The Resolution of Periodontal Inflammation Promotes Changes in Cytokine Expression in the Intestine and Gingival Tissues of Aged Rats with DSS-Induced Colitis
Abstract
1. Introduction
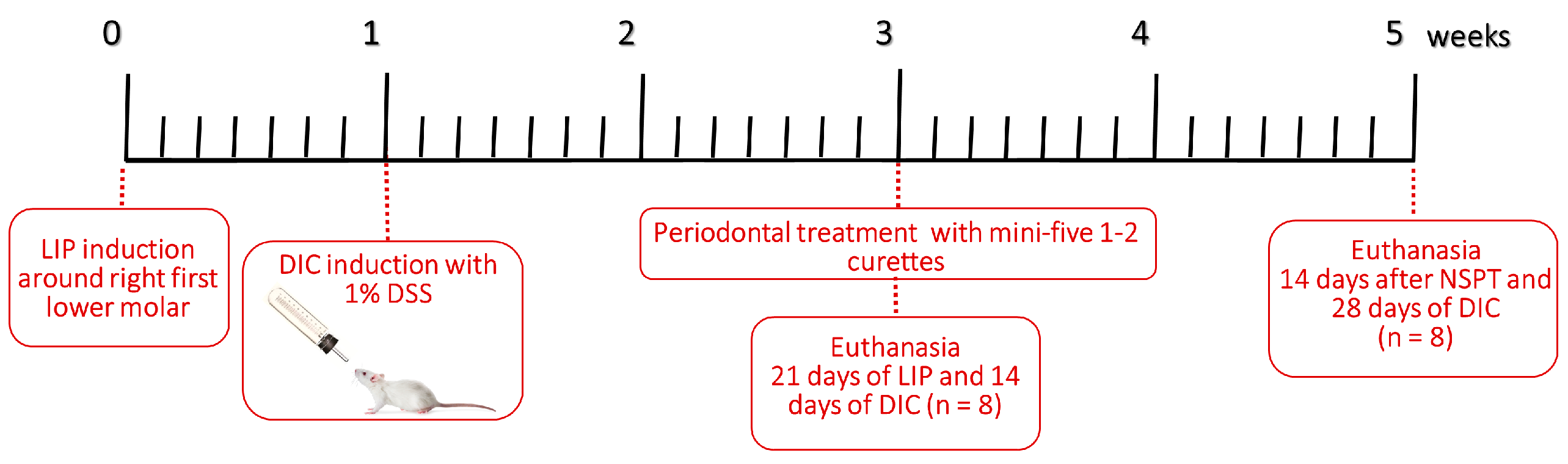
2. Materials and Methods
2.1. Animals
2.2. Ligature-Induced Periodontitis
2.3. Induction of DSS-Induced Colitis (DIC)
2.4. Resolution of Periodontal Inflammation
2.5. Clinical Assessment
2.6. Sample Collection and Euthanasia
2.7. Cytokine Analysis
2.8. Processing for Histology
2.9. Histopathological Evaluation
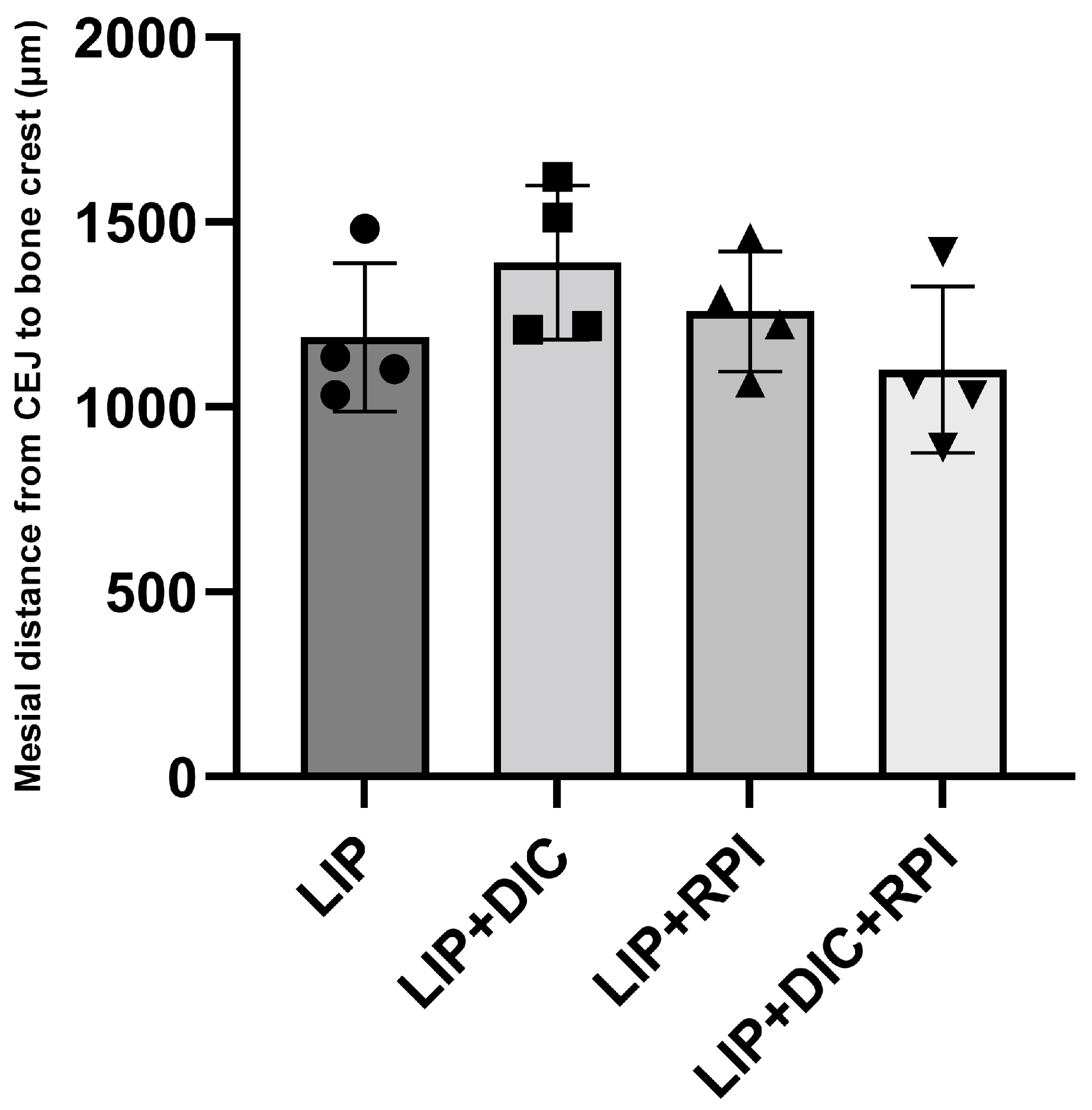
2.10. Histometric Analysis
2.11. Statistical Analysis
3. Results
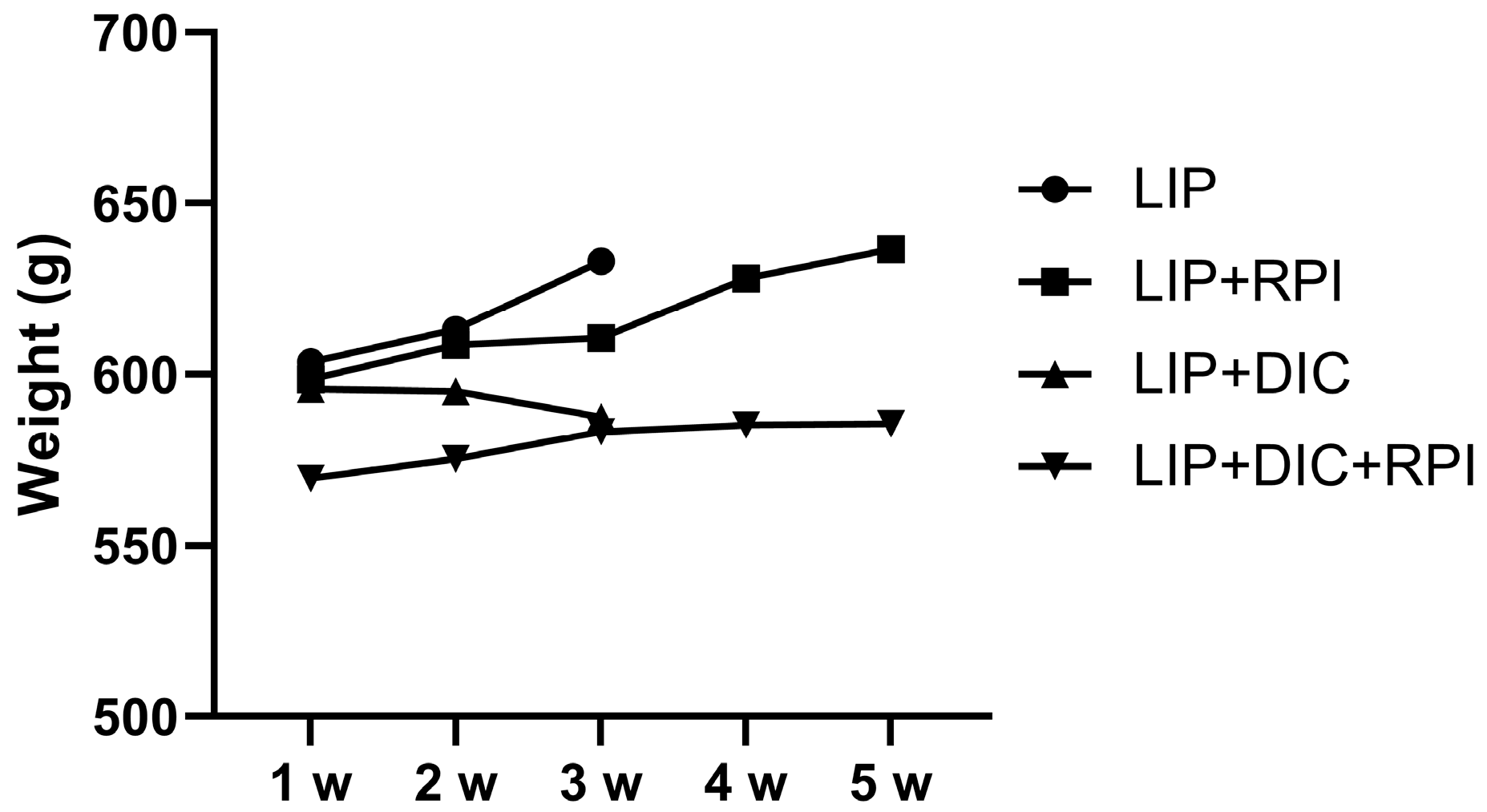
3.1. Clinical Assessment
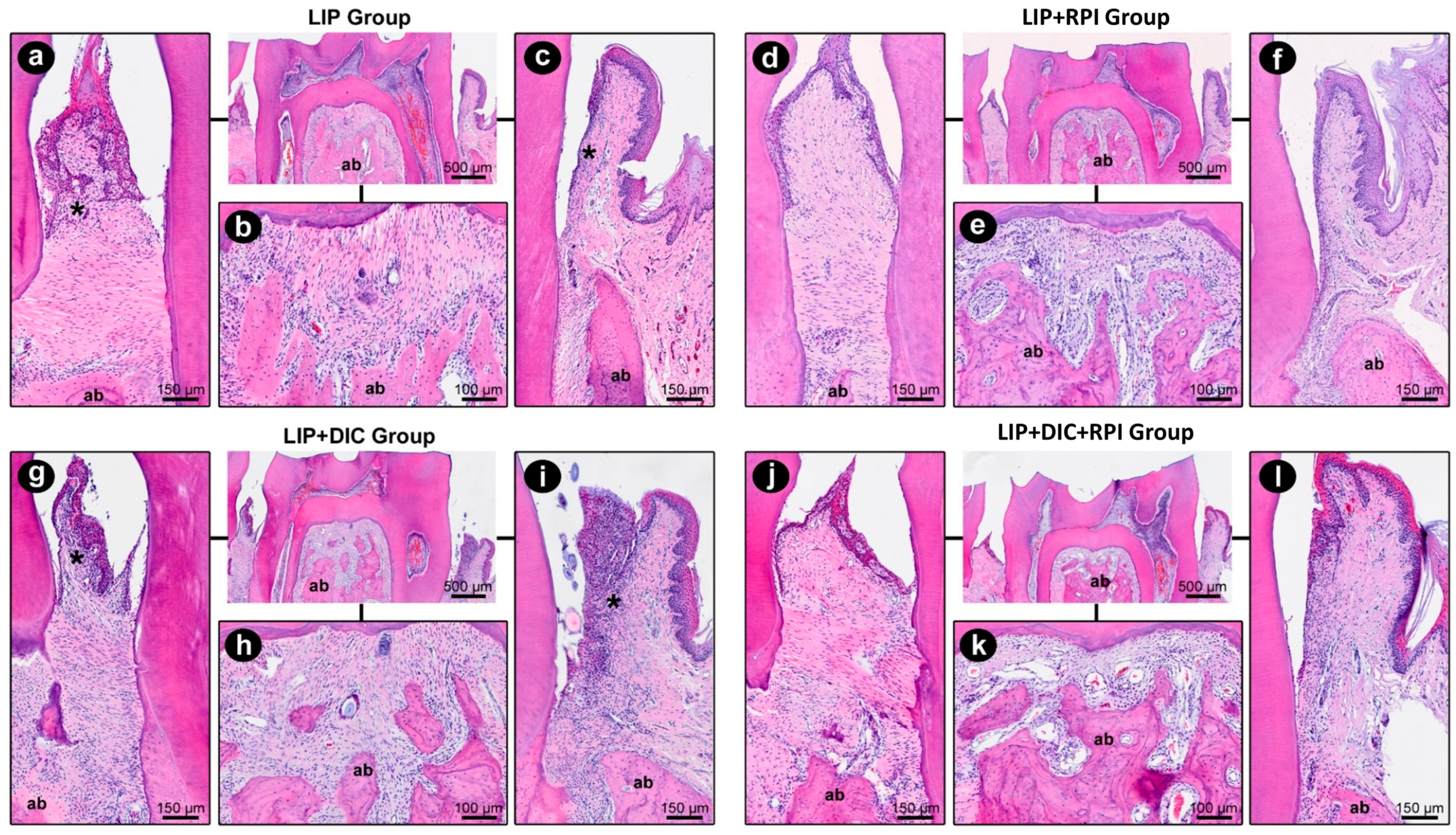
3.2. Histopathological and Histometric Analyses
3.2.1. Ligature-Induced Periodontitis
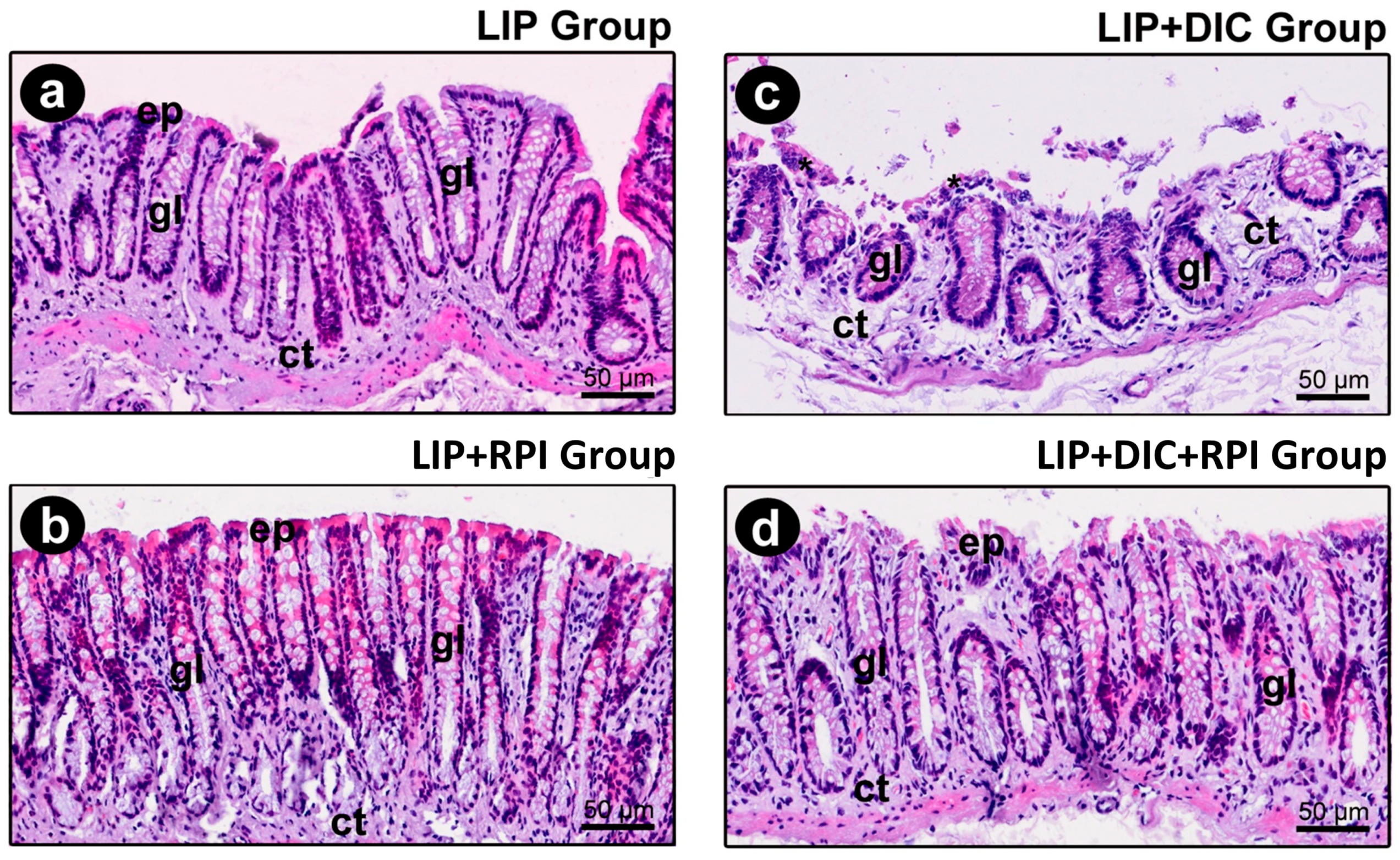
3.2.2. DSS-Induced Colitis
3.3. Immunological Analyses
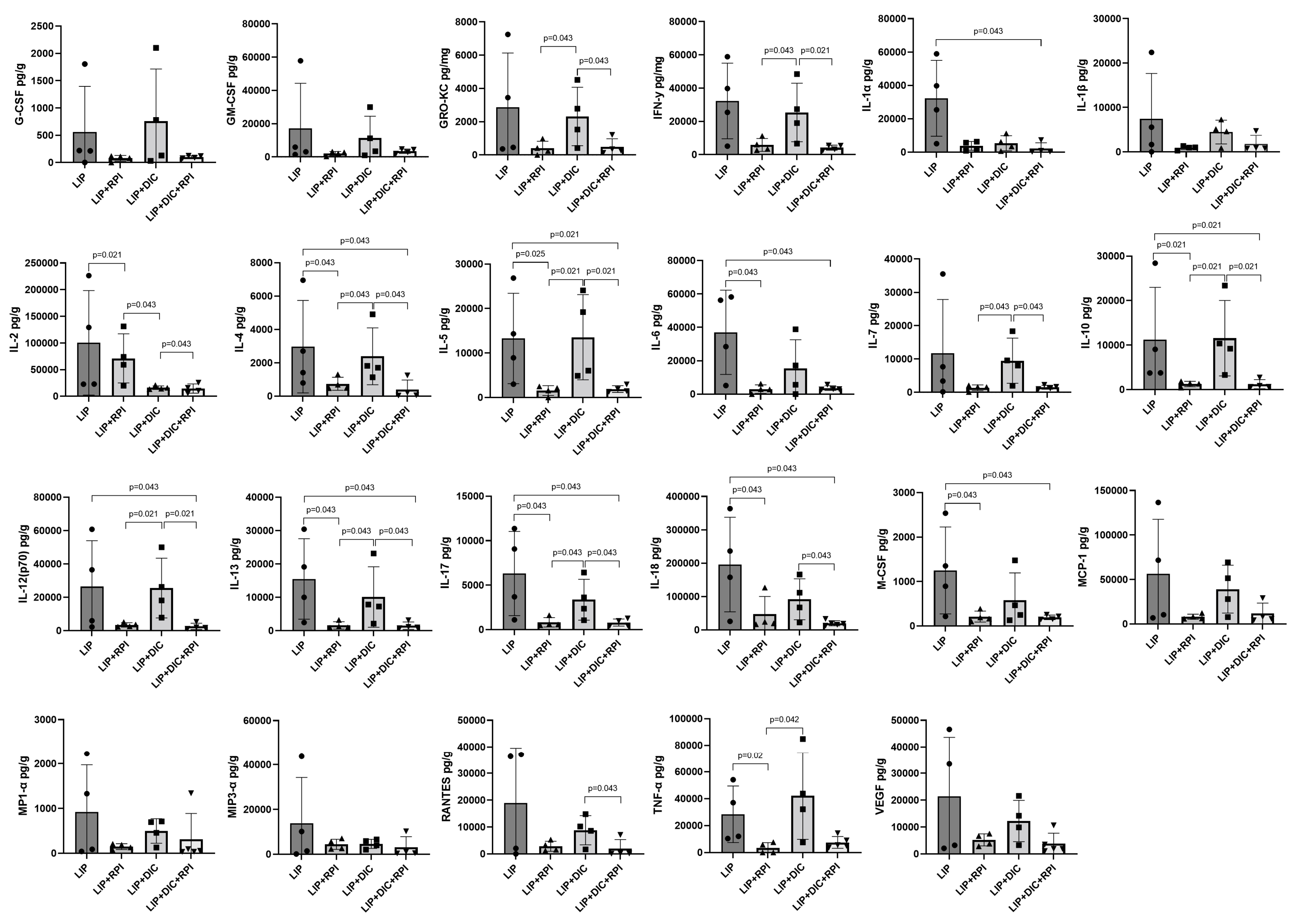
3.3.1. Gingival Cytokine Expression
3.3.2. Intestinal Cytokine Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byrd, K.M.; Gulati, A.S. The “Gum-Gut” Axis in Inflammatory Bowel Diseases: A Hypothesis-Driven Review of Associations and Advances. Front. Immunol. 2021, 12, 620124. [Google Scholar] [CrossRef] [PubMed]
