Three Doses of COVID-19 Vaccines: A Retrospective Study Evaluating the Safety and the Immune Response in Patients with Multiple Sclerosis
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Demographic and Clinical Data Collection
2.3. Statistical Analysis
2.4. Ethics
3. Results
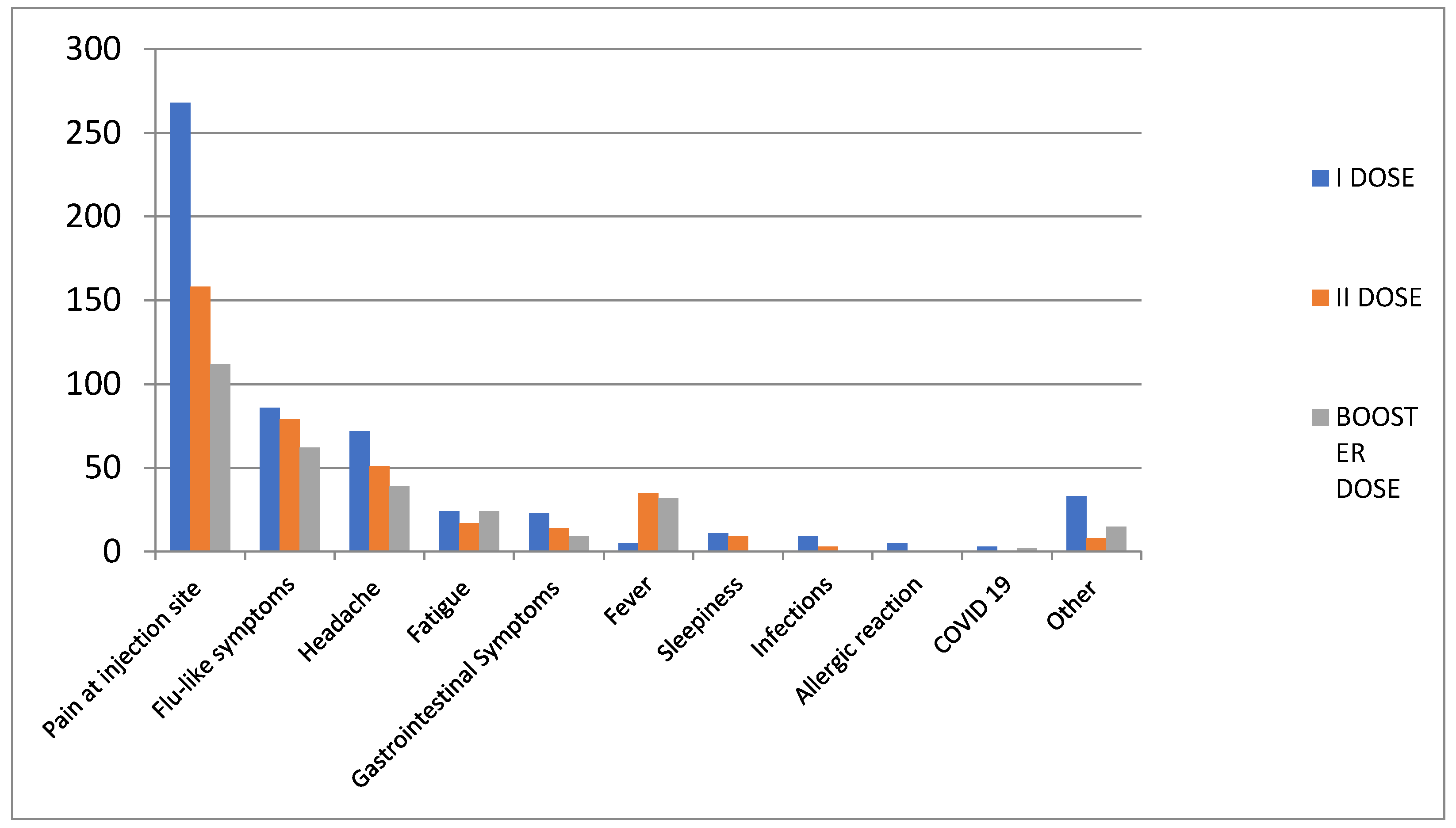
3.1. Overall Safety Results
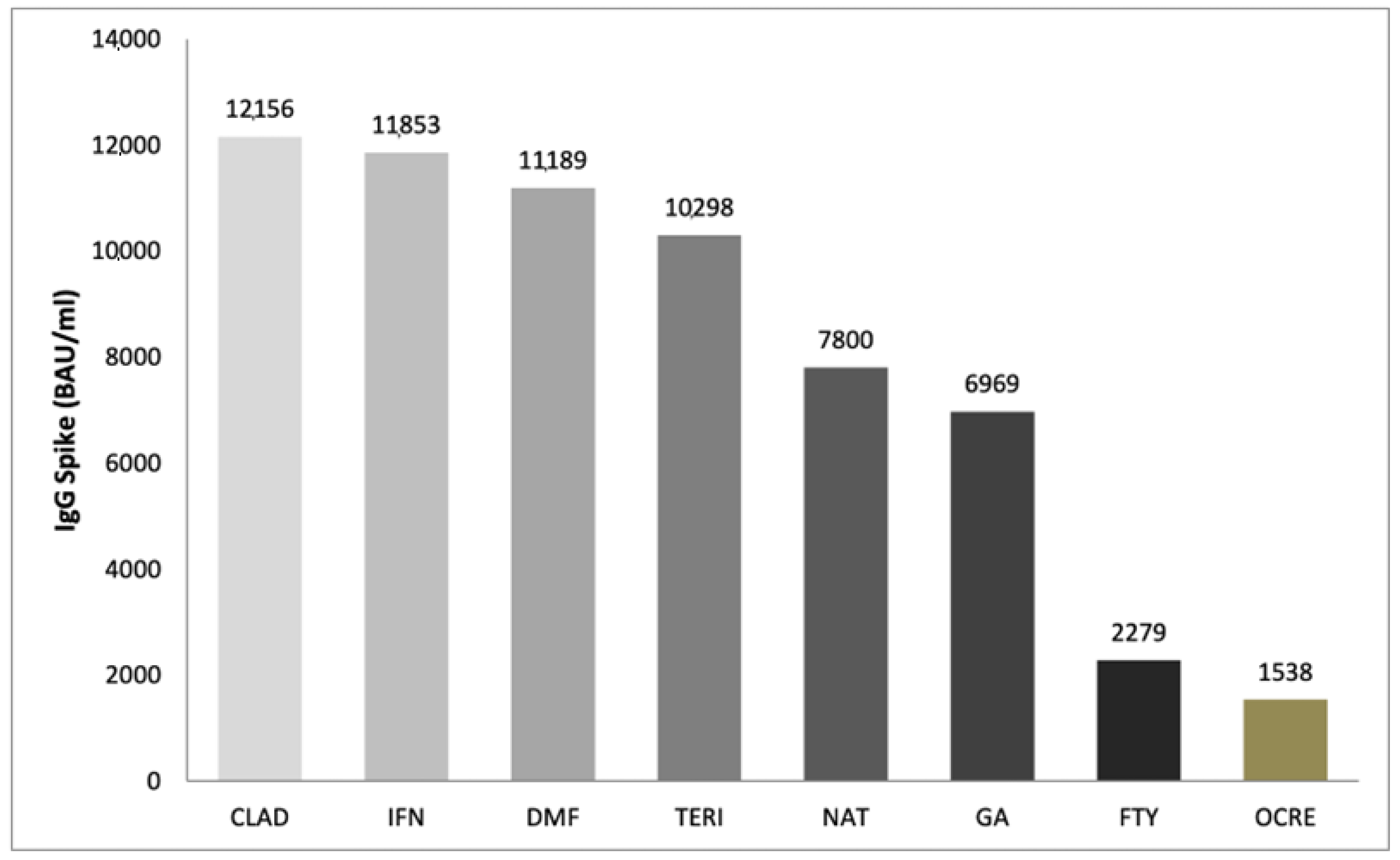
3.2. Immune Response Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raccomandazioni Aggiornate sul COVID-19 per le Persone con Sclerosi Multipla (SM)—12 Gennaio 2021 SIN ed AISM 12 Gennaio 2021 Ministero della Salute: Raccomandazioni ad Interim sui Gruppi Target della Vaccinazione Anti-SARS-CoV-2/COVID-19 8 Febbraio 2021. Available online: https://www.aism.it/sites/default/files/Raccomandazioni_COVID_SM_AISM_SIN.pdf (accessed on 16 November 2022).
