (18F)FDG PET/CT for primary mass and axillary lymph node metastasis in patients with invasive ductal carcinoma: A retrospective analysis in relation to molecular subtype, axillary lymph node metastasis and immunohistochemistry and inflammatory markers. Nucl. Med. Commun. 2019, 40, 1051–1059. [Google Scholar] [PubMed]
- Fujii, T.; Tokuda, S.; Nakazawa, Y.; Kurozumi, S.; Obayashi, S.; Yajima, R.; Shirabe, K. Relationship Between FDG Uptake and the Platelet/lymphocyte Ratio in Patients With Breast Invasive Ductal Cancer. Vivo 2020, 34, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Puppe, J.; Seifert, T.; Eichler, C.; Pilch, H.; Mallmann, P.; Malter, W. Genomic Signatures in Luminal Breast Cancer. Breast Care 2020, 15, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ha, S.; An, H.J.; Lee, J.S.; Han, W.; Im, S.-A.; Ryu, H.S.; Kim, W.H.; Chang, J.M.; Cho, N.; et al. Association between partial-volume corrected SUVmax and Oncotype DX recurrence score in early-stage, ER-positive/HER2-negative invasive breast cancer. Eur. J. Nucl. Med. 2016, 43, 1574–1584. [Google Scholar] [CrossRef]
- Tsukada, H.; Tsukada, J.; Ochi, T.; Noguchi, E.; Okamoto, T. Radiological predictive factors on preoperative mul-timodality imaging are related to Oncotype DX recurrence score in estrogen-positive/human epidermal growth factor receptor 2-negative invasive breast cancer: A cross-sectional study. Ann. Nucl. Med. 2022, 36, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Mayer, I.A.; Diamond, J.R.; Moroose, R.L.; Isakoff, S.J.; Starodub, A.N.; Shah, N.C.; O’shaughnessy, J.; Kalinsky, K.; Guarino, M.; et al. Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2017, 35, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously un-treated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Nduom, E.K.; Wei, J.; Yaghi, N.K.; Huang, N.; Kong, L.-Y.; Gabrusiewicz, K.; Ling, X.; Zhou, S.; Ivan, C.; Chen, J.Q.; et al. PD-L1 expression and prognostic impact in gli-blastoma. Neuro-Oncology 2016, 18, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Sinigaglia, M.; Assi, T.; Besson, F.L.; Ammari, S.; Edjlali, M.; Feltus, W.; Rozenblum-Beddok, L.; Zhao, B.; Schwartz, L.H.; Mokrane, F.Z.; et al. Imaging-guided precision medicine in glioblastoma patients treated with immune check-point modulators: Research trend and future directions in the field of imaging biomarkers and artificial intelligence. EJNMMI 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.T.; Natarajan, A.; Gordon, S.R.; Maute, R.L.; McCracken, M.N.; Ring, A.M.; Weissman, I.L.; Gambhir, S.S. Practical Immuno-PET Radiotracer Design Considerations for Human Immune Checkpoint Imaging. J. Nucl. Med. 2017, 58, 538–546. [Google Scholar] [CrossRef]
- Hettich, M.; Braun, F.; Bartholomä, M.D.; Schirmbeck, R.; Niedermann, G. High-Resolution PET Imaging with Therapeutic Anti-body-based PD-1/PD-L1 Checkpoint Tracers. Theranostics 2016, 6, 1629–1640. [Google Scholar] [CrossRef]
- Heskamp, S.; Hobo, W.; Molkenboer-Kuenen, J.D.; Olive, D.; Oyen, W.J.; Dolstra, H.; Boerman, O.C. Noninvasive Imaging of Tumor PD-L1 Expression Using Radiolabeled Anti–PD-L1 Antibodies. Cancer Res. 2015, 75, 2928–2936. [Google Scholar] [CrossRef]
- Kikuchi, M.; Clump, D.A.; Srivastava, R.M.; Sun, L.; Zeng, D.; Diaz-Perez, J.A.; Anderson, C.J.; Edwards, W.B.; Ferris, R.L. Preclinical immunoPET/CT imaging using Zr-89-labeled anti-PD-L1 monoclonal antibody for assessing radiation-induced PD-L1 upregulation in head and neck cancer and melanoma. OncoImmunology 2017, 6, e1329071. [Google Scholar] [CrossRef]
- Bensch, F.; Van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; Van Der Wekken, A.J.; et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef]
- Broos, K.; Keyaerts, M.; Lecocq, Q.; Renmans, D.; Nguyen, T.; Escors, D.; Liston, A.; Raes, G.; Breckpot, K.; Devoogdt, N. Non-invasive assessment of murine PD-L1 levels in syngeneic tumor models by nuclear imaging with nanobody tracers. Oncotarget 2017, 8, 41932–41946. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Appert-Collin, A.; Hubert, P.; Crémel, G.; Bennasroune, A. Role of ErbB Receptors in Cancer Cell Migration and Invasion. Front. Pharmacol. 2015, 6, 283. [Google Scholar] [CrossRef]
- Giltnane, J.M.; Moeder, C.B.; Camp, R.L.; Rimm, D.L. Quantitative multiplexed analysis of ErbB family coexpression for primary breast cancer prognosis in a large retrospective cohort. Cancer 2009, 115, 2400–2409. [Google Scholar] [CrossRef]
- Elizalde, P.V.; Russo, R.I.C.; Chervo, M.F.; Schillaci, R. ErbB-2 nuclear function in breast cancer growth, metastasis and resistance to therapy. Endocr.-Relat. Cancer 2016, 23, T243–T257. [Google Scholar] [CrossRef] [PubMed]
