Abstract
Background: Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have benefits for survival in some cancers with peritoneal metastasis. Hematologic toxicity described rate is 2 to 38%. Methods: Patients admitted to an intensive care unit (ICU) after CRS and HIPEC over 78 months. The data recorded were demographic characteristics, the severity of illness, complete blood samples, the type of cancer and extension, HIPEC drug and temperature, ICU and hospital stay and mortality, bleeding, and the need for transfusion of blood products. Results: Of the 96 patients included, 77.1% presented hematological complications: 8.3% leukopenia (<4000/mm3 leucocytes), 66.7% anemia (hemoglobin < 10 mg/dL), and 22.9% coagulopathy (INR < 1.5, or/and aPTT < 45 s, or/and platelet count < 100,000/mm3, or/and <100 mg/dL of serum fibrinogen). Leukopenia was higher in ovarian cancer or those treated with doxorubicin. Females with anemia, ovarian cancer, and those treated with cisplatin or doxorubicin had longer ICU stays. Bleeding complications were low-corrected in a conservative manner. The median ICU stay was 5 (4.0–5.0) days. The ICU mortality rate was 1.0%. Conclusions: In our study, 77.1% of patients treated with CRS and HIPEC developed hematological complications during the postoperative period; the majority of them were not severe and resolved spontaneously, without an effect on mortality or hospital stay.
1. Introduction
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have demonstrated a decrease in peritoneal recurrence and a higher survival in some cancers with peritoneal metastasis.
In this technique, CRS consists of numerous surgical procedures depending on the extent of peritoneal tumor manifestation to achieve, if possible, no visible residual tumor. Followed by HIPEC, the chemotherapy agent is typically perfused at an inflow of above 43 °C to reach an intraperitoneal temperature of 41–42 °C [1,2].
Several postoperative complications have been described after CRS and HIPEC, related to surgery, hyperthermia, and/or chemotherapy. The described morbidity rates are 25% to 51%, and up to 21–30% patients develop severe postoperative complications, requiring surgical, endoscopic, or radiological reintervention or readmittance to the intensive care unit (ICU) [2,3,4]. The described mortality rate varies between 0 and 18% [5,6].
Hematologic toxicity is a complication that is not well established because few studies have been conducted on it, and in these, the results are very different, with percentages ranging from to 2 to 38% [6,7,8].
The purpose of this study is to define the clinical impact of alteration in coagulation and other hematological alterations that occur in immediate postoperative period after CRS and HIPEC during an ICU stay.
2. Materials and Methods
We included all consecutive patients who were admitted to our ICU after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy, from 1 January 2013 to 30 June 2019. We excluded patients who refused to consent to the study. The study was approved by the Institutional Ethics and Clinical Trials Committee of University Hospital Príncipe de Asturias, and written informed consent was obtained from the patients. This study did not receive financial or logistical support from any institution outside the service or the hospital where the study was carried out; neither the researchers nor the subjects recruited in this study received any fees or material incentives for their participation.
The compiled data of every patient included the following: demographic characteristics, the severity of illness (measured by APACHE II [9]), complete blood samples (platelet count, international normalized ratio (INR), active partial thromboplastin time (aPTT), and serum fibrinogen) at admission and daily until ICU discharge, the type of cancer, Peritoneal Carcinomatosis Index (PCI) [1], HIPEC drug and temperature, ICU and hospital stay, ICU and hospital mortality, the presence of bleeding, and the need for the transfusion of blood products during surgery and ICU stay. When more than one value was available for a given day, the most abnormal value was recorded.
We defined leukopenia as a leukocyte count less than 4000/mm3 and anemia as a hemoglobin level equal to or less than 10 mg/dL. Due to the lack of a consensus regarding the precise definition of “coagulopathy”, we defined abnormal coagulation as an INR equal to or more than 1.5, or/and aPTT equal to or more than 45 s, or/and platelet count equal to or less than 100,000/mm3, or/and equal to or less than 100 mg/dL of serum fibrinogen. These criteria were applied in another study concerned with coagulation after CRS and HIPEC [7].
The management of patients during their stay in the ICU, including the transfusion of blood products, was conducted according to standard protocols. Venous thromboembolism prophylaxis with enoxaparin was used in all patients, except when a contraindication existed.
The normal distribution of variables was assessed using the Kolmogorov–Smirnov test. Quantitative variables with normal distribution are expressed as means ± S.D. and compared using the Student t test; non-normal distribution variables are shown as medians and interquartile ranges and compared using the Mann–Whitney test. Qualitative variables are shown as percentages and compared with the chi-square test.