- Maniscalco, G.T.; Scavone, C.; Moreggia, O.; Cesare, D.D.G.; Aiezza, M.L.; Guglielmi, G.; Longo, G.; Maiolo, M.; Raiola, E.; Russo, G.; et al. Flu vaccination in multiple sclerosis patients: A monocentric prospective vaccine-vigilance study. Expert Opin. Drug Saf. 2022, 21, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, G.T.; Manzo, V.; Di Battista, M.E.; Salvatore, S.; Moreggia, O.; Scavone, C.; Capuano, A. Severe Multiple Sclerosis Relapse After COVID-19 Vaccination: A Case Report. Front. Neurol. 2021, 12, 721502. [Google Scholar] [CrossRef] [PubMed]
- Ziello, A.; Scavone, C.; Di Battista, M.E.; Salvatore, S.; Cesare, D.D.G.; Moreggia, O.; Allegorico, L.; Sagnelli, A.; Barbato, S.; Manzo, V.; et al. Influenza Vaccine Hesitancy in Patients with Multiple Sclerosis: A Monocentric Observational Study. Brain Sci. 2021, 11, 890. [Google Scholar] [CrossRef]
- Maniscalco, G.T.; Scavone, C.; Mascolo, A.; Manzo, V.; Prestipino, E.; Guglielmi, G.; Aiezza, M.L.; Cozzolino, S.; Bracco, A.; Moreggia, O.; et al. The Safety Profile of COVID-19 Vaccines in Patients Diagnosed with Multiple Sclerosis: A Retrospective Observational Study. J. Clin. Med. 2022, 11, 6855. [Google Scholar] [CrossRef]
- Titus, H.E.; Chen, Y.; Podojil, J.R.; Robinson, A.P.; Balabanov, R.; Popko, B.; Miller, S.D. Pre-clinical and clinical implications of “inside-out” vs. “outside-in” paradigms in multiple sclerosis etiopathogenesis. Front. Cell. Neurosci. 2020, 14, 599717. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, L.; Shen, L.; Tang, K. Response of COVID-19 vaccination in multiple sclerosis patients following disease-modifying therapies: A meta-analysis. EBioMedicine 2022, 81, 104102. [Google Scholar] [CrossRef]
- Capuano, R.; Prosperini, L.; Altieri, M.; Lorefice, L.; Fantozzi, R.; Cavalla, P.; Guaschino, C.; Radaelli, M.; Cordioli, C.; Nociti, V.; et al. Symptomatic COVID-19 course and outcomes after three mRNA vaccine doses in multiple sclerosis patients treated with high-efficacy DMTs. Mult. Scler. J. 2023, 29, 856–865. [Google Scholar] [CrossRef]
- Harbo, H.F.; Gold, R.; Tintoré, M. Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef]
- Yamoah, P.; Mensah, K.B.; Attakorah, J.; Padayachee, N.; Oosthuizen, F.; Bangalee, V. Adverse events following immunization associated with coronavirus disease 2019 (COVID-19) vaccines: A descriptive analysis from VigiAccess. Hum. Vaccines Immunother. 2022, 18, 2109365. [Google Scholar] [CrossRef]
- Harris, T.; Nair, J.; Fediurek, J.; Deeks, S.L. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012–15. Vaccine 2017, 35, 2600–2604. [Google Scholar] [CrossRef]
