DSA-Based 2D Perfusion Measurements in Delayed Cerebral Ischemia to Estimate the Clinical Outcome in Patients with Aneurysmal Subarachnoid Hemorrhage: A Technical Feasibility Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population, In-/Exclusion Criteria, and Angiography Setting
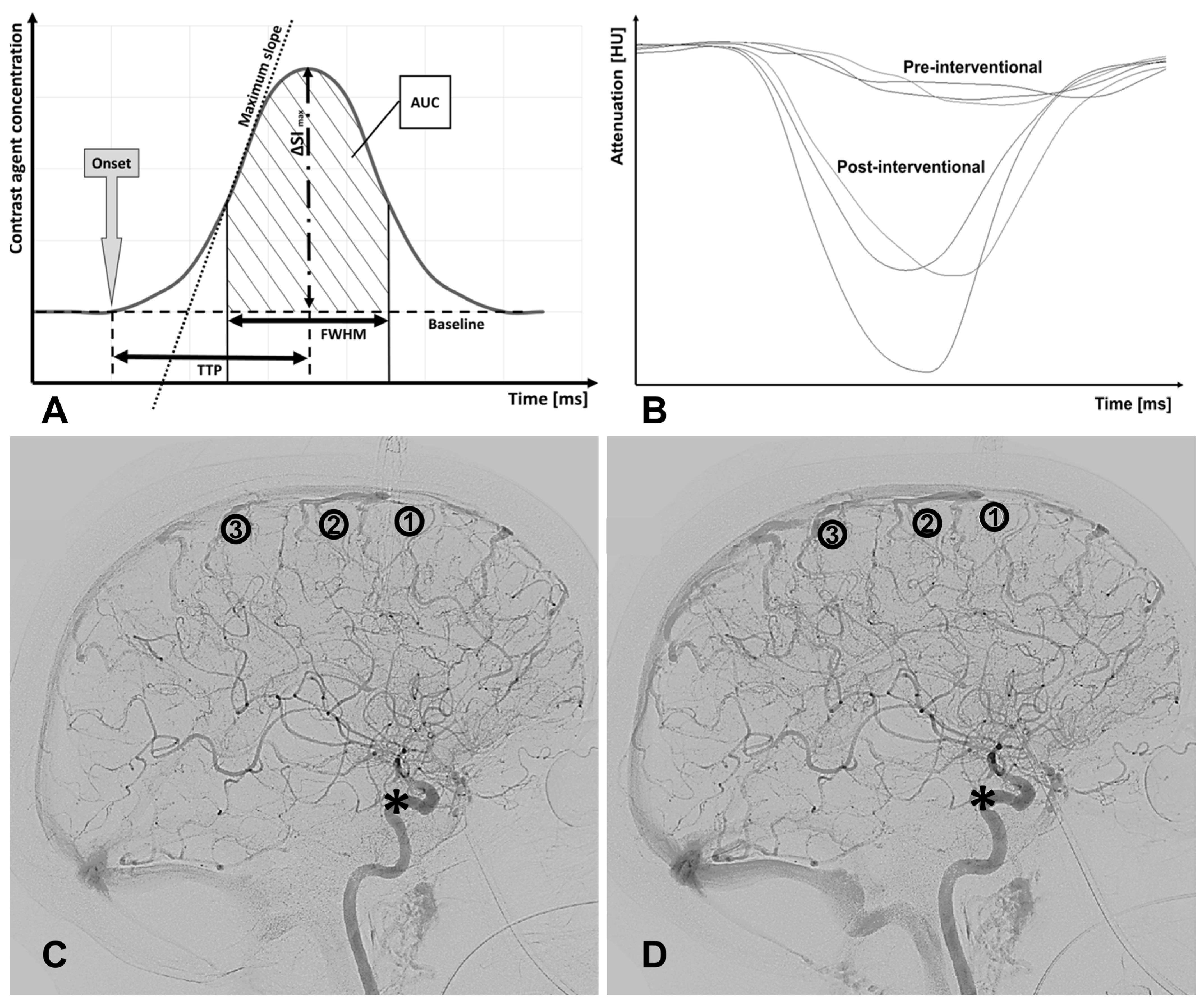
2.2. Bolus Tracking Model
- Onset (ms): first point in time of a significantly higher density (or CA concentration) compared to earlier time points.
