Comparison of Protein- or Amino Acid-Based Supplements in the Rehabilitation of Men with Severe Obesity: A Randomized Controlled Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Treatments
2.3. Metabolic-Nutritional-Psychological Rehabilitation Program
2.4. Anthropometric and Body Composition
2.5. Energy Expenditure Assessment
2.6. Biochemical and Metabolic Marker Measurements
2.7. Muscle Strength and Performance
2.8. Statistical Analysis
3. Results
3.1. Effects of Nutritional Supplements on Body Weight and Body Composition
3.2. Effect of Nutritional Supplements on Glucose Homeostasis and Lipid Metabolism
3.3. Effects of Nutritional Supplements on Physical Performance and Muscle Health
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornier, M.A. A review of current guidelines for the treatment of obesity. Am. J. Manag. Care 2022, 28, S288–S296. [Google Scholar] [PubMed]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. Obesity Management Task Force of the European Association for the Study of Obesity. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef]
- Kercher, V.M.; Kercher, K.; Levy, P.; Bennion, T.; Alexander, C.; Amaral, P.C.; Batrakoulis, A.; Chávez, L.F.J.G.; Cortés-Almanzar, P.; Haro, J.L.; et al. Fitness Trends from Around the Globe. ACSM’s Health Fit. J. 2023, 27, 19–30. [Google Scholar] [CrossRef]
- Batrakoulis, A. European Fitness Trends for 2020. ACSMs Health Fit. J. 2019, 23, 28–35. [Google Scholar] [CrossRef]
- Cava, E.; Yeat, N.C.; Mittendorfer, B. Preserving Healthy Muscle during Weight Loss. Adv. Nutr. 2017, 8, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Weinheimer, E.M.; Sands, L.P.; Campbell, W.W. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: Implications for sarcopenic obesity. Nutr. Rev. 2010, 68, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Pahor, M.; Guralnik, J.M.; Ambrosius, W.T.; Blair, S.; Bonds, D.E.; Church, T.S.; Espeland, M.A.; Fielding, R.A.; Gill, T.M.; Groessl, E.J.; et al. Effect of Structured Physical Activity on Prevention of Major Mobility Disability in Older Adults the LIFE Study Randomized Clinical Trial. JAMA 2014, 311, 2387–2396. [Google Scholar] [CrossRef] [PubMed]
- Feraco, A.; Gorini, S.; Armani, A.; Camajani, E.; Rizzo, M.; Caprio, M. Exploring the Role of Skeletal Muscle in Insulin Resistance: Lessons from Cultured Cells to Animal Models. Int. J. Mol. Sci. 2021, 22, 9327. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Moran, L.J.; Clifton, P.M.; Noakes, M.; Brinkworth, G.D. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 1281–1298. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Breen, L.; Di Donato, D.M.; Hector, A.J.; Mitchell, C.J.; Moore, D.R. Leucine supplementation of a low-protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double-blind, randomized trial. Am. J. Clin. Nutr. 2014, 99, 276–286. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.; Berg, A. Weight Loss Strategies and the Risk of Skeletal Muscle Mass Loss. Nutrients 2021, 13, 2473. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Foster-Schubert, K.E.; Alfano, C.M.; Duggan, C.R.; Xiao, L.; Campbell, K.L.; Kong, A.; Bain, C.E.; Wang, C.Y.; Blackburn, G.L.; McTiernan, A. Effect of Diet and Exercise, Alone or Combined, on Weight and Body Composition in Overweight-to-Obese Postmenopausal Women. Obesity 2012, 20, 1628–1638. [Google Scholar] [CrossRef]
- Verreijen, A.M.; Verlaan, S.; Engberink, M.F.; Swinkels, S.; de Vogel-van den Bosch, J.; Weijs, P.J.M. A high whey protein–, leucine-, and vitamin D–enriched supplement preserves muscle mass during intentional weight loss in obese older adults: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 279–286. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Burd, N.A.; Phillips, S.M. Nutritional regulation of muscle protein synthesis with resistance exercise: Strategies to enhance anabolism. Nutr. Metab. 2012, 9, 40. [Google Scholar] [CrossRef]
- Campbell, W.W.; Kim, J.E.; Amankwaah, A.F.; Gordon, S.L.; Weinheimer-Haus, E.M. Higher total protein intake and change in total protein intake affect body composition but not metabolic syndrome indexes in middle-aged overweight and obese adults who perform resistance and aerobic exercise for 36 weeks. J. Nutr. 2015, 145, 2076–2083. [Google Scholar] [CrossRef]
- Backx, E.M.; Tieland, M.; Borgonjen-van den Berg, K.J.; Claessen, P.R.; van Loon, L.J.; de Groot, L.C. Protein intake and lean body mass preservation during energy intake restriction in overweight older adults. Int. J. Obes. 2016, 40, 299–304. [Google Scholar] [CrossRef]
- Sammarco, R.; Marra, M.; Di Guglielmo, M.L.; Naccarato, M.; Contaldo, F.; Poggiogalle, E.; Donini, L.M.; Pasanisi, F. Evaluation of Hypocaloric Diet with Protein Supplementation in Middle-Aged Sarcopenic Obese Women: A Pilot Study. Obes. Facts 2017, 10, 160–167. [Google Scholar] [CrossRef]
- Buondonno, I.; Sassi, F.; Carignano, G.; Dutto, F.; Ferreri, C.; Pili, F.G.; Massaia, M.; Nisoli, E.; Ruocco, C.; Porrino, P.; et al. From mitochondria to healthy aging the role of branched-chain amino acids treatment: MATeR a randomized study. Clin. Nutr. 2020, 39, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Wu, S.J.; Wang, S.T.; Chang, Y.F.; Chang, C.S.; Kuan, T.S.; Chuang, H.Y.; Chang, C.M.; Chou, W.; Wu, C.H. Effects of enriched branched-chain amino acid supplementation on sarcopenia. Aging 2020, 12, 15091–15103. [Google Scholar] [CrossRef]
- Gwin, J.A.; Church, D.D.; Wolfe, R.R.; Ferrando, A.A.; Pasiakos, S.M. Muscle protein synthesis and wholebody protein turnover responses to ingesting essential amino acids, intact protein, and protein-containing mixed meals with considerations for energy deficit. Nutrients 2020, 12, E2457. [Google Scholar] [CrossRef]
