Aminosteroid RM-581 Decreases Cell Proliferation of All Breast Cancer Molecular Subtypes, Alone and in Combination with Breast Cancer Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Proliferation and Drug Combination Assays
2.3. Spheroids Assay (3D Culture)
2.4. RNA Isolation and Quantitative Real-Time PCR (qPCR)
3. Results
3.1. RM-581 Has an Antiproliferative Effect on Breast Cancer Cell Lines
3.1.1. RM-581 Antiproliferative Effect Found in 2D Culture
3.1.2. RM-581 Antiproliferative Effect Found in 3D Culture
3.2. RM-581 Is More Effective in Combination with Other Breast Cancer Treatments
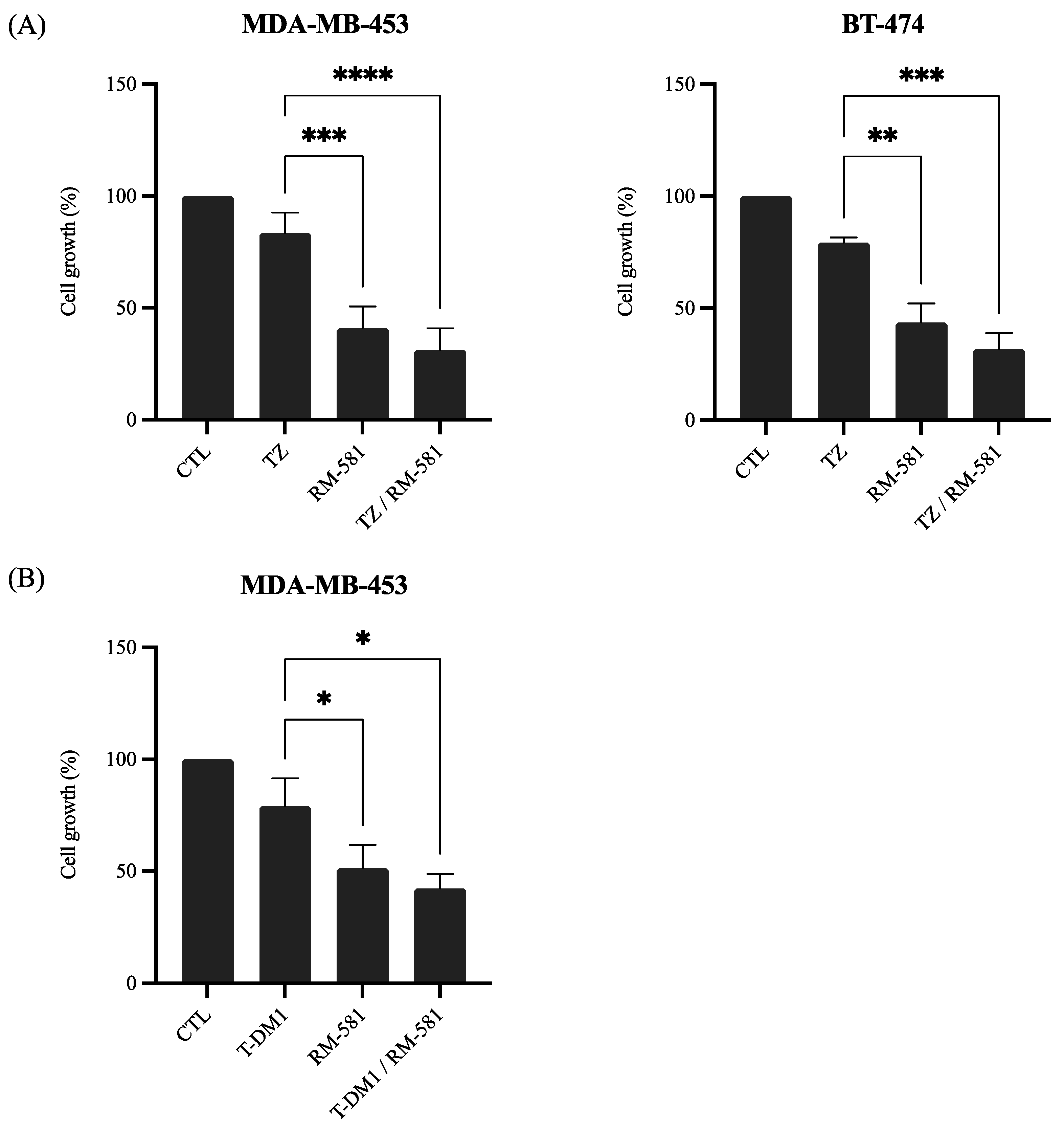
3.2.1. RM-581 in Combination with Anti-HER2 Therapies
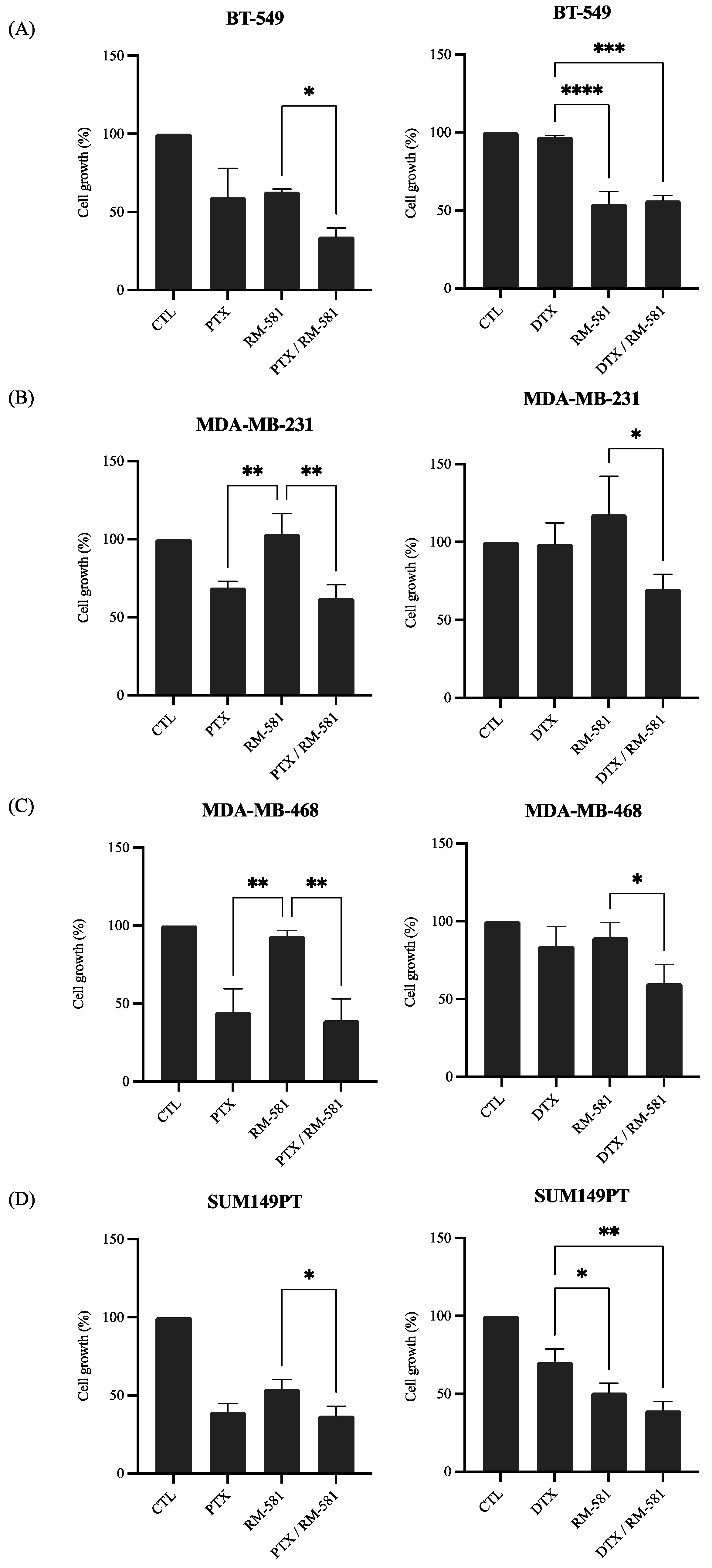
3.2.2. RM-581 in Combination with Chemotherapy
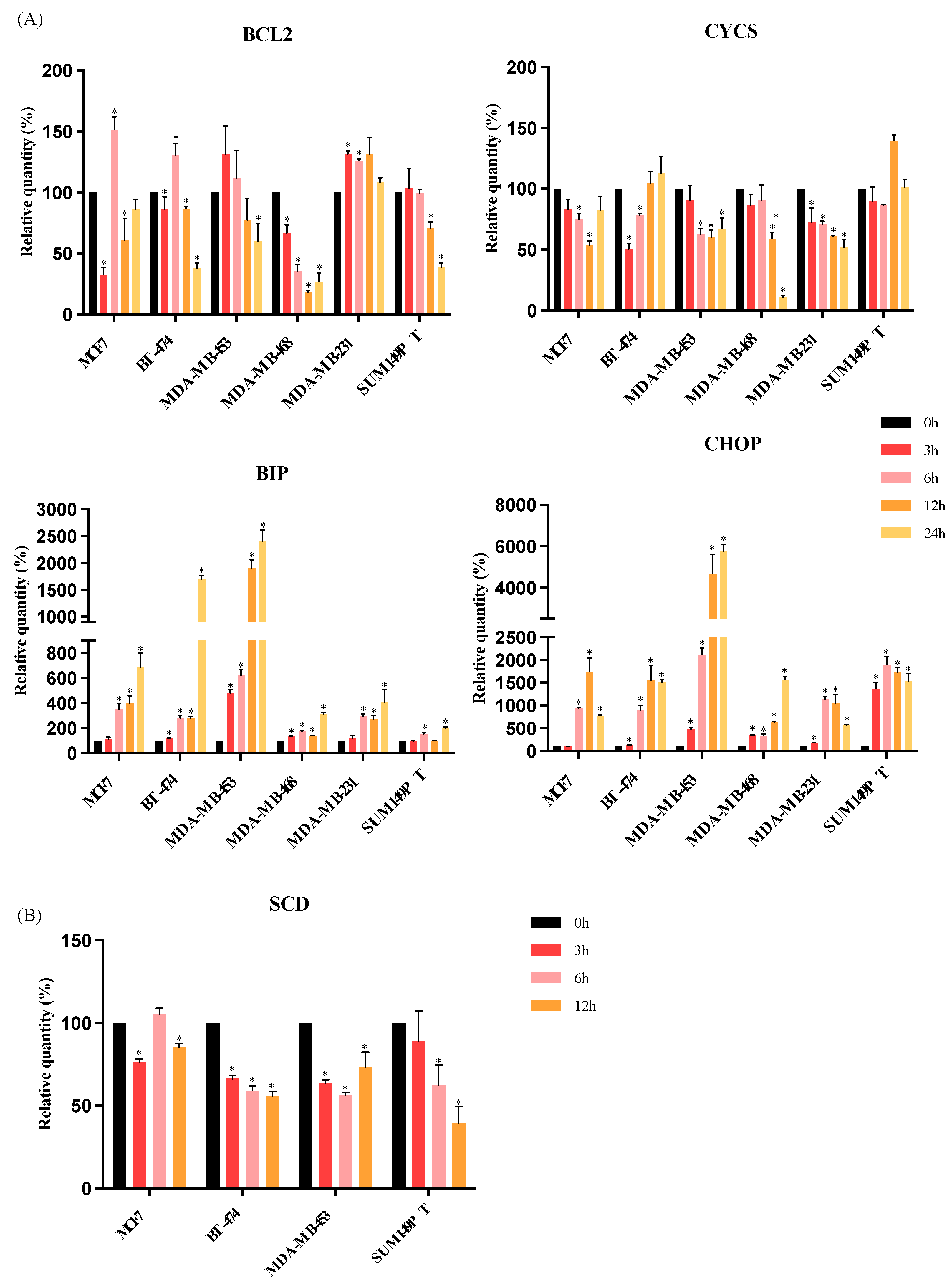
3.3. RM-581 Increases the Expression of Endoplasmic Reticulum Stress Apoptosis Markers
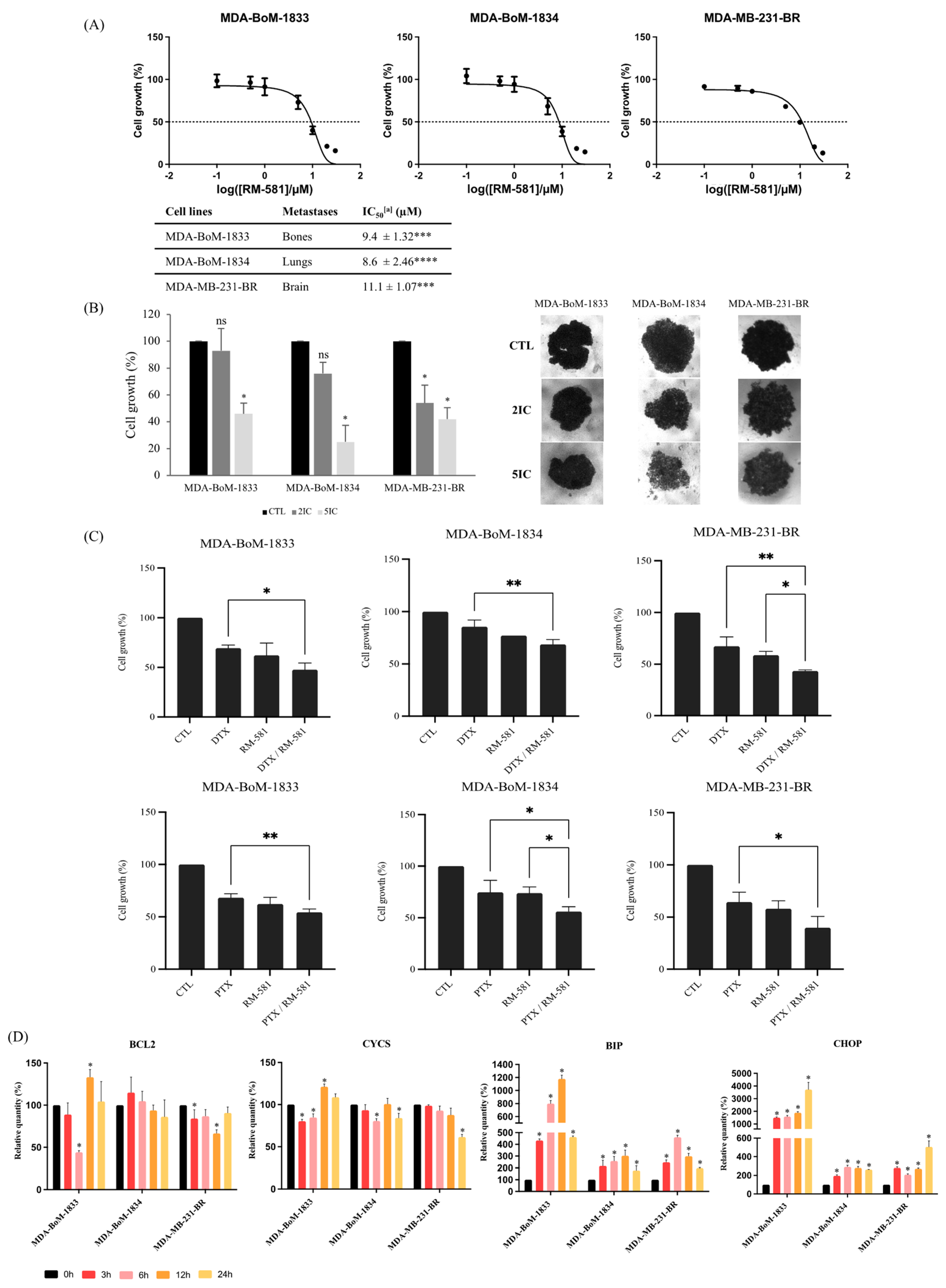
3.4. RM-581 Is Effective against TNBC Derivative Metastasis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Gnant, M. Breast Cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Cronin, K.A.; Kurian, A.W.; Andridge, R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-Negative Breast Cancer: Challenges and Opportunities of a