The EUPEMEN (EUropean PErioperative MEdical Networking) Protocol for Bowel Obstruction: Recommendations for Perioperative Care
Abstract
1. Introduction
2. Bowel Obstruction
3. Enhanced Recovery after Surgery (ERAS)
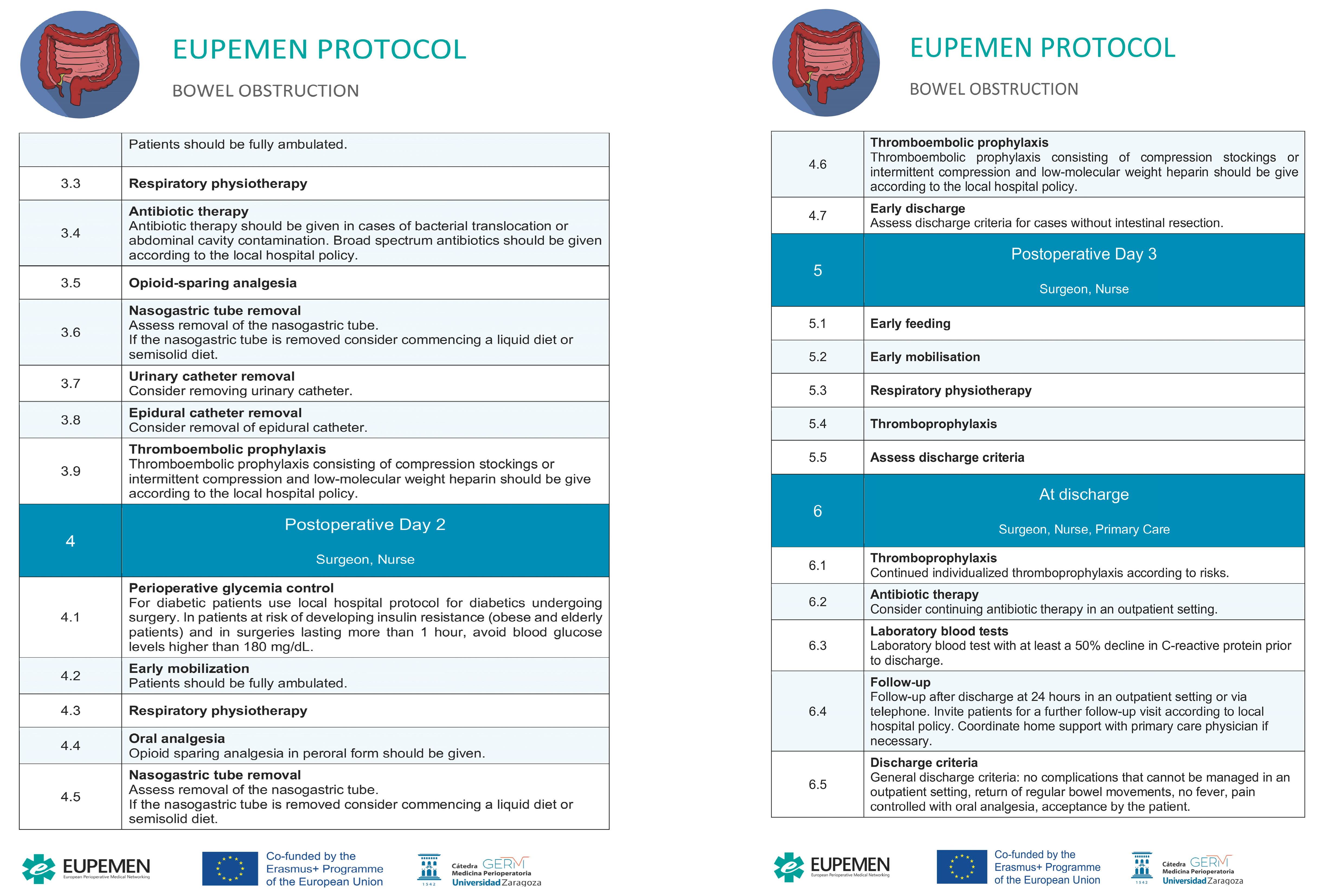
4. The EUPEMEN Bowel Obstruction Protocol (Figure 1)
4.1. Preoperative Phase
4.2. Intraoperative Phase
4.3. Immediate Postoperative Phase
4.4. First Postoperative Day
4.5. Second Postoperative Day
4.6. Third Postoperative Day
4.7. Discharge

5. ERAS in Bowel Obstruction
6. The EUPEMEN Project
- Preparation of an educational project (that included a teaching the teachers’ model);
- Implementation in a significant number of European hospitals of the evidence-based protocols in a homogeneous and standardised way;
- Collection of data about hospital stay, morbidity and mortality of European Surgical patients that once analysed through machine learning algorithm, will be of relevant interest to better know the surgical risk of an individual patient, hence to prevent perioperative complications.
- The preparation of the EUPEMEN Multimodal Rehabilitation manual with the protocols of 6 different modules:
- Bariatric Surgery;
- Oesophageal Surgery;
- Gastric Cancer Surgery;
- Colon Surgery;
- Hepatobiliary Surgery;
- Urgent abdominal surgery, including appendectomy and small bowel obstruction (SBO).
