The Efficacy of Scalp Nerve Block in Postoperative Pain Management after Microvascular Decompression: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrolment
2.2. Randomization and Blinding
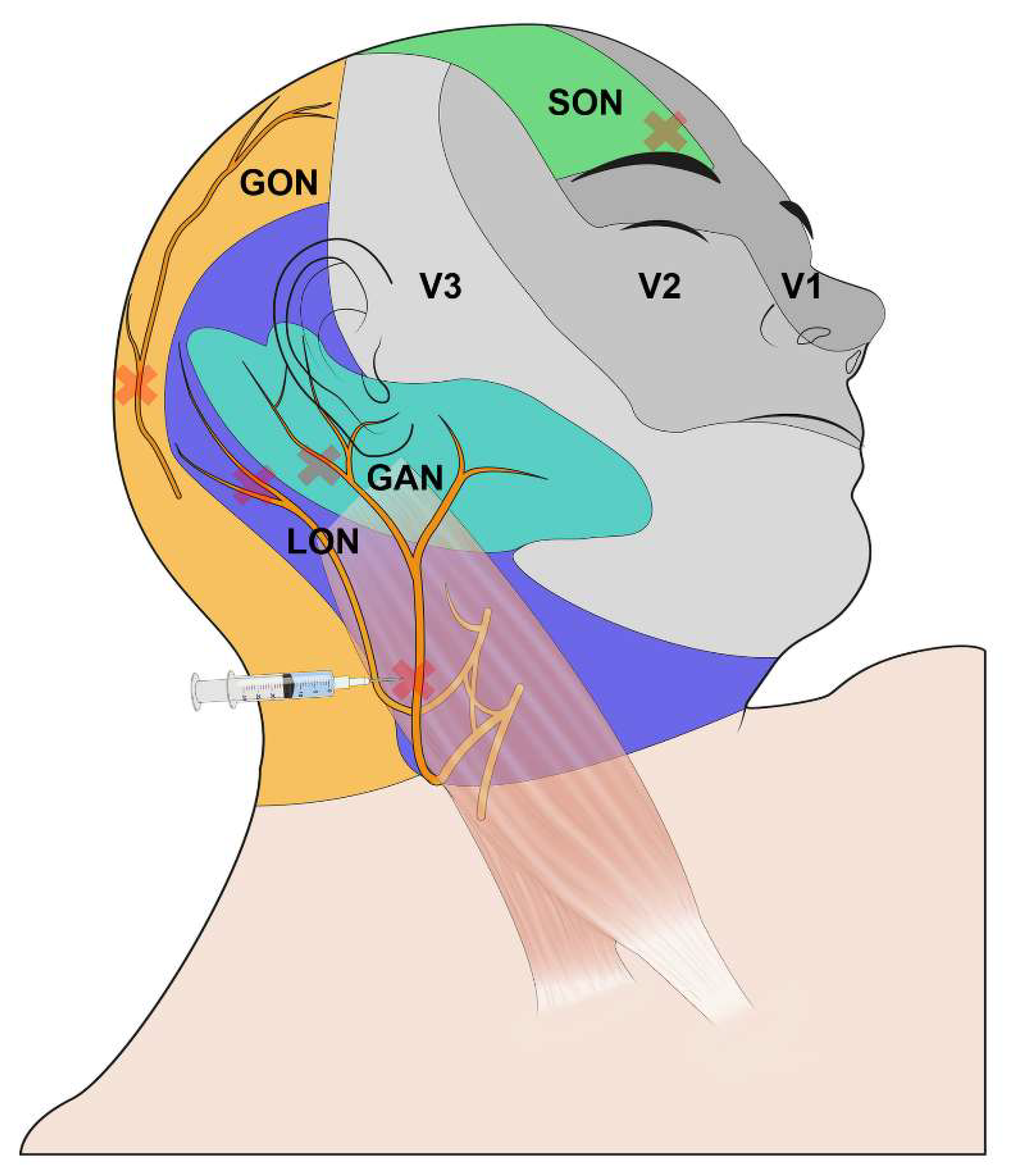
2.3. Intervention
2.4. Anesthetic Management
2.5. Postoperative Management
2.6. Outcome Variables
2.7. Statistical Analysis
3. Results
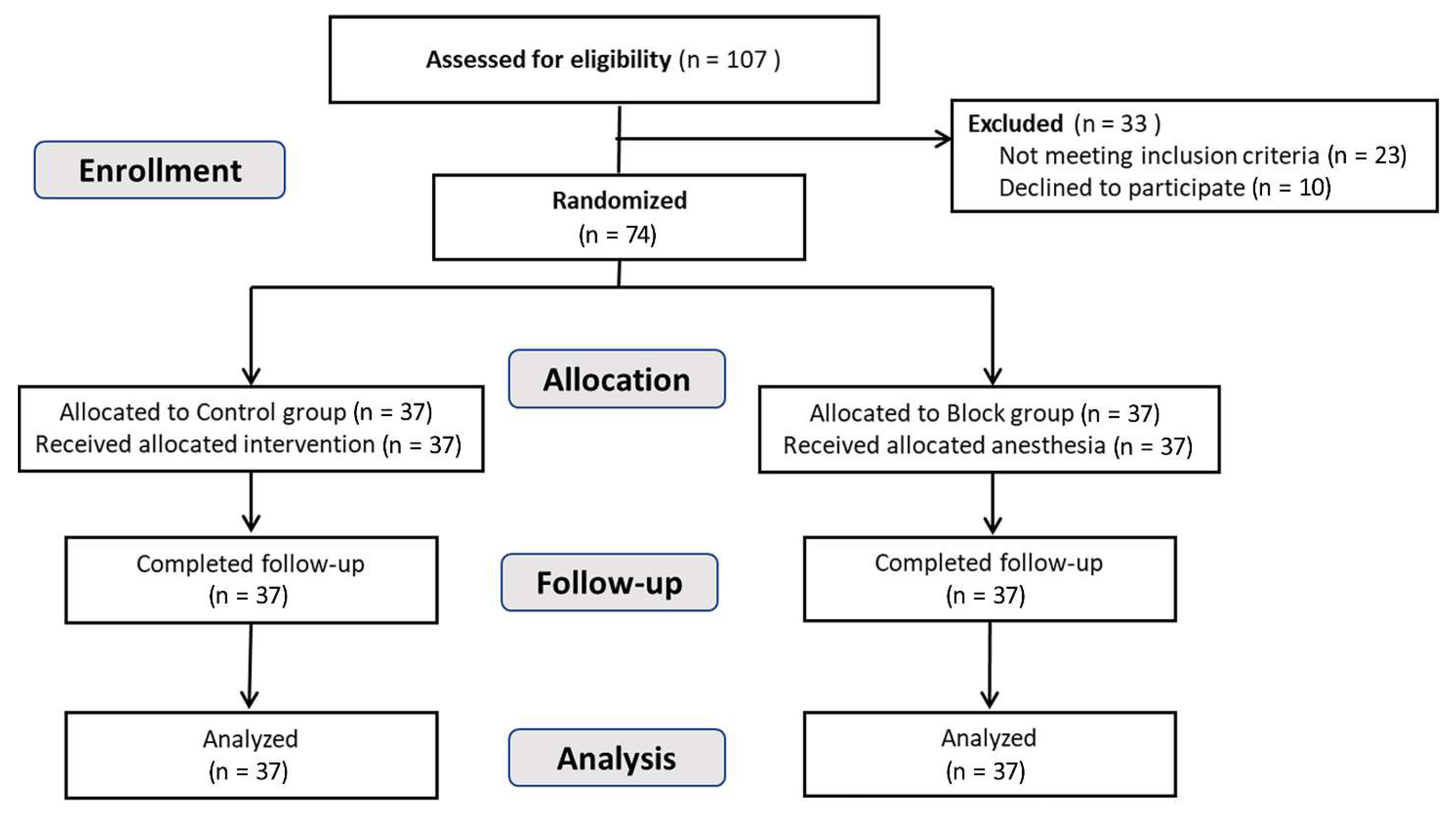
3.1. Study Participants
3.2. Primary Outcome and Opioid Consumptions
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, Y.H.; Kim, W.H.; Lee, J.J.; Yang, S.I.; Lim, S.H.; Seo, D.W.; Park, K.; Chung, I.S. Lateral spread response monitoring during microvascular decompression for hemifacial spasm. Comparison of two targets of partial neuromuscular blockade. Anaesthesist 2014, 63, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Miller, V.M. Safety and effectiveness of microvascular decompression for treatment of hemifacial spasm: A systematic review. Br. J. Neurosurg. 2012, 26, 438–444. [Google Scholar] [CrossRef]
- Barker, F.G., 2nd; Jannetta, P.J.; Bissonette, D.J.; Shields, P.T.; Larkins, M.V.; Jho, H.D. Microvascular decompression for hemifacial spasm. J. Neurosurg. 1995, 82, 201–210. [Google Scholar] [CrossRef]
- Holste, K.; Chan, A.Y.; Rolston, J.D.; Englot, D.J. Pain Outcomes Following Microvascular Decompression for Drug-Resistant Trigeminal Neuralgia: A Systematic Review and Meta-Analysis. Neurosurgery 2020, 86, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, Y.; Nagahiro, S.; Kondo, A.; Arita, K.; Date, I.; Fujii, Y.; Fujimaki, T.; Hanaya, R.; Hasegawa, M.; Hatayama, T.; et al. Microvascular Decompression for Trigeminal Neuralgia: A Prospective, Multicenter Study. Neurosurgery 2021, 89, 557–564. [Google Scholar] [CrossRef]
- Amaya Pascasio, L.; De La Casa-Fages, B.; Esteban de Antonio, E.; Grandas, F.; Garcia-Leal, R.; Ruiz Juretschke, F. Microvascular decompression for trigeminal neuralgia: A retrospective analysis of long-term outcomes and prognostic factors. Neurologia, 2021; online ahead of print. Available online: https://www.sciencedirect.com/science/article/pii/S0213485321000712 (accessed on 1 October 2020). [CrossRef]