Heterogeneous Disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Maltais, R.; Hospital, A.; Delhomme, A.; Roy, J.; Poirier, D. Chemical Synthesis, NMR Analysis and Evaluation on a Cancer Xenograft Model (HL-60) of the Aminosteroid Derivative RM-133. Steroids 2014, 82, 68–76. [Google Scholar] [CrossRef]
- Talbot, A.; Maltais, R.; Poirier, D. New Diethylsilylacetylenic Linker for Parallel Solid-Phase Synthesis of Libraries of Hydroxy Acetylenic Steroid Derivatives with Improved Metabolic Stability. ACS Comb. Sci. 2012, 14, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Jegham, H.; Maltais, R.; Roy, J.; Doillon, C.; Poirier, D. Biological Evaluation of a New Family of Aminosteroids That Display a Selective Toxicity for Various Malignant Cell Lines. Anticancer Drugs 2012, 23, 803–814. [Google Scholar] [CrossRef]
- Kenmogne, L.C.; Ayan, D.; Roy, J.; Maltais, R.; Poirier, D. The Aminosteroid Derivative RM-133 Shows In Vitro and In Vivo Antitumor Activity in Human Ovarian and Pancreatic Cancers. PLoS ONE 2015, 10, e0144890. [Google Scholar] [CrossRef]
- Maltais, R.; Perreault, M.; Roy, J.; Poirier, D. Minor Chemical Modifications of the Aminosteroid Derivative RM-581 Lead to Major Impact on Its Anticancer Activity, Metabolic Stability and Aqueous Solubility. Eur. J. Med. Chem. 2020, 188, 111990. [Google Scholar] [CrossRef]