- ΔSImax or dSI: maximum difference in grey scales/maximum amplitude of CA concentration.
- Time to peak TTP (ms): time from onset to maximal signal attenuation/CA concentration (dSI).
- Full width at half maximum FWHM (ms): time interval between incoming and outflowing contrast agent bolus when half of the maximum difference in grey scales (dSI or ΔSImax) was reached.
- Area under the curve AUC: area enclosed by the C(t)-curve from onset to the endpoint of FWHM.
2.3. Statistics
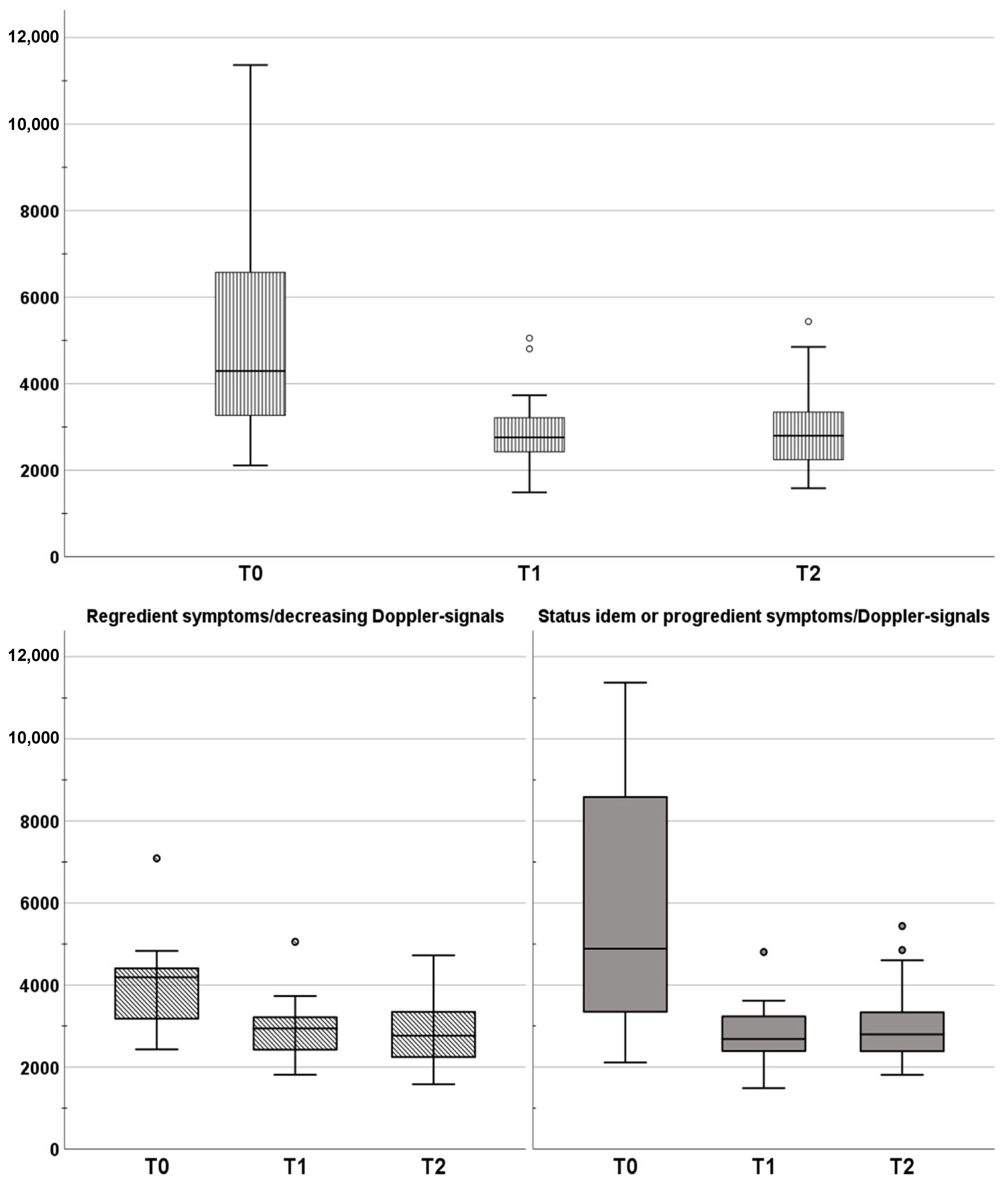
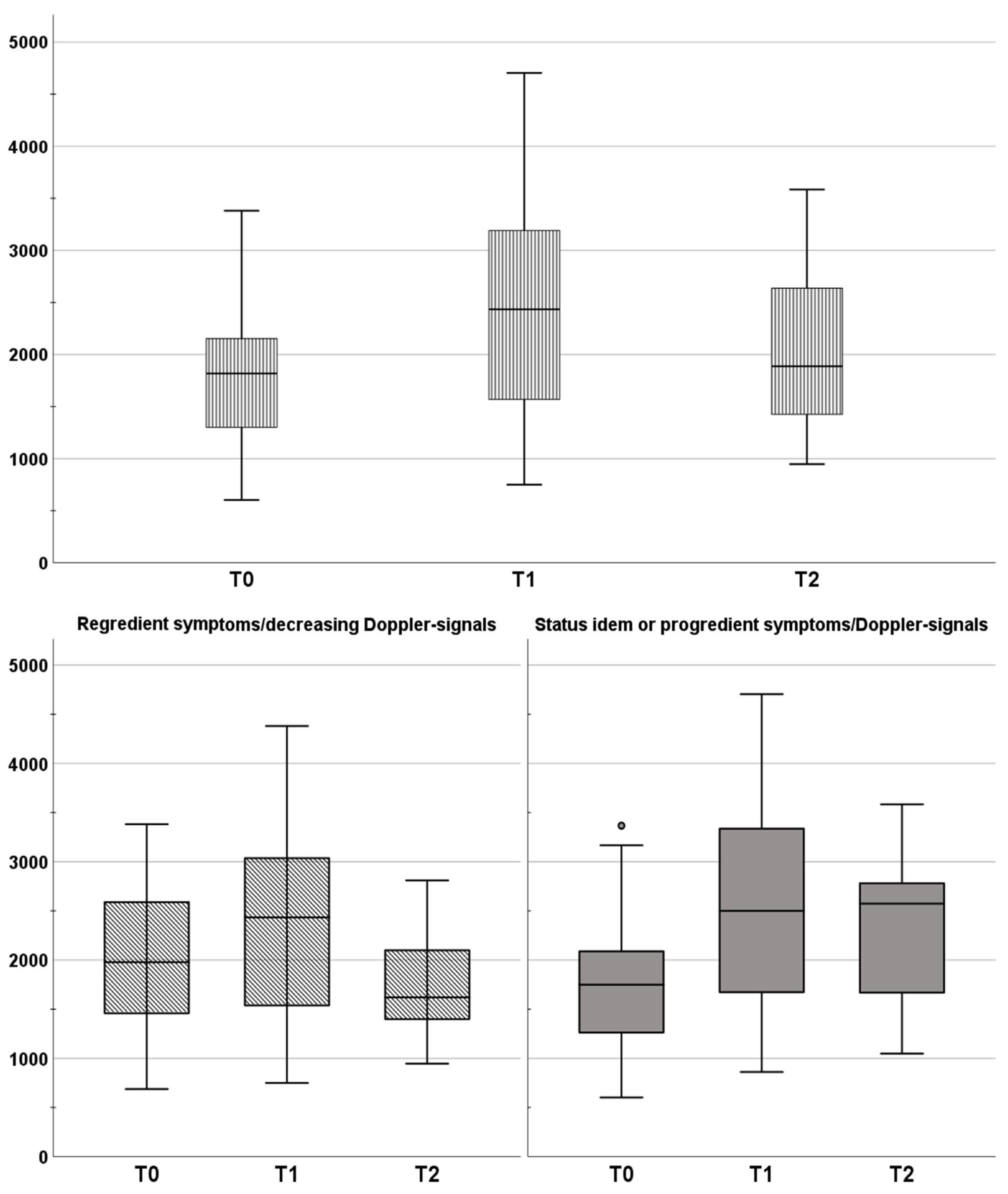
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nieuwkamp, D.J.; Setz, L.E.; Algra, A.; Linn, F.H.H.; de Rooij, N.K.; Rinkel, G.J.E. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: A meta-analysis. Lancet Neurol. 2009, 8, 635–642. [Google Scholar] [CrossRef]
- Haegens, N.M.; Gathier, C.S.; Horn, J.; Coert, B.A.; Verbaan, D.; van den Bergh, W.M. Induced Hypertension in Preventing Cerebral Infarction in Delayed Cerebral Ischemia After Subarachnoid Hemorrhage. Stroke 2018, 49, 2630–2636. [Google Scholar] [CrossRef] [Green Version]
- Feigin, V.; Norrving, B.; Sudlow, C.L.M.; Sacco, R.L. Updated Criteria for Population-Based Stroke and Transient Ischemic Attack Incidence Studies for the 21st Century. Stroke 2018, 49, 2248–2255. [Google Scholar] [CrossRef] [Green Version]
- Francoeur, C.L.; Mayer, S.A. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit. Care 2016, 20, 277. [Google Scholar] [CrossRef] [Green Version]