- Hao, W.; Cong, C.; Yuanfeng, D.; Ding, W.; Li, J.; Yongfeng, S.; Shijun, W.; Wenhua, Y. Multidata Analysis Based on an Artificial Neural Network Model for Long-Term Pain Outcome and Key Predictors of Microvascular Decompression in Trigeminal Neuralgia. World Neurosurg. 2022, 164, e271–e279. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Zhou, H.Y.; Xiao, Z.L.; Wang, W.; Li, X.L.; Chen, S.J.; Yin, X.P.; Xu, L.J. Efficacy and Safety of Gabapentin vs. Carbamazepine in the Treatment of Trigeminal Neuralgia: A Meta-Analysis. Pain Pract. 2016, 16, 1083–1091. [Google Scholar] [CrossRef]
- Sindou, M.; Leston, J.; Howeidy, T.; Decullier, E.; Chapuis, F. Micro-vascular decompression for primary Trigeminal Neuralgia (typical or atypical). Long-term effectiveness on pain; prospective study with survival analysis in a consecutive series of 362 patients. Acta Neurochir. 2006, 148, 1235–1245, discussion 1245. [Google Scholar] [CrossRef]
- Heuser, K.; Kerty, E.; Eide, P.K.; Cvancarova, M.; Dietrichs, E. Microvascular decompression for hemifacial spasm: Postoperative neurologic follow-up and evaluation of life quality. Eur. J. Neurol. 2007, 14, 335–340. [Google Scholar] [CrossRef]
- Huh, R.; Han, I.B.; Moon, J.Y.; Chang, J.W.; Chung, S.S. Microvascular decompression for hemifacial spasm: Analyses of operative complications in 1582 consecutive patients. Surg. Neurol. 2008, 69, 153–157, discussion 157. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, S.T.; Zhong, J.; Ying, T.T.; Guan, H.X.; Yang, X.S.; Zhou, Q.M.; Jiao, W. Microvascular decompression for hemifacial spasm. J. Craniofac. Surg. 2012, 23, 1385–1387. [Google Scholar] [CrossRef] [PubMed]
- Zetlaoui, P.J.; Gauthier, E.; Benhamou, D. Ultrasound-guided scalp nerve blocks for neurosurgery: A narrative review. Anaesth. Crit. Care Pain Med. 2020, 39, 876–882. [Google Scholar] [CrossRef]
- Osborn, I.; Sebeo, J. “Scalp block” during craniotomy: A classic technique revisited. J. Neurosurg. Anesthesiol. 2010, 22, 187–194. [Google Scholar] [CrossRef]