- Pasini, E.; Corsetti, G.; Aquilani, R.; Pasini, E.; Corsetti, G.; Aquilani, R. Protein-amino acid metabolism disarrangements: The hidden enemy of chronic age-related conditions. Nutrients 2018, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, C.; Segala, A.; Valerio, A.; Nisoli, E. Essential amino acid formulations to prevent mitochondrial dysfunction and oxidative stress. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 88–95. [Google Scholar] [CrossRef]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell. Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, C.; Ragni, M.; Rossi, F.; Carullo, P.; Ghini, V.; Piscitelli, F.; Cutignano, A.; Manzo, E.; Ioris, R.M.; Bontems, F.; et al. Manipulation of Dietary Amino Acids Prevents and Reverses Obesity in Mice Through Multiple Mechanisms That Modulate Energy Homeostasis. Diabetes 2020, 69, 2324–2339. [Google Scholar] [CrossRef]
- Tedesco, L.; Rossi, F.; Ragni, M.; Ruocco, C.; Brunetti, D.; Carruba, M.O.; Torrente, Y.; Valerio, A.; Nisoli, E. A Special Amino-Acid Formula Tailored to Boosting Cell Respiration Prevents Mitochondrial Dysfunction and Oxidative Stress Caused by Doxorubicin in Mouse Cardiomyocytes. Nutrients 2020, 12, 282. [Google Scholar] [CrossRef]
- Brunetti, D.; Bottani, E.; Segala, A.; Marchet, S.; Rossi, F.; Orlando, F.; Malavolta, M.; Carruba, M.O.; Lamperti, C.; Provinciali, M.; et al. Targeting Multiple Mitochondrial Processes by a Metabolic Modulator Prevents Sarcopenia and Cognitive Decline in SAMP8 Mice. Front. Pharmacol. 2020, 11, 1171. [Google Scholar] [CrossRef]
- Tedesco, L.; Rossi, F.; Ruocco, C.; Ragni, M.; Carruba, M.O.; Valerio, A.; Nisoli, E. Experimental evidence on the efficacy of two new metabolic modulators on mitochondrial biogenesis and function in mouse cardiomyocytes. J. Popul. Ther. Clin. Pharmacol. 2020, 27, e87–e96. [Google Scholar] [CrossRef]
- Al-Nimr, R.I. Optimal Protein Intake during Weight Loss Interventions in Older Adults with Obesity. J. Nutr. Gerontol. Geriatr. 2019, 38, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.S.; Chumlea, W.C.; Heymsfield, S.B.; Lukaski, H.C.; Schoeller, D.; Friedl, K.; Kuczmarski, R.J.; Flegal, K.M.; Johnson, C.L.; Hubbard, V.S. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am. J. Clin. Nutr. 2003, 77, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R.; Charlier, R.; Caspers, M.; Knaeps, S.; Mertens, E.; Lambrechts, D.; Lefevre, J.; et al. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Haidar, S.G.; Kumar, D.; Bassi, R.S.; Deshmukh, S.C. Average versus Maximum Grip Strength: Which is more consistent? J. Hand Surg. Br. 2004, 29, 82–84. [Google Scholar] [CrossRef]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef]
- Villareal, D.T.; Aguirre, L.; Burke Gurney, A.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Zamboni, M.; Rubele, S.; Rossi, A. Sarcopenia and obesity. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 13–19. [Google Scholar] [CrossRef]