- Rinne, S.S.; Orlova, A.; Tolmachev, V. PET and SPECT Imaging of the EGFR Family (RTK Class I) in Oncology. Int. J. Mol. Sci. 2021, 22, 3663. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Hung, M.-C.; Yamaguchi, H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am. J. Cancer Res. 2016, 6, 1609–1623. [Google Scholar] [PubMed]
- Masuda, H.; Zhang, D.; Bartholomeusz, C.; Doihara, H.; Hortobagyi, G.N.; Ueno, N.T. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res. Treat. 2012, 136, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Gelmon, K.; Dent, R.; Mackey, J.R.; Laing, K.; McLeod, D.; Verma, S. Targeting triple negative breast cancer: Optimising therapeutic outcomes. Ann. Oncol. 2012, 23, 2223–2234. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Iida, M.; Dunn, E.F.; Ghia, A.J.; Wheeler, D.L. Nuclear EGFR contributes to acquired resistance to cetuximab. Oncogene 2009, 28, 3801–3813. [Google Scholar] [CrossRef]
- Brand, T.M.; Iida, M.; Dunn, E.F.; Luthar, N.; Kostopoulos, K.T.; Corrigan, K.L.; Wleklinski, M.J.; Yang, D.; Wisinski, K.B.; Salgia, R.; et al. Nuclear epidermal growth factor receptor is a functional molecular target in triple-negative breast cancer. Mol. Cancer Ther. 2014, 13, 1356–1368. [Google Scholar] [CrossRef]
- Brand, T.M.; Iida, M.; Luthar, N.; Starr, M.M.; Huppert, E.J.; Wheeler, D.L. Nuclear EGFR as a molecular target in cancer. Radiother. Oncol. 2013, 108, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.L.; Iida, M.; Kruser, T.J.; Nechrebecki, M.M.; Dunn, E.F.; Armstrong, E.A.; Huang, S.; Harari, P.M. Epidermal growth factor receptor cooperates with Src family kinases in acquired resistance to cetuximab. Cancer Biol Ther. 2009, 8, 696–703. [Google Scholar] [CrossRef]
- Li, C.; Iida, M.; Dunn, E.F.; Wheeler, D.L. Dasatinib blocks cetuximab- and radiation-induced nuclear translocation of the epidermal growth factor receptor in head and neck squamous cell carcinoma. Radiother. Oncol. 2010, 97, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Shi, J.; Afari, G.; Bhattacharyya, S. Preparation of clinical-grade89Zr-panitumumab as a positron emission tomography biomarker for evaluating epidermal growth factor receptor-targeted therapy. J. Label. Compd. Radiopharm. 2013, 57, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Kurdziel, K.; Wei, L.; Riffle, L.; Kaur, G.; Hill, G.C.; Jacobs, P.M.; Tatum, J.L.; Doroshow, J.H.; Kalen, J.D. Zirconium-89 labeled panitumumab: A potential immu-no-PET probe for HER1- expressing carcinomas. Nucl. Med. Biol. 2013, 40, 451–457. [Google Scholar] [CrossRef]
- Aerts, H.J.; Dubois, L.; Perk, L.; Vermaelen, P.; van Dongen, G.A.; Wouters, B.G.; Lambin, P. Disparity Between In Vivo EGFR Expression and 89Zr-Labeled Cetuximab Uptake Assessed with PET. J. Nucl. Med. 2008, 50, 123–131. [Google Scholar] [CrossRef]
- McKnight, B.N.; Kim, S.; Boerner, J.L.; Viola, N.T. Cetuximab PET delineated changes in cellular distribution of EGFR upon dasatinib treatment in triple negative breast cancer. Breast Cancer Res. 2020, 22, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Lee, J.-S.; Han, J.-Y.; Cho, E.; Haura, E.; Lee, K.; Bauml, J.; Sanborn, R.; Curtis, M.; Attiyeh, E.; et al. JNJ-61186372 (JNJ-372), an EGFR-cMET bispecific antibody, in advanced non-small cell lung cancer (NSCLC): An update on phase I results. Ann. Oncol. 2018, 29, viii542. [Google Scholar] [CrossRef]
- Cavaliere, A.; Sun, S.; Lee, S.; Bodner, J.; Li, Z.; Huang, Y.; Moores, S.L.; Marquez-Nostra, B. Development of [89Zr]ZrDFO-amivantamab bispecific to EGFR and c-MET for PET imaging of triple-negative breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 383–394. [Google Scholar] [CrossRef]
- Hanna, W.M.; Slodkowska, E.; Lu, F.-I.; Nafisi, H.; Nofech-Mozes, S. Comparative Analysis of Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer According to 2007 and 2013 American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations. J. Clin. Oncol. 2017, 35, 3039–3045. [Google Scholar] [CrossRef]
- Muss, H.B.; Thor, A.D.; Berry, D.A.; Kute, T.; Liu, E.T.; Koerner, F.; Cirrincione, C.T.; Budman, D.R.; Wood, W.C.; Barcos, M.; et al. c-erbB-2 expression and response to adjuvant therapy in women with node-positive early breast cancer. N. Engl. J. Med. 1994, 330, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, C.; Perrone, F.; Gallo, C.; De Laurentiis, M.; Lauria, R.; Morabito, A.; Pettinato, G.; Panico, L.; D’Antonio, A.; Bianco, A.R.; et al. cerb B2 overexpression decreases the benefit of adjuvant tamoxifen in early-stage breast cancer without axillary lymph node metastases. J. Clin. Oncol. 1996, 14, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Press, M.F.; Bernstein, L.; Thomas, P.A.; Meisner, L.F.; Zhou, J.Y.; Ma, Y.; Hung, G.; Robinson, R.A.; Harris, C.; ElNaggar, A.; et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: Poor prognosis in node-negative breast carcinomas. J. Clin. Oncol. 1997, 15, 2894–2904. [Google Scholar] [CrossRef]
