Clinical Application of the HCM-AF Risk Score in the Prediction of Clinical Outcomes of Polish Patients with Hypertrophic Cardiomyopathy
Abstract
1. Introduction
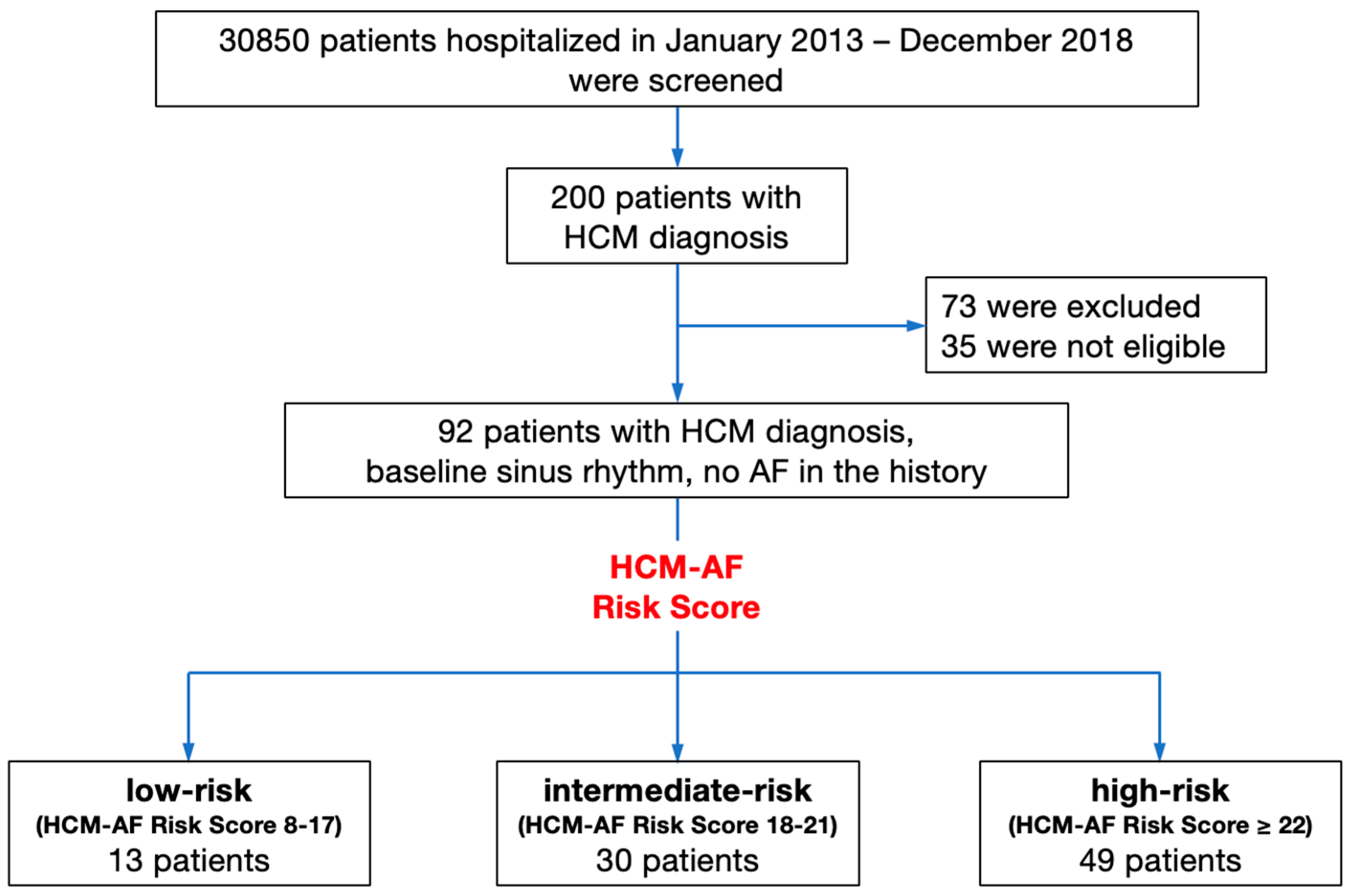
2. Materials and Methods
- AF was defined as any form of AF (paroxysmal, persistent, permanent).
- Clinical endpoints of the study:
- The primary endpoint was the AF occurrence in the 2- and 5-year follow-ups.
- The secondary endpoints were the total mortality, rehospitalisation due to HF, and the complex endpoints including both total mortality and rehospitalisation due to HF.