- She, Y.Y.; Kong, X.B.; Ge, Y.P.; Liu, Z.Y.; Chen, J.Y.; Jiang, J.W.; Jiang, H.B.; Fang, S.L. Periodontitis and inflammatory bowel disease: A meta-analysis. BMC Oral Health 2020, 20, 67. [Google Scholar] [CrossRef] [PubMed]
- Kitamoto, S.; Nagao-Kitamoto, H.; Jiao, Y.; Gillilland, M.G., 3rd; Hayashi, A.; Imai, J.; Sugihara, K.; Miyoshi, M.; Brazil, J.C.; Kuffa, P.; et al. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell 2020, 182, 447–462.e14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Fu, H.; Kuang, S.; He, F.; Zhang, M.; Shen, Z.; Qin, W.; Lin, Z.; Huang, S. Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int. J. Oral Sci. 2021, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- de Mello-Neto, J.M.; Elangovan, G.; Ervolino, E.; Johnson, N.W.; Gustafsson, A.; da Figueredo, C.M. Colitis induced by dextran sulphate sodium causes histopathological and immunological changes in the periodontal tissues of Wistar rats. J. Periodontal Res. 2022, 57, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, H.; Gu, Q.; Xu, X.; Yu, R.; Huang, H. Analysis of Th-cell subsets in local and systemic environments from experimental periodontitis rats. Mol. Oral Microbiol. 2022, 38, 83–92. [Google Scholar] [CrossRef] [PubMed]