- Lu, J.; Steeg, P.S.; Price, J.E.; Krishnamurthy, S.; Mani, S.A.; Reuben, J.; Cristofanilli, M.; Dontu, G.; Bidaut, L.; Valero, V.; et al. Breast Cancer Metastasis: Challenges and Opportunities. Cancer Res. 2009, 69, 4951–4953. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.-B.; Im, Y.-H.; Im, S.-A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Martin, M.; Symmans, W.F.; Jung, K.H.; Huang, C.S.; Thompson, A.M.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 115–126. [Google Scholar] [CrossRef]
- Santinelli, A.; Pisa, E.; Stramazzotti, D.; Fabris, G. HER-2 status discrepancy between primary breast cancer and metastatic sites. Impact on target therapy. Int. J. Cancer 2007, 122, 999–1004. [Google Scholar] [CrossRef]
- Ligthart, S.; Bidard, F.-C.; Decraene, C.; Bachelot, T.; Delaloge, S.; Brain, E.; Campone, M.; Viens, P.; Pierga, J.-Y.; Terstappen, L. Unbiased quantitative assessment of Her-2 expression of circulating tumor cells in patients with metastatic and non-metastatic breast cancer. Ann. Oncol. 2013, 24, 1231–1238. [Google Scholar] [CrossRef]
- Schrijver, W.A.M.E.; Suijkerbuijk, K.P.M.; van Gils, C.H.; van der Wall, E.; Moelans, C.B.; van Diest, P.J. Receptor conversion in distant breast cancer metastases: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2018, 110, 568–580. [Google Scholar] [CrossRef]
- Phillips, K.A.; Marshall, D.A.; Haas, J.S.; Elkin, E.B.; Liang, S.Y.; Hassett, M.J.; Ferrusi, I.; Brock, J.E.; Van Bebber, S.L. Clinical practice patterns and cost-effectiveness of HER2 testing strategies in breast cancer patients. Cancer 2009, 115, 5166–5174. [Google Scholar] [CrossRef]
- Tolmachev, V. Imaging of HER-2 Overexpression in Tumors for Guiding Therapy. Curr. Pharm. Des. 2008, 14, 2999–3019. [Google Scholar] [CrossRef]
- Massicano, A.V.F.; Marquez-Nostra, B.V.; Lapi, S.E. Targeting HER2 in nuclear medicine for imaging and therapy. Mol. Imaging 2018, 17, 1536012117745386. [Google Scholar] [CrossRef] [PubMed]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schröder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef]
- Laforest, R.; Lapi, S.E.; Oyama, R.; Bose, R.; Tabchy, A.; Marquez-Nostra, B.V.; Burkemper, J.; Wright, B.D.; Frye, J.; Frye, S.; et al. [89Zr]Trastuzumab: Evaluation of Radiation Dosimetry, Safety, and Optimal Imaging Parameters in Women with HER2-Positive Breast Cancer. Mol. Imaging Biol. 2016, 18, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, G.; Lamberts, L.E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Bensch, F.; Brouwers, A.H.; Lub-de Hooge, M.N.; de Jong, J.R.; van der Vegt, B.; Sleijfer, S.; de Vries, E.G.E.; Schröder, C.P. 89Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2300–2306. [Google Scholar] [CrossRef]
- Beylergil, V.; Morris, P.G.; Smith-Jones, P.M.; Modi, S.; Solit, D.; Hudis, C.A.; Lu, Y.; O’donoghue, J.; Lyashchenko, S.K.; Carrasquillo, J.A.; et al. Pilot study of 68Ga-DOTA-F(ab′)2-trastuzumab in patients with breast cancer. Nucl. Med. Commun. 2013, 34, 1157–1165. [Google Scholar] [CrossRef]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef]
- Mortimer, J.E.; Bading, J.R.; Park, J.M.; Frankel, P.H.; Carroll, M.I.; Tran, T.T.; Poku, E.K.; Rockne, R.C.; Raubitschek, A.A.; Shively, J.E.; et al. Tumor Uptake of 64Cu-DOTA-Trastuzumab in Patients with Metastatic Breast Cancer. J. Nucl. Med. 2018, 59, 38–43. [Google Scholar] [CrossRef]
- Damaskos, C.; Garmpis, N.; Valsami, S.; Kontos, M.; Spartalis, E.; Kalampokas, T.; Kalampokas, E.; Athanasiou, A.; Moris, D.; Daskalopoulou, A.; et al. Histone Deacetylase Inhibitors: An Attractive Therapeutic Strategy Against Breast Cancer. Anticancer Res. 2017, 37, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef] [PubMed]
- Zucchetti, B.; Shimada, A.K.; Katz, A.; Curigliano, G. The role of histone deacetylase inhibitors in metastatic breast cancer. Breast 2019, 43, 130–134. [Google Scholar] [CrossRef]
- Tago, T.; Toyohara, J. Advances in the Development of PET Ligands Targeting Histone Deacetylases for the As-sessment of Neurodegenerative Diseases. Molecules 2018, 23, 300. [Google Scholar] [CrossRef]