- Zinzi, A.; Gaio, M.; Liguori, V.; Ruggiero, R.; Tesorone, M.; Rossi, F.; Rafaniello, C.; Capuano, A. Safety Monitoring of mRNA COVID-19 Vaccines in Children Aged 5 to 11 Years by Using EudraVigilance Pharmacovigilance Database: The CoVaxChild Study. Vaccines 2023, 11, 401. [Google Scholar] [CrossRef]
- Baba, C.; Ozcelik, S.; Kaya, E.; Samedzada, U.; Ozdogar, A.T.; Cevik, S.; Dogan, Y.; Ozakbas, S. Three doses of COVID-19 vaccines in multiple sclerosis patients treated with disease-modifying therapies. Mult. Scler. Relat. Disord. 2022, 68, 104119. [Google Scholar] [CrossRef]
- Dreyer-Alster, S.; Menascu, S.; Mandel, M.; Shirbint, E.; Magalashvili, D.; Dolev, M.; Flechter, S.; Givon, U.; Guber, D.; Stern, Y.; et al. COVID-19 vaccination in patients with multiple sclerosis: Safety and humoral efficacy of the third booster dose. J. Neurol. Sci. 2022, 434, 120155. [Google Scholar] [CrossRef]
- Castaldo, M.; Waliszewska-Prosół, M.; Koutsokera, M.; Robotti, M.; Straburzyński, M.; Apostolakopoulou, L.; Capizzi, M.; Çibuku, O.; Ambat, F.D.F.; Frattale, I.; et al. Headache onset after vaccination against SARS-CoV-2: A systematic literature review and meta-analysis. J. Headache Pain 2022, 23, 41. [Google Scholar] [CrossRef]
- Scavone, C.; Mascolo, A.; Rafaniello, C.; Sportiello, L.; Trama, U.; Zoccoli, A.; Bernardi, F.F.; Racagni, G.; Berrino, L.; Castaldo, G.; et al. Therapeutic strategies to fight COVID-19: Which is the status artis? Br. J. Pharmacol. 2021, 179, 2128–2148. [Google Scholar] [CrossRef]
- Mascolo, A.; Scavone, C.; Rafaniello, C.; De Angelis, A.; Urbanek, K.; di Mauro, G.; Cappetta, D.; Berrino, L.; Rossi, F.; Capuano, A. The Role of Renin-Angiotensin-Aldosterone System in the Heart and Lung: Focus on COVID-19. Front. Pharmacol. 2021, 12, 667254. [Google Scholar] [CrossRef]
- Pitzalis, M.; Idda, M.L.; Lodde, V.; Loizedda, A.; Lobina, M.; Zoledziewska, M.; Virdis, F.; Delogu, G.; Pirinu, F.; Marini, M.G.; et al. Effect of Different Disease-Modifying Therapies on Humoral Response to BNT162b2 Vaccine in Sardinian Multiple Sclerosis Patients. Front. Immunol. 2021, 12, 781843. [Google Scholar] [CrossRef]
- Garjani, A.; Patel, S.; Bharkhada, D.; Rashid, W.; Coles, A.; Law, G.R.; Evangelou, N. Impact of mass vaccination on SARS-CoV-2 infections among multiple sclerosis patients taking immunomodulatory disease-modifying therapies in England. Mult. Scler. Relat. Disord. 2021, 57, 103458. [Google Scholar] [CrossRef]
- Torres, P.; Sancho-Saldaña, A.; Gil Sánchez, A.; Peralta, S.; Solana, M.J.; Bakkioui, S.; González-Mingot, C.; Quibus, L.; Ruiz-Fernández, E.; Pedro-Murillo, E.S.; et al. A prospective study of cellular immune response to booster COVID-19 vaccination in multiple sclerosis patients treated with a broad spectrum of disease-modifying therapies. J. Neurol. 2023, 270, 2380–2391. [Google Scholar] [CrossRef]