- de Mello-Neto, J.M.; Elangovan, G.; Ervolino, E.; Johnson, N.W.; Gustafsson, A.; da Silva Figueredo, C.M. Higher expression of Th1/Th2-related cytokines in the intestine of Wistar rats with ligature-induced periodontitis. J. Periodontal Res. 2023, 58, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liao, Y.; Luo, B.; Li, L.; Zhang, Y.; Yan, F. Non-surgical Periodontal Treatment Restored the Gut Microbiota and Intestinal Barrier in Apolipoprotein E(−/−) Mice With Periodontitis. Front. Cell Infect. Microbiol. 2020, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthi, I.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Vet. Clin. Pathol. 2012, 41, 27–31. [Google Scholar] [CrossRef] [PubMed]
- de Molon, R.S.; Park, C.H.; Jin, Q.; Sugai, J.; Cirelli, J.A. Characterization of ligature-induced experimental periodontitis. Microsc. Res. Tech. 2018, 81, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Ghattamaneni, N.K.R.; Panchal, S.K.; Brown, L. An improved rat model for chronic inflammatory bowel disease. Pharmacol. Rep. 2019, 71, 149–155. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.; Ervolino, E.; Bonfietti, L.H.; Novaes, V.C.; Theodoro, L.H.; Fernandes, L.A.; Martins, T.M.; Faleiros, P.L.; Garcia, V.G. Adjuvant Therapy With Sodium Alendronate for the Treatment of Experimental Periodontitis in Rats. J. Periodontol. 2015, 86, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Thorbert-Mros, S.; Cassel, B.; Berglundh, T. Age of onset of disease in subjects with severe periodontitis: A 9- to 34-year retrospective study. J. Clin. Periodontol. 2017, 44, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.D.; Logan, R.F. What is the peak age for onset of IBD? Inflamm. Bowel Dis. 2008, 14 (Suppl. S2), S4–S5. [Google Scholar] [CrossRef] [PubMed]


| PARAMETERS AND RESPECTIVE SCORES | % of Animals | |||
|---|---|---|---|---|
| EXPERIMENTAL GROUPS | ||||
| LIP | LIP + RPI | LIP + DIC | LIP + DIC + RPI | |
| INTENSITY OF LOCAL INFLAMMATORY INFILTRATE | ||||
| (1) absence of inflammation | - | 50% | - | 50% |
| (2) small quantity of inflammatory cells (1/3 of cells were inflammatory cells) | - | 50% | - | 50% |
| (3) moderate quantity of inflammatory cells (1/3 to 2/3 were inflammatory cells) | 100% | - | 25% | - |
| (4) large quantity of inflammatory cells (more than 2/3 were inflammatory cells) | - | - | 75% | - |
| EXTENSION OF INFLAMMATORY INFILTRATE | ||||