- Perreault, M.; Maltais, R.; Roy, J.; Dutour, R.; Poirier, D. Design of a Mestranol 2-N-Piperazino-Substituted Derivative Showing Potent and Selective in Vitro and in Vivo Activities in MCF-7 Breast Cancer Models. ChemMedChem 2017, 12, 177–182. [Google Scholar] [CrossRef]
- Perreault, M.; Maltais, R.; Roy, J.; Picard, S.; Popa, I.; Bertrand, N.; Poirier, D. Induction of Endoplasmic Reticulum Stress by Aminosteroid Derivative RM-581 Leads to Tumor Regression in PANC-1 Xenograft Model. Investig. New Drugs 2019, 37, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Maltais, R.; Jegham, H.; Poirier, D. Libraries of 2β-(N-Substituted Piperazino)-5α-Androstane-3α, 17β-Diols: Chemical Synthesis and Cytotoxic Effects on Human Leukemia HL-60 Cells and on Normal Lymphocytes. Mol. Divers. 2011, 15, 317–339. [Google Scholar] [CrossRef] [PubMed]
- Ayan, D.; Maltais, R.; Hospital, A.; Poirier, D. Chemical Synthesis, Cytotoxicity, Selectivity and Bioavailability of 5α-Androstane-3α,17β-Diol Derivatives. Bioorg. Med. Chem. 2014, 22, 5847–5859. [Google Scholar] [CrossRef] [PubMed]