- The development of the EUPEMEN online platform (https://eupemen.eu/, accessed on 24 April 2023): to host the e-learning training course and a collaborative area to improve and to participate in the protocols;
- The training of the trainers to teach the future teachers the different protocol to be able to teach them in the different hospitals;
- The dissemination of the results in 5 Multiplier events, one per partner, to promote the protocols;
- The organization of 4 transnational meetings, one per country;
- The translation into English of the Recovery Intensification for optimal Care in Adult’s surgery—RICA from the Spanish de Recuperación Intensificada en Cirugía del Adulto (RICA).
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.J.; Baldini, G.; Fearon, K.C.H.; Feldheiser, A.; Feldman, L.S.; Gan, T.J.; Ljungqvist, O.; Lobo, D.N.; Rockall, T.A.; Schricker, T.; et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 1: Pathophysiological considerations. Acta Anaesthesiol. Scand. 2015, 59, 1212–1231. [Google Scholar] [CrossRef]
- Catena, F.; De Simone, B.; Coccolini, F.; Di Saverio, S.; Sartelli, M.; Ansaloni, L. Bowel obstruction: A narrative review for all physicians. World J. Emerg. Surg. 2019, 14, 20. [Google Scholar] [CrossRef]
- Gore, R.M.; Silvers, R.I.; Thakrar, K.H.; Wenzke, D.R.; Mehta, U.K.; Newmark, G.M.; Berlin, J.W. Bowel Obstruction. Radiol. Clin. N. Am. 2015, 53, 1225–1240. [Google Scholar] [CrossRef]
- Johnson, W.R.; Hawkins, A.T. Large Bowel Obstruction. Clin. Colon. Rectal Surg. 2021, 34, 233–241. [Google Scholar] [CrossRef]
- Long, B.; Robertson, J.; Koyfman, A. Emergency Medicine Evaluation and Management of Small Bowel Obstruction: Evidence-Based Recommendations. J. Emerg. Med. 2019, 56, 166–176. [Google Scholar] [CrossRef]
- Detz, D.J.; Podrat, J.L.; Muniz Castro, J.C.; Lee, Y.K.; Zheng, F.; Purnell, S.; Pei, K.Y. Small bowel obstruction. Curr. Probl. Surg. 2021, 58, 100893. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.; Ali, N.S.; Leshchinskiy, S.; Cherukuri, A.R.; Tam, J.K. Small bowel obstruction and the gastrografin challenge. Abdom. Radiol. 2018, 43, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Aka, A.A.; Wright, J.P.; DeBeche-Adams, T. Small Bowel Obstruction. Clin. Colon. Rectal Surg. 2021, 34, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Bower, K.L.; Lollar, D.I.; Williams, S.L.; Adkins, F.C.; Luyimbazi, D.T.; Bower, C.E. Small Bowel Obstruction. Surg. Clin. N. Am. 2018, 98, 945–971. [Google Scholar] [CrossRef]
- Diamond, M.; Lee, J.; LeBedis, C.A. Small Bowel Obstruction and Ischemia. Radiol. Clin. N. Am. 2019, 57, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Scott, M.J.; Schwenk, W.; Demartines, N.; Roulin, D.; Francis, N.; McNaught, C.E.; MacFie, J.; Liberman, A.S.; Soop, M.; et al. Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin. Nutr. 2012, 31, 783–800. [Google Scholar] [CrossRef]
- Nygren, J.; Thacker, J.; Carli, F.; Fearon, K.C.; Norderval, S.; Lobo, D.N.; Ljungqvist, O.; Soop, M.; Ramirez, J. Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin. Nutr. 2012, 31, 801–816. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Hubner, M.; Nygren, J.; Demartines, N.; Francis, N.; Rockall, T.A.; Young-Fadok, T.M.; Hill, A.G.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019, 43, 659–695. [Google Scholar] [CrossRef] [PubMed]
- Bisch, S.P.; Jago, C.A.; Kalogera, E.; Ganshorn, H.; Meyer, L.A.; Ramirez, P.T.; Dowdy, S.C.; Nelson, G. Outcomes of enhanced recovery after surgery (ERAS) in gynecologic oncology—A systematic review and meta-analysis. Gynecol. Oncol. 2021, 161, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Noba, L.; Rodgers, S.; Chandler, C.; Balfour, A.; Hariharan, D.; Yip, V.S. Enhanced Recovery After Surgery (ERAS) Reduces Hospital Costs and Improve Clinical Outcomes in Liver Surgery: A Systematic Review and Meta-Analysis. J. Gastrointest. Surg. 2020, 24, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yu, J.; Doumouras, A.G.; Li, J.; Hong, D. Enhanced recovery after surgery (ERAS) versus standard recovery for elective gastric cancer surgery: A meta-analysis of randomized controlled trials. Surg. Oncol. 2020, 32, 75–87. [Google Scholar] [CrossRef]
- Ye, Z.; Chen, J.; Shen, T.; Yang, H.; Qin, J.; Zheng, F.; Rao, Y. Enhanced recovery after surgery (ERAS) might be a standard care in radical prostatectomy: A systematic review and meta-analysis. Ann. Palliat. Med. 2020, 9, 746–758. [Google Scholar] [CrossRef]
- Noba, L.; Rodgers, S.; Doi, L.; Chandler, C.; Hariharan, D.; Yip, V. Costs and clinical benefits of enhanced recovery after surgery (ERAS) in pancreaticoduodenectomy: An updated systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2023. [Google Scholar] [CrossRef]
- Grupo de Trabajo. Vía Clínica de Recuperación Intensificada en Cirugía Abdominal (RICA). Vía Clínica de Recupe- Ración Intensificada en Cirugía Abdominal (RICA) Ministerio de Sanidad, Servicios Sociales e Igualdad. Instituto Aragonés de Ciencias de la Salud. 2014. Available online: https://portal.guiasalud.es/wp-content/uploads/2019/01/vc_1_viaclinica-rica.pdf (accessed on 24 April 2023).