Logistic regression models were used for the analysis of factors associated with a longer ICU stay (defined as more than 5 days). A univariate analysis of the main variables registered at ICU admission (age, sex, type of cancer, and APACHE II) and during ICU stay (the transfusion of blood products during ICU stay, and bleeding) was performed. For the analysis, some variables (APACHE II, ICU stay, and SOFA score) were dichotomized based on the median. Predictor variables that were statistically significant (set to p < 0.10) when evaluated individually were included in the forward stepwise multiple logistic regression analysis. The results are presented as odds ratios with 95% confidence intervals.
The level of statistical significance was set to a p ≤ 0.05, using bilateral contrast, and the results are expressed with a 95% confidence interval.
3. Results
During the study period, 97 patients were admitted to our ICU after CRS and HIPEC. One patient refused to consent to the study, so 96 patients were finally included in the study.
The mean age was 60.7 ± 9.7 years; 50% of patients were male.
In 78 patients (81.3%), the cancer had a digestive origin (43 (44.8%) patients had colorectal cancer, 33 (34.4%) had gastric cancer, and 2 (2.1%) had pseudomyxoma); in 18 patients (18.8%), the origin was ovarian, with a median peritoneal carcinomatosis index of 5.0 (1.2–10.7).
The median duration of surgery was 10.0 (8.5–11.0) h, during which 16 patients (16.7%) needed blood transfusion (a mean of two red blood cell concentrates).
The mean APACHE II [9] at ICU admission was 9.3 ± 3.9, with a mean SOFA score [10] of 1.0 (0.25–3.0). At ICU admission, 5 patients (5.2%) were under mechanical ventilation, and 11 (11.6%) had vasoactive support during or at ICU admission.
During admission, 74 patients (77.1%) presented some kind of hematological complications: 8 (8.3%) patients developed leukopenia, 64 (66.7%) developed anemia, and 22 (22.9%) developed coagulopathy.
Of the 74 patients included in the study, 4 patients presented bleeding through surgical drainages, 8 presented hematuria, and 1 presented digestive hemorrhage, but only 3 patients needed a transfusion of blood products. All bleeding complications were corrected in a conservative manner.
A total of 19 (19.8%) patients needed a transfusion of blood products due to progressive anemia without seeing active bleeding, probably related to the received chemotherapy treatment (only one patient had hematuria, one bled through peritoneal drainages, and one had low digestive hemorrhage).
The median ICU stay was 5 (4.0–5.0) days; the median hospitalization was 10.0 (9.0–13.0) days. One patient (1.0%) died during their ICU stay due to massive pulmonary embolism; none did so during their hospital stay after ICU admission.
There were no differences between the patients who developed coagulopathy or not, even in the development of bleeding complications (Table 1).

Table 1.
Characteristics of patients with coagulopathy.
We found that female patients, those who had ovarian cancer, or those who were treated with cisplatin or doxorubicin developed anemia more frequently. Obviously, they received more transfusion of blood products during their ICU stay. Although they did not have a higher incidence of bleeding complications, these patients had a longer ICU stay (Table 2).

Table 2.
Characteristics of patients with anemia.
Anemia appears on day 3 postoperatively (Figure 1).
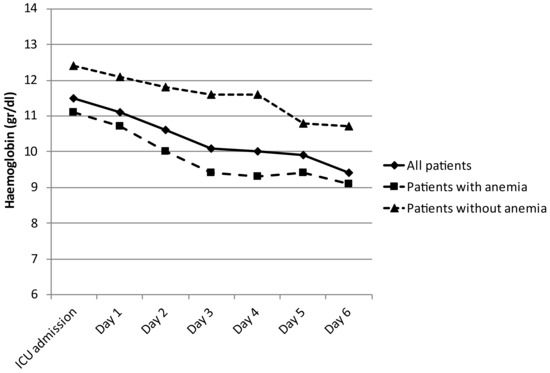
Figure 1.
Hemoglobin evolution during first postoperative days.
Leukopenia developed more frequently among patients with ovarian cancer or those that received doxorubicin as intraperitoneal chemotherapy (Table 3).

Table 3.
Characteristics of patients with leukopenia.
Logistic regression analysis was performed to assess the effect of clinical variables and hematological toxicity with a long ICU stay (defined as more than 5 days). Only age was found to have a trend towards a longer ICU stay (Table 4), and no factor was correlated with ICU mortality (Table 5).

Table 4.