- Maltais, R.; Roy, J.; Perreault, M.; Sato, S.; Lévesque, J.-C.; Poirier, D. Induction of Endoplasmic Reticulum Stress-Mediated Apoptosis by Aminosteroid RM-581 Efficiently Blocks the Growth of PC-3 Cancer Cells and Tumors Resistant or Not to Docetaxel. Int. J. Mol. Sci. 2021, 22, 11181. [Google Scholar] [CrossRef]
- Schröder, M.; Kaufman, R.J. The Mammalian Unfolded Protein Response. Annu. Rev. Biochem. 2005, 74, 739–789. [Google Scholar] [CrossRef]
- Riha, R.; Gupta-Saraf, P.; Bhanja, P.; Badkul, S.; Saha, S. Stressed Out—Therapeutic Implications of ER Stress Related Cancer Research. Oncomedicine 2017, 2, 156–167. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of Natural Products on Developing New Anti-Cancer Agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef]
- Kelloff, G.J.; Crowell, J.A.; Steele, V.E.; Lubet, R.A.; Malone, W.A.; Boone, C.W.; Kopelovich, L.; Hawk, E.T.; Lieberman, R.; Lawrence, J.A.; et al. Progress in Cancer Chemoprevention: Development of Diet-Derived Chemopreventive Agents. J. Nutr. 2000, 130, 467S–471S. [Google Scholar] [CrossRef]
- Kim, C.; Song, H.-S.; Park, H.; Kim, B. Activation of ER Stress-Dependent MiR-216b Has a Critical Role in Salvia Miltiorrhiza Ethanol-Extract-Induced Apoptosis in U266 and U937 Cells. Int. J. Mol. Sci. 2018, 19, 1240. [Google Scholar] [CrossRef]