- Pascoal, T.A.; Chamoun, M.; Lax, E.; Wey, H.-Y.; Shin, M.; Ng, K.P.; Kang, M.S.; Mathotaarachchi, S.; Benedet, A.L.; Therriault, J.; et al. [11C]Martinostat PET analysis reveals reduced HDAC I availability in Alzheimer’s disease. Nat. Commun. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Lumachi, F.; Santeufemia, D.A.; Basso, S.M. Current medical treatment of estrogen receptor-positive breast cancer. World J. Biol. Chem. 2015, 6, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gong, C.; Liu, S.; Zhang, Y.; Zhang, Y.; Xu, X.; Yuan, H.; Wang, B.; Yang, Z. 18F-FES PET/CT Influences the Staging and Management of Patients with Newly Diagnosed Estrogen Receptor-Positive Breast Cancer: A Retrospective Comparative Study with 18F-FDG PET/CT. Oncologist 2019, 24, e1277–e1285. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Jhaveri, K.; Chandarlapaty, S.; Hatzoglou, V.; Riedl, C.C.; Lewis, J.S.; Mauguen, A. Head-to-Head Evaluation of 18F-FES and 18F-FDG PET/CT in Metastatic Invasive Lobular Breast Cancer. J. Nucl. Med. 2021, 62, 326–331. [Google Scholar] [CrossRef]
- Kufe, D.W. MUC1-C oncoprotein as a target in breast cancer: Activation of signaling pathways and therapeutic approaches. Oncogene 2012, 32, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, M.; Maeda, T.; Mehrotra, N.; Jin, C.; Alam, M.; Bouillez, A.; Hata, T.; Tagde, A.; Keating, A.; Kharbanda, S.; et al. Targeting MUC1-C suppresses BCL2A1 in triple-negative breast cancer. Signal Transduct. Target. Ther. 2018, 3, 13. [Google Scholar] [CrossRef]
- Duffy, M.J.; Shering, S.; Sherry, F.; McDermott, E.; O’Higgins, N. CA 15-3: A prognostic marker in breast cancer. Int. J. Biol. Markers 2001, 15, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Z.; Zhang, S.; Zhu, P.; Ko, J.K.-S.; Yung, K.K.-L. MUC1: Structure, Function, and Clinic Application in Epithelial Cancers. Int. J. Mol. Sci. 2021, 22, 6567. [Google Scholar] [CrossRef] [PubMed]
- Thie, H.; Toleikis, L.; Li, J.; von Wasielewski, R.; Bastert, G.; Schirrmann, T.; Esteves, I.T.; Behrens, C.K.; Fournes, B.; Fournier, N.; et al. Rise and Fall of an Anti-MUC1 Specific Antibody. PLoS ONE 2011, 6, e15921. [Google Scholar] [CrossRef] [PubMed]
- Okarvi, S. Preparation and evaluation of a tumor-associated antigen mucin (MUC1) and tumor-associated monoclonal anti-body-derived hybrid peptide as a breast cancer imaging agent. J. Nucl. Med. 2010, 51 (Suppl. 2), 1521. [Google Scholar]
- Okarvi, S.M.; Al Jammaz, I. Preparation and evaluation of the tumor-specific antigen-derived synthetic mucin 1 peptide: A potential candidate for the targeting of breast carcinoma. Nucl. Med. Biol. 2016, 43, 403–409. [Google Scholar] [CrossRef]
- Stergiou, N.; Nagel, J.; Pektor, S.; Heimes, A.-S.; Jäkel, J.; Brenner, W.; Schmidt, M.; Miederer, M.; Kunz, H.; Roesch, F.; et al. Evaluation of a novel monoclonal antibody against tumor-associated MUC1 for diagnosis and prognosis of breast cancer. Int. J. Med. Sci. 2019, 16, 1188–1198. [Google Scholar] [CrossRef]
- Al Jammaz, I.; Al-Otaibi, B.; Al-Malki, Y.; Abousekhrah, A.; Okarvi, S.M. Fast Fluorine-18 labeling and preclinical evaluation of novel Mucin1 and its Folate hybrid peptide con-jugate for targeting breast carcinoma. EJNMMI Radiopharm. Chem. 2021, 6, 12. [Google Scholar] [CrossRef]
- Brossart, P.; Heinrich, K.S.; Stuhler, G.; Behnke, L.; Reichardt, V.L.; Stevanovic, S.; Muhm, A.; Rammensee, H.G.; Kanz, L.; Brugger, W. Identification of HLA-A2–Restricted T-Cell Epitopes Derived From the MUC1 Tumor Antigen for Broadly Applicable Vaccine Therapies. Blood 1999, 93, 4309–4317. [Google Scholar] [CrossRef]
- Lakshminarayanan, V.; Thompson, P.; Wolfert, M.A.; Buskas, T.; Bradley, J.M.; Pathangey, L.B.; Madsen, C.S.; Cohen, P.A.; Gendler, S.J.; Boons, G.-J. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc. Natl. Acad. Sci. USA 2011, 109, 261–266. [Google Scholar] [CrossRef]
- Knutson, K.L.; Schiffman, K.; Disis, M. Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J. Clin. Investig. 2001, 107, 477–484. [Google Scholar] [CrossRef]
- Pegram, M.D.; Borges, V.F.; Ibrahim, N.; Fuloria, J.; Shapiro, C.; Perez, S.; Wang, K.; Stark, F.S.; Luck, N.C. Phase I dose escalation pharmacokinetic assessment of intravenous humanized anti-MUC1 antibody AS1402 in patients with advanced breast cancer. Breast Cancer Res. 2009, 11, R73. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.K.; Yariz, K.O.; Bondarenko, I.; Manikhas, A.; Semiglazov, V.; Alyasova, A.; Komisarenko, V.; Shparyk, Y.; Murray, J.L.; Jones, D.; et al. Randomized Phase II Trial of Letrozole plus Anti-MUC1 Antibody AS1402 in Hormone Receptor–Positive Locally Advanced or Metastatic Breast Cancer. Clin. Cancer Res. 2011, 17, 6822–6830. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, L.; Qiu, Y.; Sun, W.; Ren, T.; Lv, Y.; Liu, M.; Wang, X.; Tao, H.; Zhao, L.; et al. A novel humanized MUC1 antibody–drug conjugate for the treatment of trastuzumab-resistant breast cancer. Acta Biochim. Biophys. Sin. 2021, 53, 1625–1639. [Google Scholar] [CrossRef]