Factors related to long ICU stay (more than 5 days).

Table 5.
Factors related to ICU mortality.
4. Discussion
In our study, we found that hematological complications during the immediate postoperative period in the ICU stay were frequent (up to 77.1% of patients), although not severe, nor were they associated with the development of bleeding complications, a longer ICU stay, or ICU mortality, even in those who developed coagulopathy. The development of anemia was associated with a longer ICU stay and more transfusions of blood products.
We found that 66.7% of patients developed anemia, which was associated with a longer ICU stay and a greater need for transfusions, although without a statistically significant association with the presence of bleeding complications. A possible explanation for this fact is that in the group of patients with anemia, they received more blood transfusions and were kept in the ICU longer to ensure the absence of bleeding and/or the stability of hemoglobin levels after the blood transfusion.
As other authors have described, we found a progressive decrease in hemoglobin levels during the postoperative period, not due to bleeding complications [11,12,13,14]. There have been few studies focused on anemia after CRS and HIPEC. Falcon-Araña [11] found that there was only a slight decrease in hemoglobin rate during surgery—not related to visible bleeding or hemodilution—so they concluded that it may be explained by hemolysis due to hyperthermia or chemotherapy. Schmidt et al. [12] showed a reduction of 24% in hemoglobin rate during surgery, which recovered slowly during the postoperative period; in 6 of the 78 patients (7.7%) studied, a median of 0.3 (0.3–1.2) L of packed red blood cells was given in the postoperative period. Cooksley et al. [13] showed a statistically significant decrease in hemoglobin in the postoperative period with respect to the preoperative level, although only 15 patients (21%) required a transfusion of red blood cells in the postoperative period in the ICU. Somashekhar et al. [14] described an incidence of anemia (defined as <8.0 gr/dL) of 21.4% in 56 patients with peritoneal surface malignancies. Cripe et al. [15] described a median hemoglobin nadir of 8.6 gr/dL on day 5 postoperatively, including patients transfused intraoperatively and/or postoperatively, without a relation to prior chemotherapy exposure, in patients with recurrent ovarian carcinoma treated with CRS and HIPEC.
In our study, nearly 20% of patients needed a transfusion of red blood cells. The rates described varied from 7.7% postoperatively to 36% [7,12]. Interestingly, Nizri et al. [16] studied 231 patients with diffuse malignant peritoneal mesothelioma and 273 patients with pseudomyxoma peritonei who were operated on in their units (CRS and HIPEC). A total of 74% of them were transfused during perioperative period, with a median of two packed red blood cells. They found that red blood cell transfusion, even minimal, had a significant adverse effect on both short- and long-term outcomes in cytoreductive surgery, and, therefore, suggested focusing on transfusion reduction in these patients.
In these patients, thrombocytopenia is due to the cytotoxic drugs used and dilutional coagulopathy and blood loss [17]. Similar to our study, several studies have shown that the platelet count decreased to noncritical levels between postoperative days 1 and 3, and normalized at day 7 [7,18,19,20,21], without the need for platelet transfusion after surgery.
The describes rates of thrombocytopenia depend on the chosen cut-off point. The majority of studies have focused on the management of these patients with epidural analgesia, so the cut-off point taken is 100.000/mm3. Furthermore, most studies have defined coagulopathy as a mixture of platelet level and coagulation times (as a platelet count <100.000/mm3, INR > 1.5, or aPTT > 45 s); this makes it difficult to make a comparison between our study and those previously described. With this cut-off point, the described level of thrombocytopenia is around 17% [7], lower than in our study. Only two studies— one involving 18 patients treated with intravenous ifosfamide and HIPEC and the other involving 50 patients treated with CRS and HIPEC—described an incidence of 61.1 and 36%, respectively, with a cut-off of 150,000/mm3 as in our study [22,23]. Another described an incidence of thrombocytopenia (defined as lower than 140,000/mm3) of 37.9% in 235 CRS and HIPEC patients; these authors found that increased age (>60 years) and previous chemotherapy were independent risk factors associated with the development of thrombocytopenia [24]. Although we did not reach such a finding, severe thrombopenia (less than 50,000/mm3) is described in 0.6–11% of patients [7,19,25].
Coagulopathy was present in 22.9% of the patients included in our study. With respect to coagulation times, Cooksley et al. [13] described the postoperative critical care of 69 patients treated with CRS and HIPEC; they found a prolongation of coagulation laboratory tests (INR and aPTT) after the surgery, with a peak 24 h postoperatively, although the correction of abnormal coagulation with fresh frozen plasma was only indicated in one patient. Similar results have been described by other authors, who also reported that at postoperative day 3, INR and aPTT normalized without the need for fresh plasma transfusion [7,20,21,25,26].