- Cha, J.; Song, H.-S.; Kang, B.; Park, M.; Park, K.; Kim, S.-H.; Shim, B.-S.; Kim, B. MiR-211 Plays a Critical Role in Cnidium Officinale Makino Extract-Induced, ROS/ER Stress-Mediated Apoptosis in U937 and U266 Cells. Int. J. Mol. Sci. 2018, 19, 865. [Google Scholar] [CrossRef]
- Clarke, R.; Cook, K.L.; Hu, R.; Facey, C.O.B.; Tavassoly, I.; Schwartz, J.L.; Baumann, W.T.; Tyson, J.J.; Xuan, J.; Wang, Y.; et al. Endoplasmic Reticulum Stress, the Unfolded Protein Response, Autophagy, and the Integrated Regulation of Breast Cancer Cell Fate. Cancer Res. 2012, 72, 1321–1331. [Google Scholar] [CrossRef]
- Ko, E.-Y.; Moon, A. Natural Products for Chemoprevention of Breast Cancer. J. Cancer Prev. 2015, 20, 223–231. [Google Scholar] [CrossRef]
- Kamiya, T.; Nishihara, H.; Hara, H.; Adachi, T. Ethanol Extract of Brazilian Red Propolis Induces Apoptosis in Human Breast Cancer MCF-7 Cells through Endoplasmic Reticulum Stress. J. Agric. Food Chem. 2012, 60, 11065–11070. [Google Scholar] [CrossRef]
- Shi, J.-M.; Bai, L.-L.; Zhang, D.-M.; Yiu, A.; Yin, Z.-Q.; Han, W.-L.; Liu, J.-S.; Li, Y.; Fu, D.-Y.; Ye, W.-C. Saxifragifolin D Induces the Interplay between Apoptosis and Autophagy in Breast Cancer Cells through ROS-Dependent Endoplasmic Reticulum Stress. Biochem. Pharmacol. 