- Smith, G.I.; Commean, P.K.; Reeds, D.N.; Klein, S.; Mittendorfer, B. Effect of Protein Supplementation During Diet-Induced Weight Loss on Muscle Mass and Strength: A Randomized Controlled Study. Obesity 2018, 26, 854–861. [Google Scholar] [CrossRef]
- Yin, Y.H.; Liu, J.Y.W.; Välimäki, M. Effectiveness of non-pharmacological interventions on the management of sarcopenic obesity: A systematic review and meta-analysis. Exp. Gerontol. 2020, 135, 110937. [Google Scholar] [CrossRef]
- Børsheim, E.; Bui, Q.U.; Tissier, S.; Kobayashi, H.; Ferrando, A.A.; Wolfe, R.R. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin. Nutr. 2008, 27, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Dillon, E.L.; Sheffield-Moore, M.; Paddon-Jones, D.; Gilkison, C.; Sanford, A.P.; Casperson, S.L.; Jiang, J.; Chinkes, D.L.; Urban, R.J. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J. Clin. Endocrinol. Metab. 2009, 94, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Ooi, D.S.Q.; Ling, J.Q.R.; Sadananthan, S.A.; Velan, S.S.; Ong, F.Y.; Khoo, C.M.; Tai, E.S.; Henry, C.J.; Leow, M.K.; Khoo, E.Y.; et al. Branched-Chain Amino Acid Supplementation Does Not Preserve Lean Mass or Affect Metabolic Profile in Adults with Overweight or Obesity in a Randomized Controlled Weight Loss Intervention. J. Nutr. 2021, 151, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Bruckbauer, A. Effects of a Leucine and Pyridoxine-Containing Nutraceutical on Fat Oxidation, and Oxidative and Inflammatory Stress in Overweight and Obese Subjects. Nutrients 2012, 4, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; Harber, M.P.; Shrivastava, C.R.; Burant, C.F.; Horowitz, J.F. Improved insulin sensitivity after weight loss and exercise training is mediated by a reduction in plasma fatty acid mobilization, not enhanced oxidative capacity. J. Physiol. 2009, 587 Pt 20, 4949–4961. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; González-Lucán, M.; Fernández-Fernández, C.; Carneiro-Freire, N.; Seco-Filgueira, M.; Pedre-Piñeiro, A.M. Effect of diet composition on insulin sensitivity in humans. Clin. Nutr. ESPEN 2019, 33, 29–38. [Google Scholar] [CrossRef]
- Smith, G.I.; Yoshino, J.; Kelly, S.C.; Reeds, D.N.; Okunade, A.; Patterson, B.W.; Klein, S.; Mittendorfer, B. High-Protein Intake during Weight Loss Therapy Eliminates the Weight-Loss-Induced Improvement in Insulin Action in Obese Postmenopausal Women. Cell Rep. 2016, 17, 849–861. [Google Scholar] [CrossRef]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef]
- Uddin, G.M.; Karwi, Q.G.; Pherwani, S.; Gopal, K.; Wagg, C.S.; Biswas, D.; Atnasious, M.; Wu, Y.; Wu, G.; Zhang, L.; et al. Deletion of BCATm increases insulin-stimulated glucose oxidation in the heart. Metab. Clin. Exp. 2021, 124, 154871. [Google Scholar] [CrossRef]