- Other data on the clinical outcomes of HF include the progression of HF defined as increase ≥1 of stage in the NYHA scale; the regression of HF defined as decrease ≥1 of stage in the NYHA scale.
3. Results
3.1. Participants’ Clinical Characteristics
3.2. Registered AF Incidence and HCM-AF Risk Score
3.3. Outcome Data
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New Perspectives on the Prevalence of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- European Commission; Eurostat. Eurostat Regional Yearbook—2022 Edition; Publications Office: Brussels, Belgium, 2022. [Google Scholar] [CrossRef]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.; Khan, S.; Arora, S.; Kumar, V.; Naraparaju, V.; Lahewala, S.; Sharma, P.; Atti, V.; Jain, V.; Shah, M.; et al. Burden and trends of arrhythmias in hypertrophic cardiomyopathy and its impact of mortality and resource utilization. J. Arrhythm. 2019, 35, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Rowin, E.J.; Hausvater, A.; Link, M.S.; Abt, P.; Gionfriddo, W.; Wang, W.; Rastegar, H.; Estes, N.A.M.; Maron, M.S.; Maron, B.J. Clinical profile and consequences of atrial fibrillation in hypertrophic cardiomyopathy. Circulation 2017, 136, 2420–2436. [Google Scholar] [CrossRef] [PubMed]
- Siontis, K.C.; Geske, J.B.; Ong, K.; Nishimura, R.A.; Ommen, S.R.; Gersh, B.J. Atrial Fibrillation in Hypertrophic Cardiomyopathy: Prevalence, Clinical Correlations, and Mortality in a Large High-Risk Population. J. Am. Coll. Cardiol. 2014, 3, e001002. [Google Scholar] [CrossRef] [PubMed]
- Derejko, P.; Polańska, M.; Chojnowska, L.; Michałowska, I.; Wójcik, A.; Piotrowicz, E.; Lech, A.; Kłopotowski, M.; Baranowski, R.; Przybylski, A.; et al. Catheter ablation of atrial fibrillation in patients with hypertrophic cardiomyopathy: Atrial fibrillation type determines the success rate. Kardiol. Pol. 2013, 71, 17–24. [Google Scholar] [PubMed]
- Carrick, R.T.; Maron, M.S.; Adler, A.; Wessler, B.; Hoss, S.; Chan, R.H.; Sridharan, A.; Huang, D.; Cooper, C.; Drummond, J.; et al. Development and Validation of a Clinical Predictive Model for Identifying Hypertrophic Cardiomyopathy Patients at Risk for Atrial Fibrillation: The HCM-AF Score. Circ. Arrhythm. Electrophysiol. 2021, 14, e009796. [Google Scholar] [CrossRef] [PubMed]
- Ntusi, N.A.; Sliwa, K. Associations of Race and Ethnicity With Presentation and Outcomes of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2021, 78, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Eberly, L.A.; Day, S.M.; Ashley, E.A.; Jacoby, D.L.; Jefferies, J.L.; Colan, S.D.; Rossano, J.W.; Semsarian, C.; Pereira, A.C.; Olivotto, I.; et al. Association of Race With Disease Expression and Clinical Outcomes Among Patients With Hypertrophic Cardiomyopathy. JAMA Cardiol. 2020, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Y.; Sun, K.; Wang, J.; Zou, Y.; Zhang, W.; Bao, J.; Zhu, L.; Shen, H.; Hui, R.; et al. Clinical profile and prognostic significance of atrial fibrillation in hypertrophic cardiomyopathy. Cardiology 2013, 126, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Dragasis, S.; Vlachos, K.; Kariki, O.; Koskina, S.; Zygouri, A.; Patsiotis, I.G.; Anastasakis, A.; Athanasopoulos, G.; Ritsatos, K.; Letsas, K.; et al. Atrial fibrillation in hypertrophic cardiomyopathy—A contemporary mini-review. Hell. J. Cardiol. 2022, 67, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Authors/Task Force Members; Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [PubMed]