| (1) absence of inflammation | - | 50% | - | 50% |
| (2) partial extension of connective tissue | - | 50% | - | 50% |
| (3) entire extension of connective tissue, without reaching bone tissue | 100% | - | 25% | - |
| (4) entire extension of connective tissue and bone tissue | - | - | 75% | - |
| EXTERNAL RADICULAR RESORPTION (CEMENTUM AND DENTIN) | ||||
| (1) absence | - | - | - | - |
| (2) only inactive reabsorption areas | - | 50% | - | 50% |
| (3) minor active reabsorption areas | 75% | 50% | 25% | 50% |
| (4) several active reabsorption areas | 25% | - | 75% | - |
| ALVEOLAR BONE RESORPTION | ||||
| (1) within normality patterns | - | - | - | - |
| (2) small amount of resorption bone areas | - | 100% | - | 100% |
| (3) moderate amount of resorption bone areas | 75% | - | 25% | - |
| (4) large amount of resorption bone areas | 25% | - | 75% | - |
| CELLULAR PATTERN AND CONNECTIVE TISSUE STRUCTURE | ||||
| (1) moderate number of fibroblasts and large amount of collagen fibres (dense connective tissue) | - | 25% | - | - |
| (2) moderate amount of both fibroblasts and collagen fibres | - | 75% | - | 100% |
| (3) small amount of both fibroblasts and collagen fibres | 100% | - | 100% | - |