- Weiss, M.; Albanna, W.; Conzen-Dilger, C.; Kastenholz, N.; Seyfried, K.; Ridwan, H.; Wiesmann, M.; Veldeman, M.; Schmidt, T.P.; Megjhani, M.; et al. Intraarterial Nimodipine Versus Induced Hypertension for Delayed Cerebral Ischemia: A Modified Treatment Protocol. Stroke 2022, 53, 2607–2616. [Google Scholar] [CrossRef]
- Budohoski, K.P.; Guilfoyle, M.; Helmy, A.; Huuskonen, T.; Czosnyka, M.; Kirollos, R.; Menon, D.K.; Pickard, J.D.; Kirkpatrick, P.J. The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Vergouwen, M.D.; Vermeulen, M.; Coert, B.A.; Stroes, E.S.; Roos, Y.B. Microthrombosis after aneurysmal subarachnoid hemorrhage: An additional explanation for delayed cerebral ischemia. J. Cereb. Blood Flow Metab. 2008, 28, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.J.S. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef] [Green Version]
- Gölitz, P.; Hoelter, P.; Rösch, J.; Roessler, K.; Knossalla, F.; Doerfler, A.J.C.N. Ultra-early detection of microcirculatory injury as predictor of developing delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Clin. Neuroradiol. 2018, 28, 501–507. [Google Scholar] [CrossRef]
- Mees, S.D.; Rinkel, G.J.; Feigin, V.L.; Algra, A.; van den Bergh, W.M.; Vermeulen, M.; van Gijn, J. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst. Rev. 2007, 2007, CD000277. [Google Scholar]
- Adami, D.; Berkefeld, J.; Platz, J.; Konczalla, J.; Pfeilschifter, W.; Weidauer, S.; Wagner, M. Complication rate of intraarterial treatment of severe cerebral vasospasm after subarachnoid hemorrhage with nimodipine and percutaneous transluminal balloon angioplasty: Worth the risk? J. Neuroradiol. 