- Nitori, N.; Ino, Y.; Nakanishi, Y.; Yamada, T.; Honda, K.; Yanagihara, K.; Kosuge, T.; Kanai, Y.; Kitajima, M.; Hirohashi, S. Prognostic Significance of Tissue Factor in Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2005, 11, 2531–2539. [Google Scholar] [CrossRef]
- Chen, Z.; Sager, R. Differential Expression of Human Tissue Factor in Normal Mammary Epithelial Cells and in Carcinomas. Mol. Med. 1995, 1, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Contrino, J.; Hair, G.; Kreutzer, D.L.; Rickles, F.R. In situ detection of tissue factor in vascular endothelial cells: Correlation with the malignant phenotype of human breast disease. Nat. Med. 1996, 2, 209–215. [Google Scholar] [CrossRef]
- Ruf, W.; Disse, J.; Carneiro-Lobo, T.C.; Yokota, N.; Schaffner, F. Tissue factor and cell signalling in cancer progression and thrombosis. J. Thromb. Haemost. 2011, 9, 306–315. [Google Scholar] [CrossRef]
- Jiang, X.; Bailly, M.A.; Panetti, T.S.; Cappello, M.; Konigsberg, W.H.; Bromberg, M.E. Formation of tissue factor–factor VIIa–factor Xa complex promotes cellular signaling and migration of human breast cancer cells. J. Thromb. Haemost. 2004, 2, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Yokota, N.; Zarpellon, A.; Chakrabarty, S.; Bogdanov, V.Y.; Gruber, A.; Castellino, F.J.; Mackman, N.; Ellies, L.G.; Weiler, H.; Ruggeri, Z.M.; et al. Contributions of thrombin targets to tissue factor-dependent metastasis in hyperthrombotic mice. J. Thromb. Haemost. 2013, 12, 71–81. [Google Scholar] [CrossRef]
- Koizume, S.; Miyagi, Y. Breast cancer phenotypes regulated by tissue factor-factor VII pathway: Possible therapeutic targets. World J. Clin. Oncol. 2014, 5, 908–920. [Google Scholar] [CrossRef]
- Shi, S.; Hong, H.; Orbay, H.; Graves, S.A.; Yang, Y.; Ohman, J.D.; Liu, B.; Nickles, R.J.; Wong, H.C.; Cai, W. ImmunoPET of tissue factor expression in triple-negative breast cancer with a radiolabeled antibody Fab fragment. Eur. J. Nucl. Med. 2015, 42, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Rydén, L.; Grabau, D.; Schaffner, F.; Jönsson, P.-E.; Ruf, W.; Belting, M. Evidence for tissue factor phosphorylation and its correlation with protease-activated receptor expression and the prognosis of primary breast cancer. Int. J. Cancer 2009, 126, 2330–2340. [Google Scholar] [CrossRef]
- Breij, E.C.; de Goeij, B.E.; Verploegen, S.; Schuurhuis, D.H.; Amirkhosravi, A.; Francis, J.; Miller, V.B.; Houtkamp, M.; Bleeker, W.K.; Satijn, D.; et al. An Antibody–Drug Conjugate That Targets Tissue Factor Exhibits Potent Therapeutic Activity against a Broad Range of Solid Tumors. Cancer Res. 2014, 74, 1214–1226. [Google Scholar] [CrossRef]
- Yu, J.L.; May, L.; Lhotak, V.; Shahrzad, S.; Shirasawa, S.; Weitz, J.I.; Coomber, B.L.; Mackman, N.; Rak, J.W. Oncogenic events regulate tissue factor expression in colorectal cancer cells: Implications for tumor progression and angiogenesis. Blood 2005, 105, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Ngo, C.V.; Picha, K.; McCabe, F.; Millar, H.; Tawadros, R.; Tam, S.H.; Nakada, M.T.; Anderson, G.M. CNTO 859, a humanized anti-tissue factor monoclonal antibody, is a potent inhibitor of breast cancer metastasis and tumor growth in xenograft models. Int. J. Cancer 2006, 120, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Versteeg, H.H.; Schaffner, F.; Kerver, M.; Petersen, H.H.; Ahamed, J.; Felding-Habermann, B.; Takada, Y.; Mueller, B.M.; Ruf, W. Inhibition of tissue factor signaling suppresses tumor growth. Blood 2008, 111, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Shen, R.; Campbell, A.; McMichael, E.; Yu, L.; Ramaswamy, B.; London, C.A.; Xu, T.; Carson, W.E., 3rd. Targeting Tissue Factor for Immunotherapy of Triple-Negative Breast Cancer Using a Second-Generation ICON. Cancer Immunol. Res. 2018, 6, 671–684. [Google Scholar] [CrossRef]
- Hong, H.; Zhang, Y.; Nayak, T.R.; Engle, J.W.; Wong, H.C.; Liu, B.; Barnhart, T.E.; Cai, W. Immuno-PET of Tissue Factor in Pancreatic Cancer. J. Nucl. Med. 2012, 53, 1748–1754. [Google Scholar] [CrossRef]
- Petersen, L.C.; Nørby, P.L.; Branner, S.; Sørensen, B.B.; Elm, T.; Stennicke, H.R.; Persson, E.; Bjørn, S.E. Characterization of recombinant murine factor VIIa and recombinant murine tissue factor: A human–murine species compatibility study. Thromb. Res. 2005, 116, 75–85. [Google Scholar] [CrossRef]
- Knudsen, T.; Olsen, O.H.; Petersen, L.C. Tissue factor and factor VIIa cross-species compatibility. Front. Biosci. 2011, 16, 3196–3215. [Google Scholar] [CrossRef]