- Ramirez, J.; Ruiz-López, P.; Gurumeta, A.; Arroyo-Sebastian, A.; Bruna-Esteban, M.; Sanchez, A.; Calvo-Vecino, J.; García-Erce, J.; García-Fernández, A.; Toro, M.; et al. CLINICAL PATHWAY Recovery Intensification for Optimal Care in Adult’s Surgery. 2021. Available online: https://www.researchgate.net/publication/354723117_CLINICAL_PATHWAY_Recovery_Intensification_for_optimal_Care_in_Adult%27s_surgery (accessed on 24 April 2023).
- Dalton, A.; Zafirova, Z. Preoperative Management of the Geriatric Patient: Frailty and Cognitive Impairment Assessment. Anesthesiol. Clin. 2018, 36, 599–614. [Google Scholar] [CrossRef]
- Subramaniam, S.; Aalberg, J.; Soriano, R.P.; Divino, C.M. New 5-Factor Modified Frailty Index Using American College of Surgeons NSQIP Data. J. Am. Coll. Surg. 2018, 226, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zou, Y.; Zhao, J.; Schneider, D.B.; Yang, Y.; Ma, Y.; Huang, B.; Yuan, D. The Impact of Frailty on Outcomes of Elderly Patients After Major Vascular Surgery: A Systematic Review and Meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 591–602. [Google Scholar] [CrossRef]
- Castellví Valls, J.; Borrell Brau, N.; Bernat, M.J.; Iglesias, P.; Reig, L.; Pascual, L.; Vendrell, M.; Santos, P.; Viso, L.; Farreres, N.; et al. Colorectal carcinoma in the frail surgical patient. Implementation of a Work Area focused on the Complex Surgical Patient improves postoperative outcome. Cir. Esp. 2018, 96, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Hao, Q.; Zhou, J.; Dong, B. The impact of frailty and sarcopenia on postoperative outcomes in older patients undergoing gastrectomy surgery: A systematic review and meta-analysis. BMC Geriatr. 2017, 17, 188. [Google Scholar] [CrossRef]
- Torossian, A.; Bräuer, A.; Höcker, J.; Bein, B.; Wulf, H.; Horn, E.P. Preventing inadvertent perioperative hypothermia. Clinical Practice Guideline. Dtsch. Arztebl. Int. 2015, 112, 166–172. [Google Scholar] [PubMed]
- Warttig, S.; Alderson, P.; Campbell, G.; Smith, A.F. Interventions for treating inadvertent postoperative hypothermia. Cochrane Database Syst. Rev. 2014, 11, CD009892. [Google Scholar] [CrossRef]
- Hooper, V.D.; Chard, R.; Clifford, T.; Fetzer, S.; Fossum, S.; Godden, B.; Martinez, E.A.; Noble, K.A.; O’Brien, D.; Odom-Forren, J.; et al. ASPAN’s evidence- based clinical practice guideline for the promotion of perioperative normothermia: Second edition. J. Perianesth. Nurs. 2010, 25, 346–365. [Google Scholar] [CrossRef]
- Akhtar, Z.; Hesler, B.D.; Fiffick, A.N.; Mascha, E.J.; Sessler, D.I.; Kurz, A.; Ayad, S.; Saager, L. A randomized trial of prewarming on patient satisfaction and thermal comfort in outpatient surgery. J. Clin. Anesth. 2016, 33, 376–385. [Google Scholar] [CrossRef]
- Protocolo de Trabajo del IQZ 2017. Available online: https://infeccionquirurgicazero.es/es/documentos-y-materiales/protocolos-de-trabajo (accessed on 1 June 2020).