- Guilfoyle, M.R.; Helmy, A.; Duane, D.; Hutchinson, P.J.A. Regional scalp block for postcraniotomy analgesia: A systematic review and meta-analysis. Anesth. Analg. 2013, 116, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Stumpo, V.; Staartjes, V.E.; Quddusi, A.; Corniola, M.V.; Tessitore, E.; Schroder, M.L.; Anderer, E.G.; Stienen, M.N.; Serra, C.; Regli, L. Enhanced Recovery After Surgery strategies for elective craniotomy: A systematic review. J. Neurosurg. 2021, 135, 1857–1881. [Google Scholar] [CrossRef]
- Elayat, A.; Jena, S.S.; Nayak, S.; Sahu, R.N.; Tripathy, S. Enhanced recovery after surgery—ERAS in elective craniotomies-a non-randomized controlled trial. BMC Neurol. 2021, 21, 127. [Google Scholar] [CrossRef]
- Galvin, I.M.; Levy, R.; Day, A.G.; Gilron, I. Pharmacological interventions for the prevention of acute postoperative pain in adults following brain surgery. Cochrane Database Syst. Rev. 2019, 2019, CD011931. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, J.A.; Lee, J.J.; Kim, H.S.; Shin, H.J.; Chung, I.S.; Kim, J.K.; Gwak, M.S.; Choi, S.J. Effect of preoperative skull block on pediatric moyamoya disease. J. Neurosurg. Pediatr. 2008, 2, 37–41. [Google Scholar] [CrossRef]
- Wang, D.; Fang, J.; Liu, J.; Hao, Q.; Ding, H.; Liu, B.; Liu, Z.; Song, H.; Ouyang, J.; Liu, R. Improving recovery after microvascular decompression surgery for hemifacial spasm: Experience from 530 cases with enhanced recovery after surgery (ERAS) protocol. Br. J. Neurosurg. 2021, 35, 486–491. [Google Scholar] [CrossRef]
- Peng, K.; Zeng, M.; Dong, J.; Yan, X.; Wang, D.; Li, S.; Peng, Y. Ultrasound-guided superficial cervical plexus block for analgesia in patients undergoing craniotomy via suboccipital retrosigmoid approach: Study protocol of a randomised controlled trial. BMJ Open 2020, 10, e034003. [Google Scholar] [CrossRef] [PubMed]
- Fishman, S.M.; Ballantyne, J.C.; Rathmell, J.P. Opioid analgesics. In Bonica’s Management of Pain, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; pp. 1172–1187. [Google Scholar]
- Yoon, S.; Joo, H.; Oh, Y.M.; Lee, J.; Bahk, J.H.; Lee, H.J. Validation and clinical utility of the Korean version of the quality of recovery-15 with enhanced recovery after surgery: A prospective observational cohort study. Br. J. Anaesth. 2020, 125, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, L.; Yu, P.; Gao, T.; Liu, K. Preemptive scalp infiltration with 0.5% ropivacaine and 1% lidocaine reduces postoperative pain after craniotomy. Acta Neurochir. 2015, 157, 993–998. [Google Scholar] [CrossRef]