- Pratt, J.; De Vito, G.; Narici, M.; Segurado, R.; Pessanha, L.; Dolan, J.; Conroy, J.; Boreham, C. Plasma C-Terminal Agrin Fragment as an Early Biomarker for Sarcopenia: Results from the Genofit Study. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2090–2096. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Yang, M.; Sun, J.; Zhao, Y.; Tang, D. The Effect of Irisin as a Metabolic Regulator and Its Therapeutic Potential for Obesity. Int. J. Endocrinol. 2021, 2021, 6572342. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Karim, A.; Muhammad, T.; Shah, I.; Khan, J. Prediction of sarcopenia using a battery of circulating biomarkers. Sci. Rep. 2021, 11, 8632. [Google Scholar] [CrossRef] [PubMed]

| ALL (N = 40) | Before | After | p-Value * | |
|---|---|---|---|---|
| Age (years) | 52.55 ± 5.06 | - | - | |
| Weight (kg) | 133.38 ± 21.39 | 126.24 ± 19.17 | Ns | |
| BMI (Kg/m2) | 44.45 ± 6.54 | 42.04 ± 5.83 | Ns | |
| Waist (cm) | 133.28 ± 12.47 | 125.55 ± 10.99 | 0.005 | |
| Sys P (mmHg) | 147.31 ± 18.53 | 124.62 ± 11.40 | <0.0001 | |
| Diast P (mmHg) | 87.44 ± 8.69 | 79.36 ± 8.18 | <0.0001 | |
| Heart Rate (bpm) | 80.74 ± 10.71 | 75.79 ± 10.62 | 0.05 | |
| FM (kg) | 56.71 ± 14.23 | 52.70 ± 13.62 | Ns | |
| FM (%) | 42.31 ± 4.41 | 41.07 ± 4.98 | Ns | |
| FFM (kg) | 75.61 ± 11.02 | 74.38 ± 9.59 | Ns | |
| FFM (%) | 57.57 ± 4.51 | 58.94 ± 4.98 | Ns | |
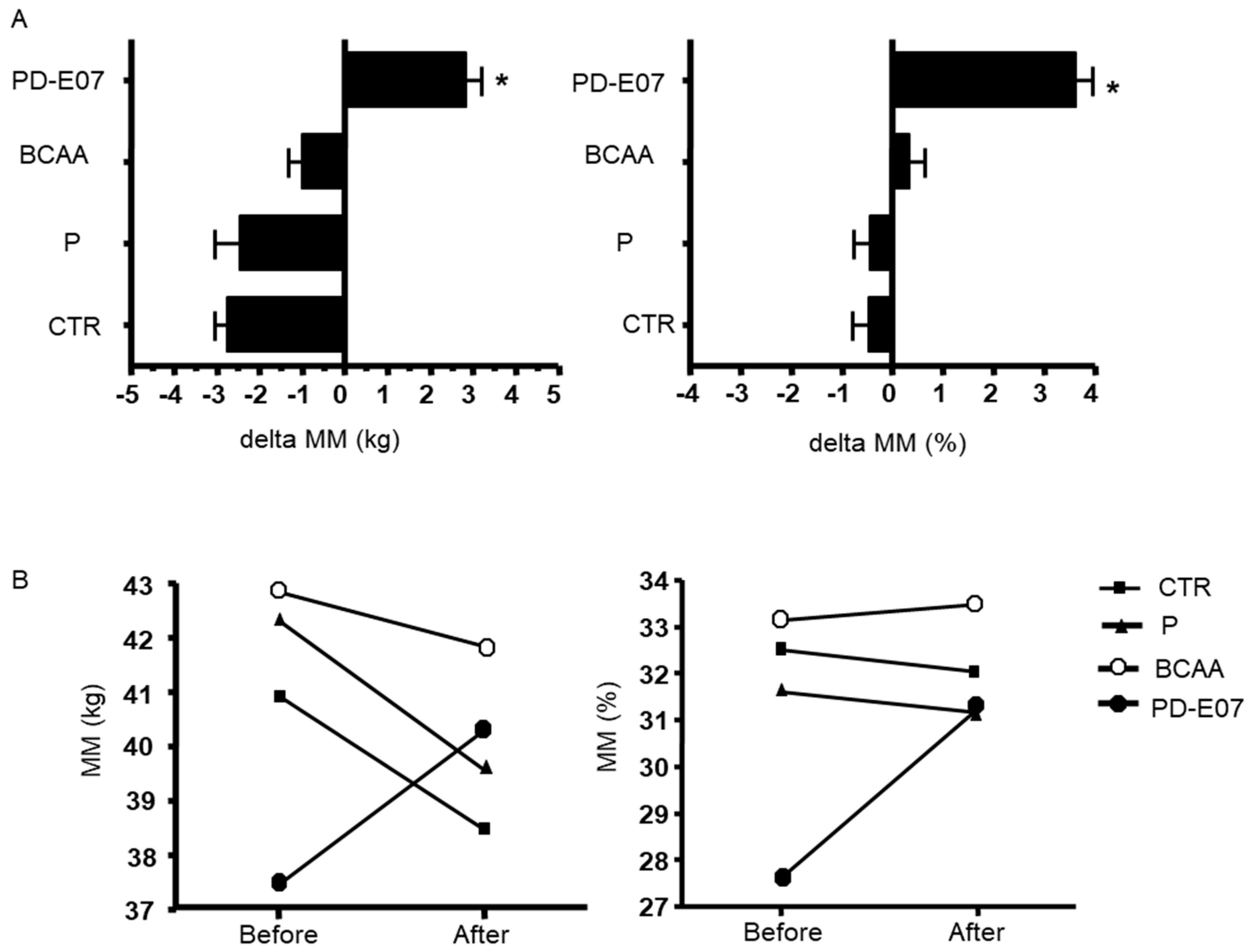
| MM (kg) | 40.89 ± 5.99 | 40.04 ± 4.64 | Ns | |
| MM (%) | 31.24 ± 4.52 | 32.00 ± 4.49 | Ns | |
| REE (Kcal/die) | 2105.64 ± 257.73 | 2022.44 ± 256.45 | Ns | |
| Glucose (mg/dL) | 97.70 ± 10.18 | 91.03 ± 8.01 | 0.002 | |
| Insulin (mU/L) | 18.79 ± 5.91 | 17.36 ± 6.96 | Ns | |
| HbA1c (%) | 5.79 ± 0.39 | 5.53 ± 0.48 | 0.01 | |
| HDL (mg/dL) | 38.65 ± 6.72 | 35.30 ± 6.05 | 0.023 | |
| LDL (mg/dL) | 132.28 ± 36.39 | 113.40 ± 29.98 | 0.01 | |
| TG (mg/dL) | 159.80 ± 42.42 | 134.33 ± 37.63 | 0.006 | |
| 6MWT (meters) | 477.06 ± 132.66 | 531.00 ± 81.68 | 0.04 | |
| HGS (right arm) | 44.70 ± 9.14 | 46.41 ± 9.77 | Ns | |
| HGS (left arm) | 41.76 ± 8.50 | 43.42 ± 9.23 | Ns | |
| CTR | P | AA | PD-E07 | p-Value † | |
|---|---|---|---|---|---|
| Weight (kg) | −6.77 (2.5) | −8.34 (4.39) | −6.12 (1.11) | −7.3 (3.03) | 0.4129 |
| Weight (%) | −5.23 (1.43) | −5.86 (2.19) | −4.67 (0.63) | −5.16 (1.5) | 0.4016 |
| BMI (kg/m2) | −2.24 (0.79) | −2.72 (1.38) | −2.1 (0.36) | −2.55 (1.07) | 0.4803 |
| BMI (%) | −5.24 (1.44) | −5.84 (2.22) | −4.77 (0.74) | −5.35 (1.51) | 0.5118 |
| Waist (cm) | −6.3 (3.43) | −11.8 (7.25) | −4.4 (5.15) | −8.4 (7.86) | 0.0648 |
| Waist (%) | −4.67 (2.34) | −8.63 (4.93) | −3.23 (3.77) | −6.08 (5.42) | 0.0491 |
| Sys P (mmHg) | −22.5 (19.76) | −12 (50.67) | −13.89 (13.18) | −24.5 (23.86) | 0.7546 |
| Diast P (mmHg) | −12 (11.83) | 2 (28.89) | −7.78 (13.25) | −4.5 (8.96) | 0.3561 |
| Heart rate (bpm) | −4.5 (15.63) | 3.4 (28.38) | −2.56 (13.53) | −7.8 (14.97) | 0.6200 |
| FM (kg) | −2.62 (4.08) | −1.87 (5.71) | −5.6 (2.19) | −5.94 (5.85) | 0.1425 |
| FM (%) | −0.14 (3.16) | 0 (4.68) | −2.52 (1.62) | −2.3 (4.09) | 0.2494 |
| FFM (kg) | −3.03 (4.03) | −4.13 (7.75) | 2.21 (4.73) | 0.04 (4.89) | 0.0582 |