- Alonso, A.; Krijthe, B.P. Simple Risk Model Predicts Incidence of Atrial Fibrillation in a Racially ang Geographically Diverse Population: The CHARGE-AF Consortium. J. Am. Heart Assoc. 2013, 2, e000102. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Friedman, S.; Reeder, C.; Di Achille, P.; Diamant, N.; Singh, P.; Harrington, L.X.; Wang, X.; Al-Alusi, M.A.; Sarma, G.; et al. ECG-Based Deep Learning and Clinical Risk Factors to Predict Atrial Fibrilation. Circulation 2022, 145, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Himmelreich, J.C.L.; Lucassen, W.A.M.; Harskamp, R.E.; Aussems, C.; Van Weert, H.C.P.M.; Nielen, M.M.J. CHARGE-AF in a national routine primary care electronic health records database in the Netherlands: Validation for 5-year risk of atrial fibrillation and implications for patient selection in atrial fibrillation screening. Open Heart 2021, 8, e001459. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-G.; Pastori, D.; Farcomeni, A.; Yang, P.-S.; Jang, E.; Joung, B.; Wang, Y.-T.; Guo, Y.-T.; Lip, G.Y.H. A Simple Clinical Risk Score (C2HEST) for Predicting Incident Atrial Fibrillation in Asian Subjects: Derivation in 471,446 Chinese Subjects, with Internal Validation and External Application in 451,199 Korean Subjects. Chest 2019, 155, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Falasconi, G.; Pannone, L.; Slavich, M.; Margonato, A.; Fragasso, G.; Spoladore, R. Atrial fibrillation in hypertrophic cardiomyopathy: Pathophysiology, diagnosis and management. Am. J. Cardiovasc. Dis. 2020, 10, 409–418. [Google Scholar] [PubMed]
- Bhattacharya, M.; Lu, D.-Y.; Ventoulis, I.; Greenland, G.V.; Yalcin, H.; Guan, Y.; Marine, J.E.; Olgin, J.E.; Zimmerman, S.L.; Abraham, T.P.; et al. Machine Learning Methods for Identifying Atrial Fibrillation Cases and Their Predictors in Patients With Hypertrophic Cardiomyopathy: The HCM-AF-Risk Model. CJC Open 2021, 3, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Sullivan, L.M.; Levy, D.; Pencina, M.J.; Massaro, J.M.; D’Agostino, R.B.; Newton-Cheh, C.; Yamamoto, J.F.; Magnani, J.W.; Tadros, T.M.; et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): A community-based cohort study. Lancet 2009, 373, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, A.M.; Agarwal, S.K.; Folsom, A.R.; Soliman, E.Z.; Chambless, L.E.; Crow, R.; Ambrose, M.; Alonso, A. A Clinical Risk Score for Atrial Fibrillation in a Biracial Prospective Cohort (from the Atherosclerosis Risk In Communities [ARIC] Study). Am. J. Cardiol. 2011, 107, 85–91. [Google Scholar] [CrossRef] [PubMed]


| Overall N = 92 (100%) | Low Risk N = 13 (14.1%) | Intermediate Risk N = 30 (32.6%) | High Risk N = 49 (53.3%) | |
|---|---|---|---|---|
| sex—male N (%) | 46 (50.0) | 7 (53.8) | 14 (46.7) | 25 (51.0) |
| age at HCM diagnosis [years] | 46.5 ± 15.9 | 31.7 ± 13.1 * | 44.2 ± 11.1 | 51.8 ± 15.4 |
| NYHA class | ||||
| class I N (%) | 29 (31.5%) | 12 (92.3%) * | 12 (40.0%) | 5 (10.2%) |
| class II N (%) | 37 (40.2%) | 1 (7.7%) * | 11 (36.7%) | 25 (51.0%) |
| class III N (%) | 21 (22.8%) | 0 (0%) * | 6 (20.0%) | 15 (30.6%) |
| class IV N (%) | 5 (5.5%) | 0 (0%) * | 1 (3.3%) | 4 (8.2%) |