- Vadivelu, N.; Kai, A.M.; Tran, D.; Kodumudi, G.; Legler, A.; Ayrian, E. Options for perioperative pain management in neurosurgery. J. Pain Res. 2016, 9, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.S.; Sun, M.Z.; Willis, S.L.; Alemnew, M.; De Jong, R.; Evans, A.S.; Duong, C.; Gopen, Q.; Yang, I. Selective scalp block decreases short term post-operative pain scores and opioid use after craniotomy: A case series. J. Clin. Neurosci. 2021, 93, 183–187. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Zhao, T.; Zhao, B.; Yu, D.; Jiang, X.; Ye, L.; Zhao, L.; Lv, W.; Zhang, Y.; et al. Safety and efficacy of a novel neurosurgical enhanced recovery after surgery protocol for elective craniotomy: A prospective randomized controlled trial. J. Neurosurg. 2018, 130, 1680–1691. [Google Scholar] [CrossRef]
- Carella, M.; Tran, G.; Bonhomme, V.L.; Franssen, C. Influence of Levobupivacaine Regional Scalp Block on Hemodynamic Stability, Intra- and Postoperative Opioid Consumption in Supratentorial Craniotomies: A Randomized Controlled Trial. Anesth. Analg. 2021, 132, 500–511. [Google Scholar] [CrossRef]
- Lee, H.H.; Kim, H.M.; Lee, J.E.; Jeon, Y.T.; Park, S.; Hwang, K.; Han, J.H. The Effect of a Transdermal Scopolamine Patch on Postoperative Nausea and Vomiting after Retromastoid Craniectomy with Microvascular Decompression: A Preliminary Single Center, Double-Blind, Randomized Controlled Trial. J. Clin. Med. 2020, 9, 156. [Google Scholar] [CrossRef]
 ) and block (
) and block ( ) groups for (A) cumulative MME, (B) cumulative MME excluding shivering, (C) the NRS of pain at rest, and (D) PONV at 6, 12, and 24 h postoperatively. The solid lines in the box indicate medians, the plus signs (+) in the box indicate mean, the boxes indicate interquartile ranges, and the whiskers indicate minimum to maximum. * significant difference between the two groups by Student’s t-test or Wilcoxon’s rank sum test (p < 0.05) in accordance with the Bonferroni correction. MME, morphine milligram equivalents; NRS, numeric rating scale; PONV, postoperative nausea and vomiting.