- Nielsen, C.H.; Erlandsson, M.; Jeppesen, T.E.; Jensen, M.M.; Kristensen, L.K.; Madsen, J.; Petersen, L.C.; Kjaer, A. Quantitative PET Imaging of Tissue Factor Expression Using 18F-Labeled Active Site–Inhibited Factor VII. J. Nucl. Med. 2015, 57, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, B.B.; Persson, E.; Freskgård, P.O.; Kjalke, M.; Ezban, M.; Williams, T.; Rao, L.V. Incorporation of an active site inhibitor in factor VIIa alters the affinity for tissue factor. J. Biol. Chem. 1997, 272, 11863–11868. [Google Scholar] [CrossRef] [PubMed]
- Loft, M.; Christensen, C.; Clausen, M.M.; Carlsen, E.A.; Hansen, C.P.; Kroman, N.; Langer, S.W.; Hoegdall, C.; Madsen, J.; Gillings, N.; et al. First-in-Humans PET Imaging of Tissue Factor in Patients with Primary and Metastatic Cancers Using18F-labeled Active-Site Inhibited Factor VII (18F-ASIS): Potential as Companion Diagnostic. J. Nucl. Med. 2022, 63, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Leroyer, A.S.; Blin, M.G.; Bachelier, R.; Bardin, N.; Blot-Chabaud, M.; Dignat-George, F. CD146 (Cluster of Differentiation 146). Arter. Thromb. Vasc. Biol. 2019, 39, 1026–1033. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, W.; Lu, D.; Yan, X. CD146, an epithelial-mesenchymal transition inducer, is associated with triple-negative breast cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 1127–1132. [Google Scholar] [CrossRef]
- de Kruijff, I.E.; Timmermans, A.M.; den Bakker, M.A.; Trapman-Jansen, A.M.A.C.; Foekens, R.; Meijer-Van Gelder, M.E.; Oomen-de Hoop, E.; Smid, M.; Hollestelle, A.; van Deurzen, C.H.M.; et al. The Prevalence of CD146 Expression in Breast Cancer Subtypes and Its Relation to Outcome. Cancers 2018, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.A.; Kang, L.; Li, C.; Kamkaew, A.; Barrett, K.E.; Aluicio-Sarduy, E.; Yang, Y.; Engle, J.W.; Jiang, D.; Cai, W. ImmunoPET of the differential expression of CD146 in breast cancer. Am. J. Cancer Res. 2021, 11, 1586–1599. [Google Scholar] [PubMed]
- Li, C.; Kang, L.; Fan, K.; Ferreira, C.A.; Becker, K.V.; Huo, N.; Liu, H.; Yang, Y.; Engle, J.W.; Wang, R.; et al. ImmunoPET of CD146 in Orthotopic and Metastatic Breast Cancer Models. Bioconjugate Chem. 2021, 32, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, M.; Younis, M.H.; Barnhart, T.E.; Jiang, D.; Sun, T.; Lang, J.M.; Engle, J.W.; Zhou, M.; Cai, W. ImmunoPET of trophoblast cell-surface antigen 2 (Trop-2) expression in pancreatic cancer. Eur. J. Nucl. Med. 2021, 49, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Xia, L.; Zhang, Z.; Ren, Y.; Pomper, M.G.; Rowe, S.P.; Li, X.; Li, N.; Zhang, N.; Zhu, H.; et al. First-in-Human Study of the Radioligand 68Ga-N188 Targeting Nectin-4 for PET/CT Imaging of Advanced Urothelial Carcinoma. Clin. Cancer Res. 2023, OF1–OF13. [Google Scholar] [CrossRef]
- Vag, T.; Steiger, K.; Rossmann, A.; Keller, U.; Noske, A.; Herhaus, P.; Ettl, J.; Niemeyer, M.; Wester, H.-J.; Schwaiger, M. PET imaging of chemokine receptor CXCR4 in patients with primary and recurrent breast carcinoma. EJNMMI Res. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Werner, R.A.; Kircher, S.; Higuchi, T.; Kircher, M.; Schirbel, A.; Wester, H.-J.; Buck, A.K.; Pomper, M.G.; Rowe, S.P.; Lapa, C. CXCR4-directed imaging in solid tumors. Front. Oncol. 2019, 9, 770. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Cavaliere, A.; Li, Z.; Huang, Y.; Marquez-Nostra, B. Preclinical Advances in Theranostics for the Different Molecular Subtypes of Breast Cancer. Front. Pharmacol. 2021, 12, 627693. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, S.; Niederstadt, L.; Koziolek, E.J.; Gómez, J.D.C.; Prasad, S.; Wagener, A.; von Hacht, J.L.; Reinicke, S.; Exner, S.; Bandholtz, S.; et al. CMKLR1-targeting peptide tracers for PET/MR imaging of breast cancer. Theranostics 2019, 9, 6719–6733. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.A.; Schubert, R.D.; Peter, R.U.; Kraut, N.; Park, J.E.; Rettig, W.J.; Garin-Chesa, P. Fibroblast Activation Protein: Differential Expression and Serine Protease Activity in Reactive Stromal Fibroblasts of Melanocytic Skin Tumors. J. Investig. Dermatol. 2003, 120, 182–188. [Google Scholar] [CrossRef]
- Kakarla, S.; Song, X.T.; Gottschalk, S. Cancer-associated fibroblasts as targets for immunotherapy. Immunotherapy 2012, 4, 1129–1138. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Zhang, H.; An, J.; Wu, P.; Zhang, C.; Zhao, Y.; Tan, D.; Shi, C.; Ge, X. The Application of [68Ga]-Labeled FAPI-04 PET/CT for Targeting and Early Detection of Pancreatic Carcinoma in Patient-Derived Orthotopic Xenograft Models. Contrast Media Mol. Imaging 2022, 2022, 6596702. [Google Scholar] [CrossRef]
- Watabe, T.; Liu, Y.; Kaneda-Nakashima, K.; Shirakami, Y.; Lindner, T.; Ooe, K.; Toyoshima, A.; Nagata, K.; Shimosegawa, E.; Haberkorn, U.; et al. Theranostics Targeting Fibroblast Activation Protein in the Tumor Stroma: 64Cu- and 225Ac-Labeled FAPI-04 in Pancreatic Cancer Xenograft Mouse Models. J. Nucl. Med. 2020, 61, 563–569. [Google Scholar] [CrossRef]