- Pontes, J.P.J.; Mendes, F.F.; Vasconcelos, M.M.; Batista, N.R. Evaluation and perioperative management of patients with diabetes mellitus. A challenge for the anesthesiologist. Rev. Bras. Anestesiol. 2018, 68, 75–86. [Google Scholar] [CrossRef]
- Akiboye, F.; Rayman, G. Management of Hyperglycemia and Diabetes in Orthopedic Surgery. Curr. Diab. Rep. 2017, 17, 13. [Google Scholar] [CrossRef]
- Dhatariya, K.; Levy, N.; Hall, G.M. The impact of glycaemic variability on the surgical patient. Curr. Opin. Anaesthesiol. 2016, 29, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Membership of the Working Party; Barker, P.; Creasey, P.E.; Dhatariya, K.; Levy, N.; Lipp, A.; Nathanson, M.H.; Penfold, N.; Watson, B.; Woodcock, T. Peri-operative management of the surgical patient with diabetes 2015: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2015, 70, 1427–1440. [Google Scholar] [PubMed]
- Allegranzi, B.; Zayed, B.; Bischoff, P.; Kubilay, N.Z.; de Jonge, S.; de Vries, F.; Gomes, S.M.; Gans, S.; Wallert, E.D.; Wu, X.; et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e288–e303. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Bischoff, P.; de Jonge, S.; Kubilay, N.Z.; Zayed, B.; Gomes, S.M.; Abbas, M.; Atema, J.J.; Gans, S.; van Rijen, M.; et al. New WHO recommendations on preoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis. 2016, 16, e276–e287. [Google Scholar] [CrossRef]
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.M.; Mitchell, S.A.; Crosby, C. Impact of surgical site infection on healthcare costs and patient outcomes: A systematic review in six European countries. J. Hosp. Infect. 2017, 96, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Proyecto Infección Quirúrgica Zero del Sistema Nacional de Salud. Sociedad Española de Medicina Preventiva, Salud Pública e Higiene. 2016. Available online: https://infeccionquirurgicazero.es/images/stories/recursos/protocolo/2017/3-1-17-documento-Protocolo-IQZ.pdf (accessed on 24 April 2023).
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; Meyer, L.A.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society recommendations—2019 update. Int. J. Gynecol. Cancer 2019, 29, 651–668. [Google Scholar] [CrossRef]
- Wongkietkachorn, A.; Wongkietkachorn, N.; Rhunsiri, P. Preoperative needs-based education to reduce anxiety, increase satisfaction, and decrease time spent in day surgery: A randomized controlled trial. World J. Surg. 2018, 42, 666–674. [Google Scholar] [CrossRef]
- Programa de Cirugía Segura del Sistema Nacional de Salud. Ministerio de Sanidad, Servi- cios Sociales e Igualdad. 2016. Available online: https://seguridaddelpaciente.sanidad.gob.es/practicasSeguras/seguridadBloqueQuirurgico/docs/Protocolo-Proyecto-Cirugia-Segura.pdf (accessed on 24 April 2023).
- De Jager, E.; McKenna, C.; Bartlett, L.; Gunnarsson, R.; Ho, Y.H. postoperative Adverse Events Inconsistently Improved by The World Health Organization Surgical Safety Checklist: A Systematic Literature Review of 25 Studies. World J. Surg. 2016, 40, 1842–1858. [Google Scholar] [CrossRef] [PubMed]
- Abbott, T.E.F.; Ahmad, T.; Phull, M.K.; Fowler, A.J.; Hewson, R.; Biccard, B.M.; Chew, M.S.; Gillies, M.; Pearse, R.M.; International Surgical Outcomes Study (ISOS) group. The surgical safety checklist and patient outcomes after surgery: Prospective observational cohort study, systematic review and meta-analysis. Br. J. Anaesth. 2018, 120, 146–155. [Google Scholar] [CrossRef]
- Biccard, B.M.; Rodseth, R.; Cronje, L.; Agaba, P.; Chikumba, E.; Du Toit, L.; Farina, Z.; Fischer, S.; Gopalan, P.D.; Govender, K.; et al. A meta-analysis of the efficacy of preoperative surgical safety checklists to improve perioperative. S. Afr. Med. J. 2016, 106, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Nagappa, M.; Wong, J.; Singh, M.; Wong, D.; Chung, F. Continuous Pulse Oximetry and Capnography Monitoring for Postoperative Respiratory Depression and Adverse Events: A Systematic Review and Meta-analysis. Anesth. Analg. 2017, 125, 2019–2029. [Google Scholar] [CrossRef]
- Frerk, C.; Mitchell, V.S.; McNarry, A.F.; Mendonca, C.; Bhagrath, R.; Patel, A.; O’Sullivan, E.P.; Woodall, N.M.; Ahmad, I. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br. J. Anaesth. 2015, 115, 827–848. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Jones, M.R.; Orhurhu, V.; Sikorsky, A.; Seifert, D.; Flores, C.; Kaye, A.D.; Viswanath, O. A Comprehensive Update of Current Anesthesia Perspectives on Therapeutic Hypothermia. Adv. Ther. 2019, 36, 2223–2232. [Google Scholar] [CrossRef]