- König, M.; Torgauten, H.M.; Tran, T.T.; Holmøy, T.; Vaage, J.T.; Lund-Johansen, F.; Nygaard, G.O. Immunogenicity and Safety of a Third SARS-CoV-2 Vaccine Dose in Patients with Multiple Sclerosis and Weak Immune Response After COVID-19 Vaccination. JAMA Neurol. 2022, 79, 307–309. [Google Scholar] [CrossRef]
- Achiron, A.; Mandel, M.; Gurevich, M.; Dreyer-Alster, S.; Magalashvili, D.; Sonis, P.; Dolev, M.; Menascu, S.; Harari, G.; Flechter, S.; et al. Immune response to the third COVID-19 vaccine dose is related to lymphocyte count in multiple sclerosis patients treated with fingolimod. J. Neurol. 2022, 269, 2286–2292. [Google Scholar] [CrossRef] [PubMed]
- Madelon, N.; Heikkilä, N.; Royo, I.S.; Fontannaz, P.; Breville, G.; Lauper, K.; Goldstein, R.; Grifoni, A.; Sette, A.; Siegrist, C.-A.; et al. Omicron-Specific Cytotoxic T-Cell Responses After a Third Dose of mRNA COVID-19 Vaccine among Patients with Multiple Sclerosis Treated with Ocrelizumab. JAMA Neurol. 2022, 79, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Sessa, M.; Rafaniello, C.; Sportiello, L.; Mascolo, A.; Scavone, C.; Maccariello, A.; Iannaccone, T.; Fabrazzo, M.; Berrino, L.; Rossi, F.; et al. Campania Region (Italy) spontaneous reporting system and preventability assessment through a case-by-case approach: A pilot study on psychotropic drugs. Expert Opin. Drug Saf. 2016, 15, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sessa, M.; Sportiello, L.; Mascolo, A.; Scavone, C.; Gallipoli, S.; Di Mauro, G.; Cimmaruta, D.; Rafaniello, C.; Capuano, A. Campania Preventability Assessment Committee (Italy): A Focus on the Preventability of Non-steroidal Anti-inflammatory Drugs’ Adverse Drug Reactions. Front. Pharmacol. 2017, 8, 305. [Google Scholar] [CrossRef]
- Capuano, A.; Scavone, C.; Racagni, G.; Scaglione, F. NSAIDs in patients with viral infections, including COVID-19: Victims or perpetrators? Pharmacol. Res. 2020, 157, 104849. [Google Scholar] [CrossRef]
| Study Cohort | Patients Who Received the Third Dose | Patients with Blood Test Collected |
|---|---|---|
| Total patients | 210 | 60 |
| Female, n (%) | 136 (64.8) | 27 (45) |
| Male, n (%) | 74 (35.2) | 33 (55) |
| Mean age (years) ± sd | 46.3 ± 13.4 | 39.6 ± 10.5 |
| MS type, n (%) | ||
| RRMS | 193 (91.9) | 59 (98.3) |
| SPMS | 12 (5.7) | 1 (1.7) |
| PPMS | 4 (1.9) | - |
| PRMS | 1 (0.5) | - |
| Disability by EDSS, n (%) | ||
| ≤3.0 | 152 (72.4) | 55 (91.7) |
| 3.5–5.5 | 34 (16.2) | 3 (5.0) |
| ≥6.0 | 24 (11.4) | 2 (3.3) |
| DMTs, n (%) | ||
| ocrelizumab | 20 (9.5) | 8 (13.4) |
| fingolimod | 22 (10.5) | 7 (11.7) |
| interferon beta 1-a | 36 (17.1) | 9 (15) |
| natalizumab | 33 (15.7) | 11 (18.3) |