) groups for (A) cumulative MME, (B) cumulative MME excluding shivering, (C) the NRS of pain at rest, and (D) PONV at 6, 12, and 24 h postoperatively. The solid lines in the box indicate medians, the plus signs (+) in the box indicate mean, the boxes indicate interquartile ranges, and the whiskers indicate minimum to maximum. * significant difference between the two groups by Student’s t-test or Wilcoxon’s rank sum test (p < 0.05) in accordance with the Bonferroni correction. MME, morphine milligram equivalents; NRS, numeric rating scale; PONV, postoperative nausea and vomiting.
 ) and block (
) and block ( ) groups for (A) cumulative MME, (B) cumulative MME excluding shivering, (C) the NRS of pain at rest, and (D) PONV at 6, 12, and 24 h postoperatively. The solid lines in the box indicate medians, the plus signs (+) in the box indicate mean, the boxes indicate interquartile ranges, and the whiskers indicate minimum to maximum. * significant difference between the two groups by Student’s t-test or Wilcoxon’s rank sum test (p < 0.05) in accordance with the Bonferroni correction. MME, morphine milligram equivalents; NRS, numeric rating scale; PONV, postoperative nausea and vomiting.
) groups for (A) cumulative MME, (B) cumulative MME excluding shivering, (C) the NRS of pain at rest, and (D) PONV at 6, 12, and 24 h postoperatively. The solid lines in the box indicate medians, the plus signs (+) in the box indicate mean, the boxes indicate interquartile ranges, and the whiskers indicate minimum to maximum. * significant difference between the two groups by Student’s t-test or Wilcoxon’s rank sum test (p < 0.05) in accordance with the Bonferroni correction. MME, morphine milligram equivalents; NRS, numeric rating scale; PONV, postoperative nausea and vomiting.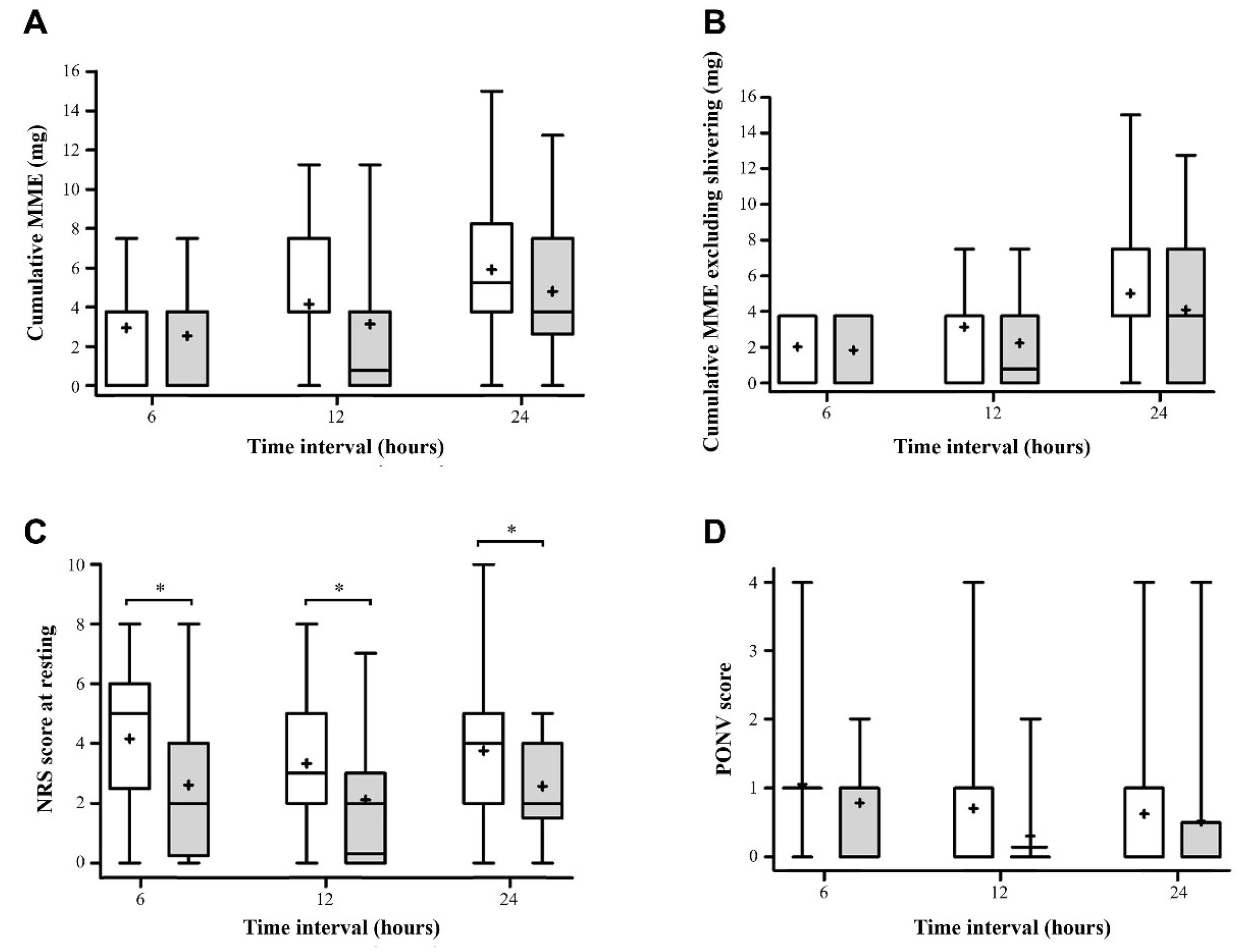
| Parameter | Control Group (n = 37) | Block Group (n = 37) |
|---|---|---|
| Sex (female) | 26 (70.3%) | 21 (56.8%) |
| Age (year) | 51.0 (46.0, 58.0) | 53.0 (44.0, 60.0) |
| Height (cm) | 158.0 (153.6, 165.6) | 164.5 (156.0, 172.0) |