| (4) severe tissue disorganisation with necrosis areas | - | - | - | - |
| PATTERN OF STRUCTURATION OF THE BONE ALVEOLAR | ||||
| (1) bone trabeculae with regular contours coated with active osteoblasts, including areas of new bone formation | - | - | - | - |
| (2) bone trabeculae with irregular contours coated with active osteoblasts and osteoclasts | - | 100% | - | 100% |
| (3) bone trabeculae with irregular contours coated with active osteoclasts | 100% | - | 100% | - |
| (4) partial tissue breakdown with areas of bone necrosis | - | - | - | - |
| HISTOPATHOLOGICAL ANALYSES | ||||
|---|---|---|---|---|
| PARAMETERS AND SCORES | PERCENTAGE OF SPECIMENS | |||
| EXPERIMENTAL GROUPS | ||||
| LIP | LIP + RPI | LIP + DIC | LIP + DIC + RPI | |
| CELLULARITY PATTERN OF THE INTESTINAL MUCOSAL EPITHELIUM | ||||
| (1) full integrity of epithelial tissue | 100% | 100% | - | - |
| (2) punctual foci of discontinuity of the epithelial tissue (commitment <10% of the circumference of the intestinal mucosa) | - | - | 75% | 100% |
| (3) punctual foci of discontinuity of the epithelial tissue (commitment >10% and <20% of the circumference of the intestinal mucosa) | - | - | 25% | - |
| (4) large areas of discontinuity of the epithelial tissue (commitment >20% of the circumference of the intestinal mucosa) | - | - | - | - |