- Calvo Vecino, J.M.; Casans Francés, R.; Ripollés Melchor, J.; Marín Zaldívar, C.; Gómez Ríos, M.A.; Pérez Ferrer, A.; Zaballos Bustingorri, J.M.; Abad Gurumeta, A.; Grupo de trabajo de la GPC de Hipotermia Perioperatoria No Intencionada de la SEDAR. Clinical practice guideline. Unintentional perioperative hypothermia. Rev. Esp. Anestesiol. Reanim. 2018, 65, 564–588. [Google Scholar] [CrossRef]
- Madden, L.K.; Hill, M.; May, T.L.; Human, T.; Guanci, M.M.; Jacobi, J.; Moreda, M.V.; Badjatia, N. The Implementation of Targeted Temperature Management: An Evidence-Based Guideline from the Neurocritical Care Society. Neurocrit. Care 2017, 27, 468–487. [Google Scholar] [CrossRef]
- Makaryus, R.; Miller, T.E.; Gan, T.J. Current concepts of fluid management in enhanced recovery pathways. Br. J. Anaesth. 2018, 120, 376–383. [Google Scholar] [CrossRef]
- Joosten, A.; Delaporte, A.; Ickx, B.; Touihri, K.; Stany, I.; Barvais, L.; Van Obbergh, L.; Loi, P.; Rinehart, J.; Cannesson, M.; et al. Crystalloid versus colloid for intraoperative goal-directed fluid therapy using a closed-loop system: A randomized, double-blinded, controlled trial in major abdominal surgery. Anesthesiology 2018, 128, 55–66. [Google Scholar] [CrossRef]
- Kapoor, P.M.; Magoon, R.; Rawat, R.S.; Mehta, Y.; Taneja, S.; Ravi, R.; Hote, M.P. Goal-directed therapy improves the outcome of high-risk cardiac patients undergoing off-pump coronary artery bypass. Ann. Card. Anaesth. 2017, 20, 83–89. [Google Scholar] [CrossRef]
- Bacchin, M.R.; Ceria, C.M.; Giannone, S.; Ghisi, D.; Stagni, G.; Greggi, T.; Bonarelli, S. Goal-direted fluid therapy based on stroke volume variation in patients undergoing major spine surgery in the prone position: A cohort study. Spine 2016, 41, E1131–E1137. [Google Scholar] [CrossRef]
- Salicath, J.H.; Yeoh, E.C.Y.; Bennett, M.H. Epidural analgesia versus patient-controlled intravenous analgesia for pain following intra-abdominal surgery in adults. Cochrane Database Syst. Rev. 2018, 8, CD010434. [Google Scholar] [CrossRef]
- Guay, J.; Nishimori, M.; Kopp, S. Epidural local anaesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting and pain after abdominal surgery. Cochrane Database Syst. Rev. 2016, 7, CD001893. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Läärä, E.; Koivuranta, M.; Greim, C.A.; Roewer, N. A simplified risk scorefor predicting postoperative nausea and vomiting: Conclusions from cross-validations between two centers. Anesthesiology 1999, 91, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Philip, B.K.; Cakmakkaya, O.S.; Shilling, A.; Shi, Y.Y.; Leslie, J.B.; Allard, M.; Turan, A.; Windle, P.; Odom-Forren, J.; et al. Who is at risk for post discharge nausea and vomiting after ambulatory surgery? Anesthesiology 2012, 117, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Apfel, C.C.; Heidrich, F.M.; Jukar-Rao, S.; Jalota, L.; Hornuss, C.; Whelan, R.P.; Zhang, K.; Cakmakkaya, O.S. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br. J. Anaesth. 2012, 109, 742–753. [Google Scholar] [CrossRef]
- Nelson, G.; Altman, A.D.; Nick, A.; Meyer, L.A.; Ramirez, P.T.; Achtari, C.; Antrobus, J.; Huang, J.; Scott, M.; Wijk, L.; et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations—Part I. Gynecol Oncol. 2016, 140, 313–322. [Google Scholar] [CrossRef]
- Nelson, G.; Altman, A.D.; Nick, A.; Meyer, L.A.; Ramirez, P.T.; Achtari, C.; Antrobus, J.; Huang, J.; Scott, M.; Wijk, L.; et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations—Part II. Gynecol Oncol. 2016, 140, 323–332. [Google Scholar] [CrossRef]
- Denost, Q.; Rouanet, P.; Faucheron, J.L.; Panis, Y.; Meunier, B.; Cotte, E.; Meurette, G.; Kirzin, S.; Sabbagh, C.; Loriau, J.; et al. To Drain or Not to Drain Infraperitoneal Anas-tomosis After Rectal Excision for Cancer: The GRECCAR 5 Randomized Trial. Ann. Surg. 2017, 265, 474–480. [Google Scholar] [CrossRef]