| Weight (kg) | 65.1 ± 11.2 | 67.2 ± 12.8 |
| BMI (kg/m2) | 25.5 ± 2.9 | 25.0 ± 3.2 |
| ASA-PS | ||
| I | 12 (32.4%) | 14 (37.8%) |
| II | 25 (67.6%) | 23 (62.2%) |
| Current smoker | 5 (13.5%) | 1 (2.7%) |
| Hypertension | 7 (18.9%) | 10 (27.0%) |
| Diabetes | 3 (8.1%) | 3 (8.1%) |
| Site of operation (left) | 19 (51.4%) | 22 (59.5%) |
| QoR-15K, preoperative | 139.2 ± 12.8 | 137.2 ± 19.4 |
| Parameter | Control Group (n = 37) | Block Group (n = 37) | p-Value |
|---|---|---|---|
| Duration of induction (min) | 14.1 ± 3.88 | 19.8 ± 6.77 | <0.001 * |
| Duration of operation (min) | 102 ± 19.2 | 106 ± 24.1 | 0.388 |
| Pethidine (mg) | 30.0 (25.0, 35.0) | 30.0 (25.0, 35.0) | 0.262 |
| Propofol (mg) | 1240.0 (1100.0, 1480.0) | 1400.0 (1140.0, 1640.0) | 0.36 |
| Remifentanil (mg) | 0.9 (0.8, 1.0) | 0.8 (0.6, 1.1) | 0.295 |
| Use of ephedrine (n) | 7 (18.9%) | 14 (37.8%) | 0.122 |
| Use of nicardipine (n) | 4 (10.8%) | 3 (8.1%) | >0.99 |
| Outcome | Control Group (n = 37) | Block Group (n = 37) | p-Value |
|---|---|---|---|
| Pain score (NRS, 0–10) | |||
| 6 h postoperative § | 5 (3, 6) | 2 (1, 4) | 0.005 * |
| 12 h postoperative § | 3 (2, 5) | 2 (0, 2) | 0.007 * |
| 24 h postoperative | 4 (2, 5) | 2 (2, 4) | 0.015 * |
| Max NRS for 24 h | 7 (5, 7) | 4 (2, 6) | <0.001 * |
| AUC over 24 h | 7 (5.5, 9) | 4 (2, 6) | <0.001 * |
| Patients requiring pethidine (n %) | |||
| 6 h | 27 (73.0) | 24 (64.9) | 0.451 † |
| 12 h | 30 (81.1) | 27 (73.0) | 0.407 † |
| 24 h | 31 (83.8) | 28 (75.7) | 0.386 † |
| Frequency of pethidine administration | |||
| 6 h | 1 (0, 1) | 1 (0, 1) | 0.393 ‡ |
| 12 h | 1 (1, 2) | 1 (0, 1) | 0.081 ‡ |
| 24 h | 1 (1, 2) | 1 (0.5, 2) | 0.215 ‡ |
| Patients requiring pethidine, nonshivering (n %) | |||
| 6 h | 21 (56.8) | 18 (38.6) | 0.485 |
| 12 h | 28 (75.7) | 23 (62.2) | 0.209 |
| 24 h | 30 (81.1) | 24 (64.9) | 0.116 |
| Postoperative ketorolac (mg) | |||
| 6 h § | 6.49 ± 12.52 | 5.68 ± 13.85 | 0.792 |
| 12 h § | 12.97 ± 19.42 | 7.30 ± 14.84 | 0.486 |