Little is known about fibrinogen in these patients. Falcon Araña [11] demonstrated a decrease in fibrinogen during surgery, and it was associated with a greater need for blood product transfusions. Van Poucke et al. [18] showed a decrease in the concentration of plasmatic fibrinogen or a deterioration of its function, which recovered at day 7 and even improved compared to before surgery. We found that hypofibrinogenemia was associated with more transfusions of blood products during ICU admission; additionally, it was more frequent in patients with residual tumor after surgery.
Although several studies showed a significant increase in white blood cell count during the first 7 postoperative days, especially if a splenectomy had been performed [12,18,27,28,29], in our study, we found that eight patients (8.3%) developed leukopenia, which other authors also found. Horvath et al. [30], in their study involving 40 patients with pseudomyxoma peritonei treated with HIPEC, found that 31% of them developed leukopenia (<4000/mm3) between day 6 and day 7 postoperatively; all of them were treated with mitomycin. Hakeam et al. [22] found an incidence of 11.1% of leukopenia, which recovered spontaneously within 48 h without a need for filgrastim use. Wong et al. [24] found an incidence of leukopenia of 15.3% that recovered after 1–3 days and a 3.8% incidence of neutropenia (<2000/mm3), which was related to ICU stay; through a univariate analysis, they found that patients that received more intraoperative blood transfusions had a higher risk of postoperative leukopenia. Fefermann [31] found a 7% incidence of leukopenia (defined as <2000/mm3) in 242 patients treated with CRS and HIPEC with mitomycin C, with a median nadir on day 5 postoperatively, who had a significantly longer ICU stay with respect to patients who did not develop leukopenia (11 vs. 6 days, p < 0.05). Bartos et al. [4] found that only 2 of the 50 patients included in their study (4%) developed leukopenia; they included patients with peritoneal carcinomatosis of different origin. Wong et al. [32] found an incidence of 12.3% of leukopenia (defined as <4000/mm3) in 220 patients treated with CRS and HIPEC, with a median duration of leukopenia of 1 day (1–3 days). The difference in leukopenia rates between all these studies may be attributed in part to the difference in the protocol of intraperitoneal chemotherapy used, as well as the different definitions of leukopenia.
We also found a trend towards a longer ICU stay in patients who developed leukopenia, as already described by other authors [24,31].
We found a trend towards a higher incidence of leukopenia in the female sex. Lambert et al. [32] described the occurrence of neutropenia (defined as an absolute neutrophil count <1000/mm3) in 39% of patients treated with mitomycin C, with a median time to onset of 9 days after surgery and a median duration of 2 days; the factors related to neutropenia were the dose of mitomycin C and the female sex.
There are several limitations to our study, such as the lack of standardized anesthesia management and postoperative care. We included patients with different pathologies that received several chemotherapies. Another limitation was the lack of preoperative INR and aPTT values for all patients, although we anticipate that the majority of the patients would have shown normal test results the day before surgery. We did not measure the effect of surgery and HIPEC on renal or hepatic function, so we cannot assess the influence of renal or hepatic dysfunction on the observed hematological toxicity. We did not perform a multivariate analysis to see the factors associated with mortality or ICU stay.
The strength of our study is that it is the first involving patients with CRS and HIPEC which comprises all hematological toxicities—affecting platelets, red blood cells, leukopenia/neutropenia, fibrinogen, and coagulation times—and that it is centered on the immediate postoperative period in the ICU.
5. Conclusions
In conclusion, we found that 77.1% of patients treated with CRS and HIPEC developed hematological complications during the postoperative period; the majority of them were not severe and resolved spontaneously, without an effect on mortality or hospital stay. Only the development of anemia was associated with a longer ICU stay and more transfusions of blood products.
Author Contributions
Conceptualization and methodology: M.C.P. and A.G.C.; formal analysis: M.C.P., I.L.U., R.G.S., M.D.A., E.N.L., A.G.C., M.A.O. and M.Á.d.M.; investigation: M.C.P., I.L.U., R.G.S., M.D.A., E.N.L. and A.G.C.; writing—review and editing: M.C.P., M.D.A., E.N.L. and A.G.C.; funding acquisition: M.A.O. and M.Á.d.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was coordinated by ProA Capital, Halekulani S.L., MJR and cofinanced by the European Development Regional Fund “A way to achieve Europe”, as well as P2022/BMD-7321 (Comunidad de Madrid).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Hospital Universitario Príncipe de Asturias.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Clinical data are available upon reasonable request.