| INFLAMMATORY INFILTRATE PRESENT IN THE LAMINA PROPRIA | ||||
| (1) presence of rare inflammatory cells, compatible with the absence of inflammation | 100% | 100% | - | - |
| (2) presence of a small number of inflammatory cells (up to 1/3 of the cells are inflammatory) | - | - | 25% | 100% |
| (3) presence of a moderate number of inflammatory cells (1/3 to 2/3 of the cells are inflammatory | - | - | 75% | - |
| (4) presence of a large number of inflammatory cells (more than 2/3 of the cells are inflammatory) | - | - | - | - |
| EXTENSION OF THE INFLAMMATORY PROCESS IN THE INTESTINAL WALL | ||||
| (1) absence of inflammation | 100% | 100% | - | - |
| (2) reaching exclusively the intestinal mucosa | - | - | 100% | 100% |
| (3) reaching the mucosa and submucosa of the intestine | - | - | - | - |
| (4) reaching the mucosa, submucosa, muscularis, and serosa of the intestine | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Mello-Neto, J.M.; Ervolino, E.; Elangovan, G.; Toro, L.F.; Lee, J.; Gustafsson, A.; Figueredo, C.M.d.S. The Resolution of Periodontal Inflammation Promotes Changes in Cytokine Expression in the Intestine and Gingival Tissues of Aged Rats with DSS-Induced Colitis. J. Clin. Med. 2023, 12, 4326. https://doi.org/10.3390/jcm12134326