| 24 h | 25.14 ± 28.73 | 13.78 ± 21.90 | 0.180 |
| Postoperative acetaminophen (g) | |||
| 6 h | 0.97 ± 0.37 | 1.03 ± 0.16 | 0.422 |
| 12 h | 1.89 ± 0.52 | 1.97 ± 0.16 | 0.367 |
| 24 h | 2.89 ± 0.81 | 3.11 ± 0.39 | 0.150 |
| Postoperative nausea scores (0–4) | |||
| 6 h § | 8/21/7/0/1 | 11/22/3/0/0 | 0.337 † |
| 12 h § | 19/12/5/0/1 | 27/6/3/0/0 | 0.130 † |
| 24 h | 23/8/4/1/1 | 28/4/1/3/1 | 0.308 |
| Postoperative metoclopramide (mg) | |||
| 6 h § | 1.35 ± 3.47 | 0.54 ± 2.29 | 0.720 |
| 12 h § | 3.51 ± 5.88 | 0.81 ± 2.77 | 0.042 * |
| 24 h | 4.86 ± 7.68 | 2.43 ± 5.48 | 0.366 |
| QoR-15K, postoperative | 97.3 ± 32.9 | 107.0 ± 24.0 | 0.152 |
| Hospital stay (days) | 7.4 ± 1.0 | 7.2 ± 0.9 | 0.548 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.K.; Lee, S.; Kwon, J.-H.; Lee, S.H.; Park, S.J.; Kim, Y.; Kang, R.; Jeong, J.S.; Lee, J.J. The Efficacy of Scalp Nerve Block in Postoperative Pain Management after Microvascular Decompression: A Randomized Clinical Trial. J. Clin. Med. 2023, 12, 4242. https://doi.org/10.3390/jcm12134242
Lee EK, Lee S, Kwon J-H, Lee SH, Park SJ, Kim Y, Kang R, Jeong JS, Lee JJ. The Efficacy of Scalp Nerve Block in Postoperative Pain Management after Microvascular Decompression: A Randomized Clinical Trial. Journal of Clinical Medicine. 2023; 12(13):4242. https://doi.org/10.3390/jcm12134242
Chicago/Turabian StyleLee, Eun Kyung, Seungwon Lee, Ji-Hye Kwon, Seung Hoon Lee, Soo Jung Park, Yunghun Kim, RyungA Kang, Ji Seon Jeong, and Jeong Jin Lee. 2023. "The Efficacy of Scalp Nerve Block in Postoperative Pain Management after Microvascular Decompression: A Randomized Clinical Trial" Journal of Clinical Medicine 12, no. 13: 4242. https://doi.org/10.3390/jcm12134242
APA StyleLee, E. K., Lee, S., Kwon, J.-H., Lee, S. H., Park, S. J., Kim, Y., Kang, R., Jeong, J. S., & Lee, J. J. (2023). The Efficacy of Scalp Nerve Block in Postoperative Pain Management after Microvascular Decompression: A Randomized Clinical Trial. Journal of Clinical Medicine, 12(13), 4242. https://doi.org/10.3390/jcm12134242