- Carmichael, J.C.; Keller, D.S.; Baldini, G.; Bordeianou, L.; Weiss, E.; Lee, L.; Boutros, M.; McClane, J.; Feldman, L.S.; Steele, S.R. Clinical Practice Guidelines for Enhanced Recovery After Colon and Rectal Surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis. Colon. Rectum 2017, 60, 761–784. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Zhao, C.L.; Xie, J.; Ye, Y.W.; Sun, J.F.; Ding, Z.H.; Xu, H.N.; Ding, L. To drain or not to drain in colorectal anastomosis: A meta-analysis. Int. J. Color. Dis. 2016, 31, 951–960. [Google Scholar] [CrossRef]
- Musser, J.E.; Assel, M.; Guglielmetti, G.B.; Pathak, P.; Silberstein, J.L.; Sjoberg, D.D.; Bernstein, M.; Laudone, V.P. Impact of routine use of surgical drains on incidence of complications with robot-assisted radical prostatectomy. J. Endourol. 2014, 28, 1333–1337. [Google Scholar] [CrossRef]
- Patel, D.; Felder, S.I.; Luu, M.; Daskivich, T.J.; Zaghiyan, K.N.; Fleshner, P. Early Urinary Catheter Removal Following Pelvic Colorectal Surgery: A Prospective, Randomized, Noninferiority Trial. Dis. Colon. Rectum 2018, 61, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Alyami, M.; Lundberg, P.; Passot, G.; Glehen, O.; Cotte, E. Laparoscopic Colonic Resection Without Urinary Drainage: Is. It “Feasible”? J. Gastrointest. Surg. 2016, 20, 1388–1392. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, W.L.; Cheng, B.; Cheng, L.; Xiong, X.K.; Zeng, Y.J. A systematic review and meta- analysis comparing immediate and delayed catheter removal following uncomplicated hysterectomy. Int. Urogynecol. J. 2015, 26, 665–674. [Google Scholar] [CrossRef]
- Kotagal, M.; Symons, R.G.; Hirsch, I.B.; Umpierrez, G.E.; Farrokhi, E.T.; Flum, D.R.; SCOAP-Ceertain Collaborative. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann. Surg. 2015, 261, 97–103. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef]
- American Diabetes Association. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. S1), S173–S181. [Google Scholar] [CrossRef]
- Torossian, A. Thermal management during anaesthesia and thermoregulation standards for the prevention of inadvertent perioperative hypothermia. Best. Pract. Res. Clin. Anaesthesiol. 2008, 22, 659–668. [Google Scholar] [CrossRef]
- Warttig, S.; Alderson, P.; Lewis, S.R.; Smith, A.F. Intravenous nutrients for preventing inadvertent perioperative hypothermia in adults. Cochrane Database Syst. Rev. 2016, 11, CD009906. [Google Scholar] [CrossRef]
- Madrid, E.; Urrútia, G.; Roqué i Figuls, M.; Pardo-Hernandez, H.; Campos, J.M.; Paniagua, P.; Maestre, L.; Alonso-Coello, P. Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Database Syst. Rev. 2016, 4, CD009016. [Google Scholar] [CrossRef]
- Campbell, G.; Alderson, P.; Smith, A.F.; Warttig, S. Warming of intravenous and irrigation fluids for preventing inadvertent perioperative hypothermia. Cochrane Database Syst. Rev. 2015, 4, CD009891. [Google Scholar] [CrossRef]
- Felder, S.; Rasmussen, M.S.; King, R.; Sklow, B.; Kwaan, M.; Madoff, R.; Jensen, C. Prolonged thrombo-prophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst. Rev. 2019, 3, CD004318. [Google Scholar] [CrossRef] [PubMed]
- Vivas, D.; Roldán, I.; Ferrandis, R.; Marín, F.; Roldán, V.; Tello-Montoliu, A.; Ruiz-Nodar, J.M.; Gómez-Doblas, J.J.; Martín, A.; Llau, J.V.; et al. Perioperative Periprocedural Management of Antithrombotic Therapy: Consensus Document of, S.E.C.; SEDAR; SEACV; SECTCV; AEC; SECPRE; SEPD; SEGO; SEHH; SETH; SEMERGEN; SEMFYC; SEMG; SEMICYUC; SEMI; SEMES; SEPAR; SENEC; SEO; SEPA; SERVEI; SECOT; AEU. Rev. Esp. Cardiol. 2018, 71, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Falck-Ytter, Y.; Francis, C.W.; Johanson, N.A.; Curley, C.; Dahl, O.E.; Schulman, S.; Ortel, T.L.; Pauker, S.G.; Colwell, C.W., Jr. Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (Suppl. S2), e278S–e325S. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.R.; Morgano, G.P.; Bennett, C.; Dentali, F.; Francis, C.W.; Garcia, D.A.; Kahn, S.R.; Rahman, M.; Rajasekhar, A.; Rogers, F.B.; et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: Prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv. 2019, 3, 3898–3944. [Google Scholar] [CrossRef]
- Afshari, A.; Fenger-Eriksen, C.; Monreal, M.; Verhamme, P.; ESA VTE Guidelines Task Force. European guidelines on perioperative venous thromboembolism prophylaxis: Mechanical prophylaxis. Eur. J. Anaesthesiol. 2018, 35, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Mulier, J.P.; Dillemans, B. Anaesthetic Factors Affecting Outcome After Bariatric Surgery, a Retrospective Levelled Regression Analysis. Obes. Surg. 2019, 29, 1841–1850. [Google Scholar] [CrossRef]