- Zboralski, D.; Hoehne, A.; Bredenbeck, A.; Schumann, A.; Nguyen, M.; Schneider, E.; Ungewiss, J.; Paschke, M.; Haase, C.; von Hacht, J.L.; et al. Preclinical evaluation of FAP-2286 for fibroblast activation protein targeted radionuclide imaging and therapy. Eur. J. Nucl. Med. 2022, 49, 3651–3667. [Google Scholar] [CrossRef]
- Van Rymenant, Y.; Tanc, M.; Van Elzen, R.; Bracke, A.; De Wever, O.; Augustyns, K.; Lambeir, A.-M.; Kockx, M.; De Meester, I.; Van Der Veken, P. In Vitro and In Situ Activity-Based Labeling of Fibroblast Activation Protein with UAMC1110-Derived Probes. Front. Chem. 2021, 9, 640566. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.S.; Elvas, F.; Vliegen, G.; De Lombaerde, S.; Vangestel, C.; De Bruycker, S.; Bracke, A.; Eppard, E.; Greifenstein, L.; Klasen, B.; et al. Targeting fibroblast activation protein (FAP): Next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA5m chelators. EJNMMI Radiopharm. Chem. 2020, 5, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, P.; Burg, M.C.; Roll, W.; Büther, F.; Breyholz, H.-J.; Weigel, S.; Heindel, W.; Pixberg, M.; Barth, P.; Tio, J.; et al. Simultaneous FAPI PET/MRI Targeting the Fibroblast-Activation Protein for Breast Cancer. Radiology 2022, 302, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.J.; Roselt, P.J.; Kallur, K.G.; Tothill, R.W.; Mileshkin, L. FAPI PET/CT: Will It End the Hegemony of 18F-FDG in Oncology? J. Nucl. Med. 2020, 62, 296–302. [Google Scholar] [CrossRef]
- Calais, J.; Mona, C.E. Will FAPI PET/CT Replace FDG PET/CT in the Next Decade? Point-An Important Diagnostic, Phenotypic, and Biomarker Role. AJR. Am. J. Roentgenol. 2021, 216, 305–306. [Google Scholar] [CrossRef]
- Moradi, F.; Iagaru, A. Will FAPI PET/CT Replace FDG PET/CT in the Next Decade? Counterpoint-No, Not So Fast! AJR Am. J. Roentgenol. 2021, 216, 307–308. [Google Scholar] [CrossRef]
- Liu, Y.; Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Naka, S.; Ooe, K.; Toyoshima, A.; Nagata, K.; Haberkorn, U.; Kratochwil, C.; et al. Fibroblast activation protein targeted therapy using [177Lu]FAPI-46 compared with [225Ac]FAPI-46 in a pancreatic cancer model. Eur. J. Nucl. Med. 2021, 49, 871–880. [Google Scholar] [CrossRef]
- Capaccione, K.M.; Doubrovin, M.; Braumuller, B.; Leibowitz, D.; Bhatt, N.; Momen-Heravi, F.; Molotkov, A.; Kissner, M.; Goldner, K.; Soffing, M.; et al. Evaluating the Combined Anticancer Response of Checkpoint Inhibitor Immunotherapy and FAP-Targeted Molecular Radiotherapy in Murine Models of Melanoma and Lung Cancer. Cancers 2022, 14, 4575. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Costa, P.F.; Kessler, L.; Weber, M.; Hirmas, N.; Kostbade, K.; Bauer, S.; Schuler, M.; Ahrens, M.; Schildhaus, H.-U.; et al. Initial clinical experience with 90Y-FAPI-46 radioligand therapy for advanced stage solid tumors: A case series of nine patients. J. Nucl. Med. 2021, 63, 727–734. [Google Scholar] [CrossRef]
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C.; et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J. Pathol. 2013, 232, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, B.L.; Galon, J.; Nayler, S.; Mlecnik, B.; Fugon, A.; Benn, C.A.; Martel, M.; Cronje, T.; Smit, T.; Moosa, F.; et al. 46P Tumor infiltrating lymphocytes in breast cancer: High levels of CD3, CD8 cells and ImmunoscoreR are as-sociated with pathological CR in patients receiving neo-adjuvant chemotherapy. Ann. Oncol. 2020, 31 (Suppl. 2), S31–S32. [Google Scholar] [CrossRef]
- Larimer, B.M.; Wehrenberg-Klee, E.; Caraballo, A.; Mahmood, U. Quantitative CD3 PET Imaging Predicts Tumor Growth Response to Anti-CTLA-4 Therapy. J. Nucl. Med. 2016, 57, 1607–1611. [Google Scholar] [CrossRef]
- Kist de Ruijter, L.; van de Donk, P.P.; Hooiveld-Noeken, J.S.; Giesen, D.; Elias, S.G.; Lub-de Hooge, M.N.; Oosting, S.F.; Jalving, M.; Timens, W.; Brouwers, A.H.; et al. Whole-body CD8+ T cell visualization before and during cancer immunotherapy: A phase 1/2 trial. Nat. Med. 2022, 28, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Shankar, L.K.; Schöder, H.; Sharon, E.; Wolchok, J.; Knopp, M.V.; Wahl, R.L.; Ellingson, B.M.; Hall, N.C.; Yaffe, M.J.; Towbin, A.J.; et al. Harnessing imaging tools to guide immunotherapy trials: Summary from the National Cancer Institute Cancer Imaging Steering Committee workshop. Lancet Oncol. 2023, 24, e133–e143. [Google Scholar] [CrossRef]
- Poh, A.R.; Ernst, M. Targeting Macrophages in Cancer: From Bench to Bedside. Front. Oncol. 2018, 8, 49. [Google Scholar] [CrossRef]
- Li, C.; Xu, X.; Wei, S.; Jiang, P.; Xue, L.; Wang, J. Tumor-associated macrophages: Potential therapeutic strategies and future prospects in cancer. J. Immunother. Cancer 2021, 9, e001341. [Google Scholar] [CrossRef]