| BMI [kg/m2] | 26.1 ± 5.3 | 24.9 ± 3.8 * | 24.5 ± 5.5 | 29.1 ± 4.6 |
| TTE baseline parametes: | ||||
| LV EDD [mm] | 48.1 ± 7.8 | 45.8 ± 5.3 * | 46.2 ± 8.2 | 49.0 ± 7.5 |
| IVSd [mm] | 17.5 ± 5.2 | 18.5 ± 7.7 | 17.9 ± 5.0 | 17.9 ± 4.0 |
| PWd [mm] | 11.3 ± 2.7 | 10.3 ± 2.7 | 11.6 ± 2.6 | 11.8 ± 2.7 |
| LVMI [g/m2] | 160.0 ± 54.1 | 153.8 ± 70.4 | 144.7 ± 53.4 | 172.8 ± 48.1 |
| LV EF (%) | 52.3 ± 13.1 | 56.2 ± 3.5 | 57.7 ± 9.1 | 50.6 ± 13.6 |
| LA diameter [mm] | 43.8 ± 8.8 | 36 ± 6.4 * | 39.7 ± 4.3 | 49.9 ± 9.6 |
| LA area [cm2] | 26.9 ± 8.2 | 20.5 ± 4.2 * | 27.1 ± 8.0 | 32.3 ± 8.1 |
| nsVT N (%) | 22 (23.9%) | 5 (38.5%) * | 5 (16.7%) | 6 (12.4%) |
| ICD implantation during follow-up | 30 (31.6%) | 4 (75.0%) * | 5 (16.7%) | 21 (42.9%) |
| Subgroup | HCM-AF Estimated Risk | Registered AF Incidence | |
|---|---|---|---|
| Low risk (N = 13) | 2-year follow-up | 1.1% | 7.7% |
| 5-year follow-up | 2.6% | 15.4% | |
| Intermediate Risk (N = 30) | 2-year follow-up | 3.5% | 16.7% |
| 5-year follow-up | 8.3% | 40.0% | |
| High risk (N = 49) | 2-year follow-up | 13.6% | 46.9% |
| 5-year follow-up | 29.0% | 51.0% |
| Overall (N = 92) | Low Risk (N = 13) | Intermediate Risk (N = 30) | High Risk (N = 49) | ||
|---|---|---|---|---|---|
| Primary endpoint | AF at 2-year | 29 (31.5%) | 1 (7.7%) * | 5 (16.7%) | 23 (46.9%) |
| AF at 5-year | 40 (43.5%) | 2 (15.4%) * | 12 (40.0%) | 25 (51.0%) | |
| Secondary endpoints | total mortality | 23 (42.6%) | 2 (15.4%) * | 6 (20.0%) | 21 (42.9%) |
| rehospitalisation due to AF | 54 (58.7%) | 3 (23.1%) * | 16 (53.3%) | 35 (71.4%) | |
| complex: total mortality and rehospitalisation due to HF | 59 (64.1%) | 3 (23.1%) * | 18 (60.0%) | 38 (77.6%) | |
| Other data on clinical outcomes | HF progression | 25 (27.2%) | 0 (0.0%) * | 7 (23.3%) | 18 (36.7%) |
| no change in HF | 51 (55.4%) | 13 (100.0%) * | 17 (56.7%) | 21 (42.9%) | |
| HF regression | 16 (17.4%) | 0 (0.0%) | 6 (20.0%) | 10 (20.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stec, M.; Suleja, A.; Gondko, D.; Kuczmik, W.; Roman, J.; Dziadosz, D.; Szydło, K.; Mizia-Stec, K. Clinical Application of the HCM-AF Risk Score in the Prediction of Clinical Outcomes of Polish Patients with Hypertrophic Cardiomyopathy. J. Clin. Med. 2023, 12, 4484. https://doi.org/10.3390/jcm12134484
Stec M, Suleja A, Gondko D, Kuczmik W, Roman J, Dziadosz D, Szydło K, Mizia-Stec K. Clinical Application of the HCM-AF Risk Score in the Prediction of Clinical Outcomes of Polish Patients with Hypertrophic Cardiomyopathy. Journal of Clinical Medicine. 2023; 12(13):4484. https://doi.org/10.3390/jcm12134484
Chicago/Turabian StyleStec, Maria, Agata Suleja, Daniel Gondko, Wiktoria Kuczmik, Jakub Roman, Dominika Dziadosz, Krzysztof Szydło, and Katarzyna Mizia-Stec. 2023. "Clinical Application of the HCM-AF Risk Score in the Prediction of Clinical Outcomes of Polish Patients with Hypertrophic Cardiomyopathy" Journal of Clinical Medicine 12, no. 13: 4484. https://doi.org/10.3390/jcm12134484
APA StyleStec, M., Suleja, A., Gondko, D., Kuczmik, W., Roman, J., Dziadosz, D., Szydło, K., & Mizia-Stec, K. (2023). Clinical Application of the HCM-AF Risk Score in the Prediction of Clinical Outcomes of Polish Patients with Hypertrophic Cardiomyopathy. Journal of Clinical Medicine, 12(13), 4484. https://doi.org/10.3390/jcm12134484