- Frauenknecht, J.; Kirkham, K.R.; Jacot-Guillarmod, A.; Albrecht, E. Analgesic impact of intra- operative opioids vs. opioid-free anaesthesia: A systematic review and meta-analysis. Anaesthesia 2019, 74, 651–662. [Google Scholar] [CrossRef]
- Mulier, J.P.; Wouters, R.; Dillemans, B.; Deckock, M.L. A Randomized Controlled, Double-Blind Trial Evaluating the Effect of Opioid-Free Versus Opioid General Anaesthesia on Postoperative Pain and Discomfort Measured by the QoR-40. J. Clin. Anesth. Pain Med. 2018, 2, 2–6. [Google Scholar]
- Mulier, J.P.; Dillemans, B. Deep Neuromuscular Blockade versus Remifentanil or Sevoflurane to Augment Measurable Laparoscopic Workspaceduring Bariatric Surgery Analysed by a Randomissed Controlled Trial. J. Clin. Anesth. Pain Med. 2018, 7, 2–4. [Google Scholar]
- Castelino, T.; Fiore, J.F., Jr.; Niculiseanu, P.; Landry, T.; Augustin, B.; Feldman, L.S. The effect of early mobilization protocols on postoperative outcomes following abdominal and thoracic surgery: A systematic review. Surgery 2016, 159, 991–1003. [Google Scholar] [CrossRef]
- de Almeida, E.P.M.; de Almeida, J.P.; Landoni, G.; Galas, F.R.B.G.; Fukushima, J.T.; Fominskiy, E.; de Brito, C.M.M.; Cavichio, L.B.L.; de Almeida, L.A.A.; Ribeiro, U., Jr.; et al. Early mobilization programme improves functional capacity after major abdominal cancer surgery: A randomized controlled trial [with consumer summary]. Br. J. Anaesth. 2017, 119, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Schaller, S.J.; Anstey, M.; Blobner, M.; Edrich, T.; Grabitz, S.D.; Gradwohl-Matis, I.; Heim, M.; Houle, T.; Kurth, T.; Latronico, N.; et al. Early, goal-directed mobilization in the surgical intensive care unit: A randomized controlled trial. Lancet 2016, 388, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Fiore, J.F., Jr.; Castelino, T.; Pecorelli, N.; Niculiseanu, P.; Balvardi, S.; Hershorn, O.; Liberman, S.; Charlebois, P.; Stein, B.; Carli, F.; et al. Ensuring early mobilization within an enhanced recovery program for colorectal surgery: A randomized controlled trial. Ann. Surg. 2017, 266, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Wolk, S.; Linke, S.; Bogner, A. Use of Activity Tracking in Major. Visceral Surgery-the Enhanced Perioperative Mobilization Trial: A Randomized Controlled Trial. J. Gastrointest. Surg. 2019, 23, 1218. [Google Scholar] [CrossRef] [PubMed]
- Katsura, M.; Kuriyama, A.; Takeshima, T.; Fukuhara, S.; Furukawa, T.A. Preoperative inspiratory muscle training for postoperative pulmonary complications in adults undergoing cardiac and major abdominal surgery. Cochrane Database Syst. Rev. 2015, 5, CD010356. [Google Scholar] [CrossRef]
- Kalil-Filho, F.A.; Campos, A.C.L.; Tambara, E.M.; Tomé, B.K.A.; Treml, C.J.; Kuretzki, C.H.; Furlan, F.L.S.; Albuquerque, J.P.; Malafaia, O. Physiotherapeutic approaches and the effects on inspiratory muscle force in patients with chronic obstructive pulmonary disease in the pre-operative preparation for abdominal surgical procedures. Arq. Bras. Cir. Dig. 2019, 32, e1439. [Google Scholar] [CrossRef]
- Kendall, F.; Oliveira, J.; Peleteiro, B.; Pinho, P.; Bastos, P.T. Inspiratory muscle training is effective to reduce postoperative pulmonary complications and length of hospital stay: A systematic review and meta-analysis. Disabil. Rehabil. 2018, 40, 864–882. [Google Scholar] [CrossRef]
- Alaparthi, G.K.; Augustine, A.J.; Anand, R.; Mahale, A. Comparison of Diaphragmatic Breathing Exercise, Volume and Flow Incentive Spirometry, on Diaphragm Excursion and Pulmonary Function in Patients Undergoing Laparoscopic Surgery: A Randomized Controlled Trial. Minim. Invasive Surg. 2016, 2016, 1967532. [Google Scholar] [CrossRef]
- Karlsson, E.; Farahnak, P.; Franzén, E.; Nygren-Bonnier, M.; Dronkers, J.; van Meeteren, N.; Rydwik, E. Feasibility of preoperative supervised home-based exercise in older adults undergoing colorectal cancer surgery-A randomized controlled design. PLoS ONE 2019, 14, e0219158. [Google Scholar] [CrossRef]
- Martinez, V.; Beloeil, H.; Marret, E.; Fletcher, D.; Ravaud, P.; Trinquart, L. Non-opioid analgesics in adults after major surgery: Systematic review with network meta-analysis of randomized trials. Br. J. Anaesth. 2017, 118, 22–31. [Google Scholar] [CrossRef]