2013, 85, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Burguin, A.; Furrer, D.; Ouellette, G.; Jacob, S.; Diorio, C.; Durocher, F. Trastuzumab Effects Depend on HER2 Phosphorylation in HER2-Negative Breast Cancer Cell Lines. PLoS ONE 2020, 15, e0234991. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Kim, C.; Wolmark, N. HER2 Status and Benefit from Adjuvant Trastuzumab in Breast Cancer. N. Engl. J. Med. 2008, 358, 1409–1411. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody-Cytotoxic Drug Conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Burguin, A.; Diorio, C.; Durocher, F. Breast Cancer Treatments: Updates and New Challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef]
- Koeberle, A.; Löser, K.; Thürmer, M. Stearoyl-CoA Desaturase-1 and Adaptive Stress Signaling. Biochim. Biophys. Acta 2016, 1861, 1719–1726. [Google Scholar] [CrossRef]
- Bergin, A.R.T.; Loi, S. Triple-Negative Breast Cancer: Recent Treatment Advances. F1000Research 2019, 8, 1342. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Siegel, P.M.; Shu, W.; Drobnjak, M.; Kakonen, S.M.; Cordón-Cardo, C.; Guise, T.A.; Massagué, J. A Multigenic Program Mediating Breast Cancer Metastasis to Bone. Cancer Cell 2003, 3, 537–549. [Google Scholar] [CrossRef]