| dimethyl fumarate | 34 (16.2) | 11 (18.3) |
| cladribine | 12 (5.7) | 6 (10) |
| glatiramer acetate | 15 (7.1) | 3 (5) |
| teriflunomide | 23 (11) | 5 (8.3) |
| interferon beta 1-b | 6 (2.9) | - |
| methotrexate | 1 (0.5) | - |
| rituximab | 3 (1.4) | - |
| alemtuzumab | 1 (0.5) | - |
| Untreated | 4 (1.9) | - |
| Total | First Dose | Second Dose | Booster Dose | |
|---|---|---|---|---|
| Study population | 310 | 310 | 288 | 210 |
| All AEFIs, n | 1207 | 539 | 374 | 294 |
| Short-term AEFIs, n (%) | 1057 (87.6) | 438 (81.3) | 352 (94.1) | 267 (90.8) |
| Long-term AEFIs, n (%) | 150 (12.4) | 101(18.7) | 22 (5.9) | 27 (9.2) |
| Short-Term AEFIs | Long-Term AEFIs | |
|---|---|---|
| Any adverse events, n (%) | 267 | 27 |
| Pain at injection site | 111 (41.6) | 1 (3.7) |
| Flu-like symptoms | 56 (20.9) | 6 (22.2) |
| Headache | 34 (12.7) | 5 (18.5) |
| Fever | 32 (12) | - |
| Fatigue | 17 (6.4) | 7 (25.9) |
| Gastrointestinal symptoms | 8 (3) | 1 (3.7) |
| Other symptoms | 9 (3.4) | 5 (18.5) |
| Infection with SARS-CoV-2 after vaccination | - | 2 (7.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniscalco, G.T.; Di Giulio Cesare, D.; Liguori, V.; Manzo, V.; Prestipino, E.; Salvatore, S.; Di Battista, M.E.; Moreggia, O.; Ziello, A.R.; Andreone, V.; et al. Three Doses of COVID-19 Vaccines: A Retrospective Study Evaluating the Safety and the Immune Response in Patients with Multiple Sclerosis. J. Clin. Med. 2023, 12, 4236. https://doi.org/10.3390/jcm12134236
Maniscalco GT, Di Giulio Cesare D, Liguori V, Manzo V, Prestipino E, Salvatore S, Di Battista ME, Moreggia O, Ziello AR, Andreone V, et al. Three Doses of COVID-19 Vaccines: A Retrospective Study Evaluating the Safety and the Immune Response in Patients with Multiple Sclerosis. Journal of Clinical Medicine. 2023; 12(13):4236. https://doi.org/10.3390/jcm12134236
Chicago/Turabian StyleManiscalco, Giorgia Teresa, Daniele Di Giulio Cesare, Valerio Liguori, Valentino Manzo, Elio Prestipino, Simona Salvatore, Maria Elena Di Battista, Ornella Moreggia, Antonio Rosario Ziello, Vincenzo Andreone, and et al. 2023. "Three Doses of COVID-19 Vaccines: A Retrospective Study Evaluating the Safety and the Immune Response in Patients with Multiple Sclerosis" Journal of Clinical Medicine 12, no. 13: 4236. https://doi.org/10.3390/jcm12134236
APA StyleManiscalco, G. T., Di Giulio Cesare, D., Liguori, V., Manzo, V., Prestipino, E., Salvatore, S., Di Battista, M. E., Moreggia, O., Ziello, A. R., Andreone, V., Scavone, C., & Capuano, A. (2023). Three Doses of COVID-19 Vaccines: A Retrospective Study Evaluating the Safety and the Immune Response in Patients with Multiple Sclerosis. Journal of Clinical Medicine, 12(13), 4236. https://doi.org/10.3390/jcm12134236