- Jørgensen, H.; Wetterslev, J.; Møiniche, S.; Dahl, J.B. Epidural local anaesthetics versus opioid-based analgesic regimens on postoperative gastrointestinal paralysis, PONV and pain after abdominal surgery. Cochrane Database Syst. Rev. 2000, 4, CD001893. [Google Scholar]
- McDaid, C.; Maund, E.; Rice, S.; Wright, K.; Jenkins, B.; Woolacott, N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery: A systematic review. Health Technol. Assess. 2010, 14, 1–153. [Google Scholar] [CrossRef] [PubMed]
- MacFater, W.S.; Rahiri, J.-L.; Lauti, M.; Su’a, B.; Hill, A.G. Intravenous lignocaine in colorectal surgery: A systematic review. ANZ J. Surg. 2017, 87, 879–885. [Google Scholar] [CrossRef]
- Weibel, S.; Jokinen, J.; Pace, N.L.; Schnabel, A.; Hollmann, M.W.; Hahnenkamp, K.; Eberhart, L.H.; Poepping, D.M.; Afshari, A.; Kranke, P. Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: A systematic review with trial sequential analysis. Br. J. Anaesth. 2016, 116, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Weibel, S.; Jelting, Y.; Pace, N.L.; Helf, A.; Eberhart, L.H.; Hahnenkamp, K.; Hollmann, M.W.; Poepping, D.M.; Schnabel, A.; Kranke, P. Continuous intra- venous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst. Rev. 2018, 6, CD009642. [Google Scholar] [PubMed]
- Baeriswyl, M.; Zeiter, F.; Piubellini, D.; Kirkham, K.R.; Albrecht, E. The analgesic efficacy of trans- verse abdominis plane block versus epidural analgesia: A systematic review with meta-analysis. Medicine 2018, 97, e11261. [Google Scholar] [CrossRef]
- Willcutts, K.F.; Chung, M.C.; Erenberg, C.L.; Finn, K.L.; Schirmer, B.D.; Byham-Gray, L.D. Early oral feeding as compared with traditional timing of oral feeding after upper gastrointestinal surgery. Ann. Surg. 2016, 264, 54–63. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef]
- Sharma, J.; Kumar, N.; Huda, F.; Payal, Y.S. Enhanced Recovery After Surgery Protocol in Emergency Laparotomy: A Randomized Control Study. Surg. J. 2021, 7, e92–e99. [Google Scholar] [CrossRef]
- Shida, D.; Tagawa, K.; Inada, K.; Nasu, K.; Seyama, Y.; Maeshiro, T.; Miyamoto, S.; Inoue, S.; Umekita, N. Modified enhanced recovery after surgery (ERAS) protocols for patients with obstructive colorectal cancer. BMC Surg. 2017, 17, 18. [Google Scholar] [CrossRef]
- Miao, X.; Tao, L.; Huang, L.; Li, J.; Pan, S. Application of Laparoscopy Combined with Enhanced Recovery after Surgery (ERAS) in Acute Intestinal Obstruction and Analysis of Prognostic Factors: A Retrospective Cohort Study. Biomed. Res. Int. 2022, 2022, 5771526. [Google Scholar] [CrossRef] [PubMed]
- Saurabh, K.; Sureshkumar, S.; Mohsina, S.; Mahalakshmy, T.; Kundra, P.; Kate, V. Adapted ERAS Pathway Versus Standard Care in Patients Undergoing Emergency Small Bowel Surgery: A Randomized Controlled Trial. J. Gastrointest. Surg. 2020, 24, 2077–2087. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidis, O.; Ramirez, J.M.; Ubieto, J.M.; Feo, C.V.; Arroyo, A.; Kocián, P.; Sánchez-Guillén, L.; Bellosta, A.P.; Whitley, A.; Enguita, A.B.; et al. The EUPEMEN (EUropean PErioperative MEdical Networking) Protocol for Bowel Obstruction: Recommendations for Perioperative Care. J. Clin. Med. 2023, 12, 4185. https://doi.org/10.3390/jcm12134185
Ioannidis O, Ramirez JM, Ubieto JM, Feo CV, Arroyo A, Kocián P, Sánchez-Guillén L, Bellosta AP, Whitley A, Enguita AB, et al. The EUPEMEN (EUropean PErioperative MEdical Networking) Protocol for Bowel Obstruction: Recommendations for Perioperative Care. Journal of Clinical Medicine. 2023; 12(13):4185. https://doi.org/10.3390/jcm12134185
Chicago/Turabian StyleIoannidis, Orestis, Jose M. Ramirez, Javier Martínez Ubieto, Carlo V. Feo, Antonio Arroyo, Petr Kocián, Luis Sánchez-Guillén, Ana Pascual Bellosta, Adam Whitley, Alejandro Bona Enguita, and et al. 2023. "The EUPEMEN (EUropean PErioperative MEdical Networking) Protocol for Bowel Obstruction: Recommendations for Perioperative Care" Journal of Clinical Medicine 12, no. 13: 4185. https://doi.org/10.3390/jcm12134185
APA StyleIoannidis, O., Ramirez, J. M., Ubieto, J. M., Feo, C. V., Arroyo, A., Kocián, P., Sánchez-Guillén, L., Bellosta, A. P., Whitley, A., Enguita, A. B., Teresa, M., & Anestiadou, E. (2023). The EUPEMEN (EUropean PErioperative MEdical Networking) Protocol for Bowel Obstruction: Recommendations for Perioperative Care. Journal of Clinical Medicine, 12(13), 4185. https://doi.org/10.3390/jcm12134185