Acknowledgments
We wanted to thank all staff and patients of Principe de Asturias University Hospital that have worked or collaborated selflessly in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sugarbaker, P.H. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin. Surg. Oncol. 1998, 14, 254–261. [Google Scholar] [CrossRef]
- Glockzin, G.; Schlitt, H.J.; Piso, P. Peritoneal carcinomatosis: Patients selection, perioperative complications and quality of life related to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J. Surg. Oncol. 2009, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Wisselink, D.D.; Braakhuis, L.L.F.; Gallo, G.; van Grevenstein, W.M.; van Dieren, S.; Kok, N.F.; de Reuver, P.R.; Tanis, P.J.; de Hingh, I.H. Systematic review of published literature on oxaliplatin and mitomycin C as chemotherapeutic agents for hyperthermic intraperitoneal chemotherapy in patients with peritoneal metastases from colorectal cancer. Crit. Rev. Oncol. Hematol. 2019, 142, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Bartos, A.; Bartos, D.; Susnea, R.; Mitre, C.; Hadade, A.; Iancu, I.; Cioltean, C.; Iancu, C.; Militaru, C.; Părău, A.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for the Treatment of Peritoneal Carcinomatosis: Our Initial Experience. Chirurgia 2019, 114, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.D.; Halabi, W.J.; Stamos, M.J.; Creutzenberg, M.; Piso, P.; Hobbhahn, J. Surgical outcomes of hyperthermic intraperitoneal chemotherapy: Analysis of the American college of surgeons national surgical quality improvement program. JAMA Surg. 2014, 149, 170–175. [Google Scholar] [CrossRef]
- Yan, T.D.; Black, D.; Savady, R.; Sugarbaker, P.H. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann. Surg. Oncol. 2007, 14, 484–492. [Google Scholar] [CrossRef]
- Hurdle, H.; Bishop, G.; Walker, A.; Tan, G.H.C.; Kumar, M.; Teo, M. Coagulation after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A retrospective cohort analysis. Can. J. Anaesth. 2017, 64, 1144–1152. [Google Scholar] [CrossRef]
- Robella, M.; Vaira, M.; Cinquegrana, A.; De Simone, M. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Morbidity and postoperative outcomes. Minerva Chir. 2019, 74, 195–202. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Vincent, J.L.; de Mendonca, A.; Cantraine, F.; Bhagwandin, S.; Kimos, J. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
- Falcon Arana, L.; Fuentes-Garcia, D.; Roca Calvo, M.J.; Bernard, J.L.; Casanova, V. Alterations in hemostasis during cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis. Cir. Esp. 2015, 93, 496–501. [Google Scholar] [CrossRef]
- Schmidt, C.; Creutzenberg, M.; Piso, P.; Hobbhahn, J.; Bucher, M. Peri-operative anaesthetic management of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Anaesthesia 2008, 63, 389–395. [Google Scholar] [CrossRef]
- Cooksley, T.J.; Haji-Michael, P. Post-operative critical care management of patients undergoing cytoreductive surgery and heated intraperitoneal chemotherapy (HIPEC). World J. Surg. Oncol. 2011, 9, 169. [Google Scholar] [CrossRef]
- Somashekhar, S.P.; Prasanna, G.; Jaka, R.; Rauthan, A.; Murthy, H.S.; Karanth, S. Hyperthermic intraperitoneal chemotherapy for peritoneal surface malignancies: A single institution Indian experience. Natl. Med. J. India 2016, 29, 262–266. [Google Scholar] [PubMed]
- Cripe, J.; Tseng, J.; Eskander, R.; Fader, A.N.; Tanner, E.; Bristow, R. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for recurrent ovarian carcinoma: Analysis of 30-day morbidity and mortality. Ann. Surg. Oncol. 2015, 22, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Nizri, E.; Kusamura, S.; Fallabrino, G.; Guaglio, M.; Baratti, D.; Deraco, M. Dose-Dependent Effect of Red Blood Cells Transfusion on Perioperative and Long-Term Outcomes in Peritoneal Surface Malignancies Treated with Cytoreduction and HIPEC. Ann. Surg. Oncol. 2018, 25, 3264–3270. [Google Scholar] [CrossRef]
- Gupta, N.; Kumar, V.; Garg, R.; Bharti, S.J.; Mishra, S.; Bhatnagar, S. Anesthetic implications in hyperthermic intraperitoneal chemotherapy. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, 3–11. [Google Scholar] [CrossRef]