de Mello-Neto JM, Ervolino E, Elangovan G, Toro LF, Lee J, Gustafsson A, Figueredo CMdS. The Resolution of Periodontal Inflammation Promotes Changes in Cytokine Expression in the Intestine and Gingival Tissues of Aged Rats with DSS-Induced Colitis. Journal of Clinical Medicine. 2023; 12(13):4326. https://doi.org/10.3390/jcm12134326
Chicago/Turabian Stylede Mello-Neto, João Martins, Edilson Ervolino, Gayathiri Elangovan, Luan Felipe Toro, Jaehee Lee, Anders Gustafsson, and Carlos Marcelo da Silva Figueredo. 2023. "The Resolution of Periodontal Inflammation Promotes Changes in Cytokine Expression in the Intestine and Gingival Tissues of Aged Rats with DSS-Induced Colitis" Journal of Clinical Medicine 12, no. 13: 4326. https://doi.org/10.3390/jcm12134326
APA Stylede Mello-Neto, J. M., Ervolino, E., Elangovan, G., Toro, L. F., Lee, J., Gustafsson, A., & Figueredo, C. M. d. S. (2023). The Resolution of Periodontal Inflammation Promotes Changes in Cytokine Expression in the Intestine and Gingival Tissues of Aged Rats with DSS-Induced Colitis. Journal of Clinical Medicine, 12(13), 4326. https://doi.org/10.3390/jcm12134326