- Fernandes, B.; Feltes, P.K.; Luft, C.; Nazario, L.R.; Jeckel, C.M.M.; Antunes, I.F.; Elsinga, P.H.; de Vries, E.F.J. Potential PET tracers for imaging of tumor-associated macrophages. EJNMMI Radiopharm. Chem. 2022, 7, 1–19. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Liu, J.-W.; Lu, C.; Wei, J.-F. CAR-T Cell Therapy for Breast Cancer: From Basic Research to Clinical Application. Int. J. Biol. Sci. 2022, 18, 2609–2626. [Google Scholar] [CrossRef]
- Woodham, A.W.; Zeigler, S.H.; Zeyang, E.L.; Kolifrath, S.C.; Cheloha, R.W.; Rashidian, M.; Chaparro, R.J.; Seidel, R.D.; Garforth, S.J.; Dearling, J.L.; et al. In vivo detection of antigen-specific CD8+ T cells by immuno-positron emission tomography. Nat. Methods 2020, 17, 1025–1032. [Google Scholar] [CrossRef]
- Larimer, B.M.; Wehrenberg-Klee, E.; Dubois, F.; Mehta, A.; Kalomeris, T.; Flaherty, K.; Boland, G.; Mahmood, U. Granzyme B PET Im-aging as a Predictive Biomarker of Immunotherapy Response. Cancer Res. 2017, 77, 2318–2327. [Google Scholar] [CrossRef] [PubMed]
- Simonetta, F.; Alam, I.S.; Lohmeyer, J.K.; Sahaf, B.; Good, Z.; Chen, W.; Xiao, Z.; Hirai, T.; Scheller, L.; Engels, P.; et al. Molecular Imaging of Chimeric Antigen Receptor T Cells by ICOS-ImmunoPET. Clin. Cancer Res. 2021, 27, 1058–1068. [Google Scholar] [CrossRef]
- Skovgard, M.S.; Hocine, H.R.; Saini, J.K.; Moroz, M.; Bellis, R.Y.; Banerjee, S.; Morello, A.; Ponomarev, V.; Villena-Vargas, J.; Adusumilli, P.S. Imaging CAR T-cell kinetics in solid tumors: Translational implications. Mol. Ther. Oncolytics 2021, 22, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Jiang, D.; Ehlerding, E.B.; Luo, Q.; Cai, W. Noninvasive PET Imaging of T cells. Trends Cancer 2018, 4, 359–373. [Google Scholar] [CrossRef]
- Prendergast, C.M.; Capaccione, K.M.; Lopci, E.; Das, J.P.; Shoushtari, A.N.; Yeh, R.; Amin, D.; Dercle, L.; De Jong, D. More than Just Skin-Deep: A Review of Imaging’s Role in Guiding CAR T-Cell Therapy for Advanced Melanoma. Diagnostics 2023, 13, 992. [Google Scholar] [CrossRef]
- Li, C.; Han, C.; Duan, S.; Li, P.; Alam, I.S.; Xiao, Z. Visualizing T-Cell Responses: The T-Cell PET Imaging Toolbox. J. Nucl. Med. 2021, 63, 183–188. [Google Scholar] [CrossRef]
- Krekorian, M.; Fruhwirth, G.O.; Srinivas, M.; Figdor, C.G.; Heskamp, S.; Witney, T.H.; Aarntzen, E.H. Imaging of T-cells and their responses during anti-cancer immunotherapy. Theranostics 2019, 9, 7924–7947. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fan, J.; Li, Y.; Fu, S.; Chen, Y.; Wu, J. Imaging of Tumor Hypoxia With Radionuclide-Labeled Tracers for PET. Front. Oncol. 2021, 11, 731503. [Google Scholar] [CrossRef]
- Horsman, M.R.; Mortensen, L.S.; Petersen, J.B.; Busk, M.; Overgaard, J. Imaging hypoxia to improve radiotherapy outcome. Nat. Rev. Clin. Oncol. 2012, 9, 674–687. [Google Scholar] [CrossRef]
- Reeves, K.M.; Song, P.N.; Angermeier, A.; Della Manna, D.; Li, Y.; Wang, J.; Yang, E.S.; Sorace, A.G.; Larimer, B.M. 18F-FMISO PET Imaging Identifies Hypoxia and Immunosuppressive Tumor Microenvironments and Guides Targeted Evofosfamide Therapy in Tumors Refractory to PD-1 and CTLA-4 Inhibition. Clin. Cancer Res. 2021, 28, 327–337. [Google Scholar] [CrossRef]
- Florea, A.; Mottaghy, F.M.; Bauwens, M. Molecular Imaging of Angiogenesis in Oncology: Current Preclinical and Clinical Sta-tus. Int. J. Mol. Sci. 2021, 22, 5544. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jong, D.; Desperito, E.; Al Feghali, K.A.; Dercle, L.; Seban, R.-D.; Das, J.P.; Ma, H.; Sajan, A.; Braumuller, B.; Prendergast, C.; et al. Advances in PET/CT Imaging for Breast Cancer. J. Clin. Med. 2023, 12, 4537. https://doi.org/10.3390/jcm12134537
de Jong D, Desperito E, Al Feghali KA, Dercle L, Seban R-D, Das JP, Ma H, Sajan A, Braumuller B, Prendergast C, et al. Advances in PET/CT Imaging for Breast Cancer. Journal of Clinical Medicine. 2023; 12(13):4537. https://doi.org/10.3390/jcm12134537
Chicago/Turabian Stylede Jong, Dorine, Elise Desperito, Karine A. Al Feghali, Laurent Dercle, Romain-David Seban, Jeeban P. Das, Hong Ma, Abin Sajan, Brian Braumuller, Conor Prendergast, and et al. 2023. "Advances in PET/CT Imaging for Breast Cancer" Journal of Clinical Medicine 12, no. 13: 4537. https://doi.org/10.3390/jcm12134537
APA Stylede Jong, D., Desperito, E., Al Feghali, K. A., Dercle, L., Seban, R.-D., Das, J. P., Ma, H., Sajan, A., Braumuller, B., Prendergast, C., Liou, C., Deng, A., Roa, T., Yeh, R., Girard, A., Salvatore, M. M., & Capaccione, K. M. (2023). Advances in PET/CT Imaging for Breast Cancer. Journal of Clinical Medicine, 12(13), 4537. https://doi.org/10.3390/jcm12134537