2019, 46, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Uchino, K.; Kolikonda, M.K.; Brown, D.; Kovi, S.; Collins, D.; Khawaja, Z.; Buletko, A.B.; Russman, A.N.; Hussain, M.S.J.S. Decline in stroke presentations during COVID-19 surge. Stroke 2020, 51, 2544–2547. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh Barfejani, A.; Rabinstein, A.A.; Wijdicks, E.F.M.; Clark, S.L. Poor Utilization of Nimodipine in Aneurysmal Subarachnoid Hemorrhage. J. Stroke Cerebrovasc. Dis. 2019, 28, 2155–2158. [Google Scholar] [CrossRef] [PubMed]
- Kapapa, T.; König, R.; Mayer, B.; Braun, M.; Schmitz, B.; Müller, S.; Schick, J.; Wirtz, C.R.; Pala, A. Adverse Events and Complications in Continuous Intra-arterial Nimodipine Infusion Therapy After Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 2022, 12, 812898. [Google Scholar] [CrossRef]
- Kieninger, M.; Flessa, J.; Lindenberg, N.; Bele, S.; Redel, A.; Schneiker, A.; Schuierer, G.; Wendl, C.; Graf, B.; Silbereisen, V. Side Effects of Long-Term Continuous Intra-arterial Nimodipine Infusion in Patients with Severe Refractory Cerebral Vasospasm after Subarachnoid Hemorrhage. Neurocrit. Care 2018, 28, 65–76. [Google Scholar] [CrossRef]
- Ott, S.; Jedlicka, S.; Wolf, S.; Peter, M.; Pudenz, C.; Merker, P.; Schürer, L.; Lumenta, C.B. Continuous Selective Intra-Arterial Application of Nimodipine in Refractory Cerebral Vasospasm due to Aneurysmal Subarachnoid Hemorrhage. Biomed. Res. Int. 2014, 2014, 970741. [Google Scholar] [CrossRef]