- Dun, M.D.; Chalkley, R.J.; Faulkner, S.; Keene, S.; Avery-Kiejda, K.A.; Scott, R.J.; Falkenby, L.G.; Cairns, M.J.; Larsen, M.R.; Bradshaw, R.A.; et al. Proteotranscriptomic Profiling of 231-BR Breast Cancer Cells: Identification of Potential Biomarkers and Therapeutic Targets for Brain Metastasis. Mol. Cell. Proteom. MCP 2015, 14, 2316–2330. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Fisusi, F.A.; Akala, E.O. Drug Combinations in Breast Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 3–23. [Google Scholar] [CrossRef]
- Wuerstlein, R.; Harbeck, N. Neoadjuvant Therapy for HER2-Positive Breast Cancer. Rev. Recent Clin. Trials 2017, 12, 81–92. [Google Scholar] [CrossRef]
- Minckwitz, G.; Procter, M.; Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- Amiri-Kordestani, L.; Blumenthal, G.M.; Xu, Q.C.; Zhang, L.; Tang, S.W.; Ha, L.; Weinberg, W.C.; Chi, B.; Candau-Chacon, R.; Hughes, P.; et al. FDA Approval: Ado-Trastuzumab Emtansine for the Treatment of Patients with HER2-Positive Metastatic Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 4436–4441. [Google Scholar] [CrossRef] [PubMed]
- Wahba, H.A.; El-Hadaad, H.A. Current Approaches in Treatment of Triple-Negative Breast Cancer. Cancer Biol. Med. 2015, 12, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Female Breast Cancer Subtypes—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/breast-subtypes.html (accessed on 5 May 2022).