- Van Poucke, S.; Huskens, D.; Van der Speeten, K.; Roest, M.; Lauwereins, B.; Zheng, M.-H.; Dehaene, S.; Penders, J.; Marcus, A.; Lancé, M. Thrombin generation and platelet activation in cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy—A prospective cohort study. PLoS ONE 2018, 13, e0193657. [Google Scholar] [CrossRef]
- Canda, A.E.; Sokmen, S.; Terzi, C.; Schlitt, H.J.; Piso, P. Complications and toxicities after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2013, 20, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, F.; Casiraghi, C.; Fumagalli, L.; Kusamura, S.; Baratti, D.; Deraco, M.; Arienti, F.; Langer, M. Epidural analgesia for cytoreductive surgery with peritonectomy and heated intraperitoneal chemotherapy. Int. J. Surg. 2015, 16, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Agyemang, P.; Soliz, J.; Hayes-Jordan, A.; Harun, N.; Gottumukkala, V. Safety of epidural analgesia in the perioperative care of patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2014, 21, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Hakeam, H.; Ayman, A.; Waleed, A.T.; Amen, T. Systemic complications of the bidirectional intraoperative chemotherapy with intravenous ifosfamide and hyperthermic intraperitoneal chemotherapy (HIPEC) using cisplatin plus doxorubicin. Pleura Peritoneum 2019, 4, 25. [Google Scholar] [CrossRef]
- Finlay, B.; Price, T.; Hewett, P. Neutropenia and thrombocytopenia after cytoreductive surgery and heated intraperitoneal chemotherapy. Pleura Peritoneum 2017, 2, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.Y.T.; Tan, G.H.C.; Kumar, M.; Teo, M.C.C. Hematological toxicities associated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Asia Pac. J. Clin. Oncol. 2019, 16, e38–e46. [Google Scholar] [CrossRef] [PubMed]
- Teoh, D.A.; Hutton, M.J.H.; Else, S.; Walker, A.; Lee, A.; Mack, L.A. Epidural analgesia? A prospective analysis of perioperative coagulation in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Am. J. Surg. 2019, 217, 887–892. [Google Scholar] [CrossRef]
- Davis, S.J.; Byrne, K.P. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy—Perioperative management at Waikato Hospital. Anaesth. Intensive Care 2019, 47, 100–101. [Google Scholar] [CrossRef]
- Desantis, M.; Bernard, J.L.; Casanova, V.; Cegarra-Escolano, M.; Benizri, E.; Rahili, A.M.; Benchimol, D.; Bereder, J.-M. Morbidity, mortality, and oncological outcomes of 401 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC). Langenbecks Arch. Surg. 2015, 400, 37–48. [Google Scholar] [CrossRef]
- Kajdi, M.E.; Beck-Schimmer, B.; Held, U.; Kofmehl, R.; Lehmann, K.; Ganter, M.T. Anaesthesia in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: Retrospective analysis of a single centre three-year experience. World J. Surg. Oncol. 2014, 12, 136. [Google Scholar] [CrossRef]
- Bell, J.C.; Rylah, B.G.; Chambers, R.W.; Peet, H.; Mohamed, F.; Moran, B.J. Perioperative management of patients undergoing cytoreductive surgery combined with heated intraperitoneal chemotherapy for peritoneal surface malignancy: A multi-institutional experience. Ann. Surg. Oncol. 2012, 19, 4244–4251. [Google Scholar] [CrossRef]
- Horvath, P.; Beckert, S.; Struller, F.; Konigsrainer, A.; Konigsrainer, I. Incidence of leukopenia after intraperitoneal vs combined intravenous/intraperitoneal chemotherapy in pseudomyxoma peritonei. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 434–439. [Google Scholar] [CrossRef]
- Feferman, Y.; Bhagwandin, S.; Kim, J.; Bharti, S.J.; Mishra, S. Conflicting Data on the Incidence of Leukopenia Neutropenia After Heated Intraperitoneal Chemotherapy with Mitomycin, C. Ann. Surg. Oncol. 2017, 24, 3831–3836. [Google Scholar] [CrossRef] [PubMed]
- Lambert, L.A.; Armstrong, T.S.; Lee, J.J.; Yan, T.D.; Black, D. Incidence risk, factors, impact of severe neutropenia after hyperthermic intraperitoneal mitomycin, C. Ann. Surg. Oncol. 2009, 16, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).