| FFM (%) | 0.12 (3.15) | 0.03 (4.68) | 3 (1.29) | 2.3 (4.09) | 0.1600 |
| MM (kg) | −2.46 (3.04) | −2.75 (5.98) | −1 (3.3) | 2.84 (3.57) | 0.0172 |
| MM (%) | −0.47 (2.28) | −0.44 (4.02) | 0.34 (2.85) | 3.63 (3.14) | 0.0172 |
| REE (kCal/die) | −116.75 (122.41) | −41.43 (78.13) | −123.7 (148.5) | −167.43 (158.24) | 0.3651 |
| Glucose (mg/dL) | −9.5 (14.19) | −4.4 (7.32) | −2.7 (7.09) | −10.1 (10.41) | 0.2856 |
| Insulin (mU/L) | −2.96 (9.55) | 1.17 (7.53) | −3.23 (4.2) | −0.89 (5.15) | 0.4695 |
| HDL (mg/dL) | −2.2 (4.98) | −3.9 (6.38) | −4.2 (3.74) | −3.1 (4.79) | 0.8156 |
| LDL (mg/dL) | −1.4 (34.63) | −18.2 (25.13) | −36.1 (34) | −19.8 (12) | 0.0694 |
| TG (mg/dL) | −27 (20.31) | −13 (40.38) | −34.5 (27.37) | −27.4 (30.23) | 0.4633 |
| HbA1c (%) | −0.33 (0.21) | −0.34 (0.6) | −0.14 (0.21) | −0.21 (0.2) | 0.5240 |
| 6MWT (meters) | 104.6 (197.4) | 54.4 (132.44) | 43.63 (37.01) | 59.33 (77.2) | 0.7532 |
| HGS (right arm, Kg) | 1.06 (2.45) | 3.63 (5.47) | 0.52 (4.4) | 1.62 (6.93) | 0.5400 |
| HGS (left arm, Kg) | 0.75 (1.91) | 3.39 (5.21) | 1.79 (4.76) | 0.59 (7.2) | 0.5976 |
| CAF (μg/mL) | 3.3 (21.91) | −5.52 (20.97) | 5.55 (12.83) | 58.92 (177.52) | 0.4521 |
| Irisin (μg/mL) | −0.86 (3.32) | 8.79 (14.78) | 3.92 (14.06) | 0.64 (3.92) | 0.2876 |
| P3NP (μg/mL) | 4.53 (13.92) | −1.53 (14.66) | −4.08 (5.31) | −0.66 (5.24) | 0.4188 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunani, A.; Cancello, R.; Gobbi, M.; Lucchetti, E.; Di Guglielmo, G.; Maestrini, S.; Cattaldo, S.; Piterà, P.; Ruocco, C.; Milesi, A.; et al. Comparison of Protein- or Amino Acid-Based Supplements in the Rehabilitation of Men with Severe Obesity: A Randomized Controlled Pilot Study. J. Clin. Med. 2023, 12, 4257. https://doi.org/10.3390/jcm12134257
Brunani A, Cancello R, Gobbi M, Lucchetti E, Di Guglielmo G, Maestrini S, Cattaldo S, Piterà P, Ruocco C, Milesi A, et al. Comparison of Protein- or Amino Acid-Based Supplements in the Rehabilitation of Men with Severe Obesity: A Randomized Controlled Pilot Study. Journal of Clinical Medicine. 2023; 12(13):4257. https://doi.org/10.3390/jcm12134257
Chicago/Turabian StyleBrunani, Amelia, Raffaella Cancello, Michele Gobbi, Elisa Lucchetti, Giulia Di Guglielmo, Sabrina Maestrini, Stefania Cattaldo, Paolo Piterà, Chiara Ruocco, Alessandra Milesi, and et al. 2023. "Comparison of Protein- or Amino Acid-Based Supplements in the Rehabilitation of Men with Severe Obesity: A Randomized Controlled Pilot Study" Journal of Clinical Medicine 12, no. 13: 4257. https://doi.org/10.3390/jcm12134257
APA StyleBrunani, A., Cancello, R., Gobbi, M., Lucchetti, E., Di Guglielmo, G., Maestrini, S., Cattaldo, S., Piterà, P., Ruocco, C., Milesi, A., Valerio, A., Capodaglio, P., & Nisoli, E. (2023). Comparison of Protein- or Amino Acid-Based Supplements in the Rehabilitation of Men with Severe Obesity: A Randomized Controlled Pilot Study. Journal of Clinical Medicine, 12(13), 4257. https://doi.org/10.3390/jcm12134257