- Minn, A.J.; Gupta, G.P.; Siegel, P.M.; Bos, P.D.; Shu, W.; Giri, D.D.; Viale, A.; Olshen, A.B.; Gerald, W.L.; Massagué, J. Genes That Mediate Breast Cancer Metastasis to Lung. Nature 2005, 436, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Sisinni, L.; Pietrafesa, M.; Lepore, S.; Maddalena, F.; Condelli, V.; Esposito, F.; Landriscina, M. Endoplasmic Reticulum Stress and Unfolded Protein Response in Breast Cancer: The Balance between Apoptosis and Autophagy and Its Role in Drug Resistance. Int. J. Mol. Sci. 2019, 20, 857. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S. GRP78 Induction in Cancer: Therapeutic and Prognostic Implications. Cancer Res. 2007, 67, 3496–3499. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wey, S.; Zhang, Y.; Ye, R.; Lee, A.S. Role of the Unfolded Protein Response Regulator GRP78/BiP in Development, Cancer, and Neurological Disorders. Antioxid. Redox Signal. 2009, 11, 2307–2316. [Google Scholar] [CrossRef]
- Lee, A.S. The ER Chaperone and Signaling Regulator GRP78/BiP as a Monitor of Endoplasmic Reticulum Stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. CHOP Induces Death by Promoting Protein Synthesis and Oxidation in the Stressed Endoplasmic Reticulum. Genes Dev. 2004, 18, 3066–3077. [Google Scholar] [CrossRef]
- Maltais, R.; Roy, J.; Poirier, D. Turning a Quinoline-Based Steroidal Anticancer Agent into Fluorescent Dye for Its Tracking by Cell Imaging. ACS Med. Chem. Lett. 2021, 12, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.R.; Schulze, A. Lipid Metabolism in Cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, G.; Plantamura, I.; Tagliabue, E.; Iorio, M.V.; Cataldo, A. Breast Cancer Drug Resistance: Overcoming the Challenge by Capitalizing on MicroRNA and Tumor Microenvironment Interplay. Cancers 2021, 13, 3691. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M.; Kapanen, A.I.; Junttila, T.; Raheem, O.; Grenman, S.; Elo, J.; Elenius, K.; Isola, J. Characterization of a Novel Cell Line Established from a Patient with Herceptin-Resistant Breast Cancer. Mol. Cancer Ther. 2004, 3, 1585–1592. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-Based Drug Screen: Considerations and Practical Approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]

| Cell Lines | Molecular Subtype | IC50 (µM) [a] |
|---|---|---|
| MCF10A | Normal | 17.1 ± 0.61 |
| MCF7 | Luminal A | 2.8 ± 1.15 **** |
| BT-474 | Luminal B | 12.0 ± 4.28 ** |
| MDA-MB-453 | HER2 | 13.4 ± 0.97 |
| JIMT-1 | HER2 | 12.3 ± 2.21 * |
| MDA-MB-231 | TNBC | 10.4 ± 2.40 *** |
| BT-549 | TNBC | 8.7 ± 0.37 **** |
| SUM159PT | TNBC | 8.5 ± 1.10 **** |
| MDA-MB-468 | TNBC | 6.9 ± 1.03 **** |
| SUM149PT | TNBC | 5.6 ± 0.96 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burguin, A.; Roy, J.; Ouellette, G.; Maltais, R.; Bherer, J.; Diorio, C.; Poirier, D.; Durocher, F. Aminosteroid RM-581 Decreases Cell Proliferation of All Breast Cancer Molecular Subtypes, Alone and in Combination with Breast Cancer Treatments. J. Clin. Med. 2023, 12, 4241. https://doi.org/10.3390/jcm12134241
Burguin A, Roy J, Ouellette G, Maltais R, Bherer J, Diorio C, Poirier D, Durocher F. Aminosteroid RM-581 Decreases Cell Proliferation of All Breast Cancer Molecular Subtypes, Alone and in Combination with Breast Cancer Treatments. Journal of Clinical Medicine. 2023; 12(13):4241. https://doi.org/10.3390/jcm12134241
Chicago/Turabian StyleBurguin, Anna, Jenny Roy, Geneviève Ouellette, René Maltais, Juliette Bherer, Caroline Diorio, Donald Poirier, and Francine Durocher. 2023. "Aminosteroid RM-581 Decreases Cell Proliferation of All Breast Cancer Molecular Subtypes, Alone and in Combination with Breast Cancer Treatments" Journal of Clinical Medicine 12, no. 13: 4241. https://doi.org/10.3390/jcm12134241
APA StyleBurguin, A., Roy, J., Ouellette, G., Maltais, R., Bherer, J., Diorio, C., Poirier, D., & Durocher, F. (2023). Aminosteroid RM-581 Decreases Cell Proliferation of All Breast Cancer Molecular Subtypes, Alone and in Combination with Breast Cancer Treatments. Journal of Clinical Medicine, 12(13), 4241. https://doi.org/10.3390/jcm12134241