- Dankbaar, J.W.; Rijsdijk, M.; van der Schaaf, I.C.; Velthuis, B.K.; Wermer, M.J.H.; Rinkel, G.J.E. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology 2009, 51, 813–819. [Google Scholar] [CrossRef] [Green Version]
- König, M.; Klotz, E.; Heuser, L. Cerebral perfusion CT: Theoretical aspects, methodical implementation and clinical experience in the diagnosis of ischemic cerebral infarction. RoFo 2000, 172, 210–218. [Google Scholar] [CrossRef]
- Brix, G.; Zwick, S.; Griebel, J.; Fink, C.; Kiessling, F. Estimation of tissue perfusion by dynamic contrast-enhanced imaging: Simulation-based evaluation of the steepest slope method. Eur. Radiol. 2010, 20, 2166–2175. [Google Scholar] [CrossRef]
- Schmitt, M.; Mohrs, O.; Petersen, S.; Kreitner, K.-F.; Voigtländer, T.; Wittlinger, T.; Horstick, G.; Ziegler, S.; Meyer, J.; Thelen, M.; et al. Bestimmung der myokardialen Perfusionsreserve bei KHK-Patienten mit der kontrastmittelverstärkten MRT: Ein Vergleich zwischen semiquantitativer und quantitativer Auswertung. RoFo 2002, 174, 187–195. [Google Scholar] [CrossRef]
- Gao, X. Nonparametric statistics. Encycl. Res. Des. 2010, 175, 916–921. [Google Scholar] [CrossRef] [Green Version]
- Nahm, F.S. Nonparametric statistical tests for the continuous data: The basic concept and the practical use. Korean J. Anesthesiol. 2016, 69, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Napierala, M.A. What is the Bonferroni correction? Aaos Now 2012, 6, 40–41. Available online: https://docs.ufpr.br/~giolo/LivroADC/Material/S3_Bonferroni%20Correction.pdf (accessed on 16 June 2023).
- VanderWeele, T.J.; Mathur, M.B. Some desirable properties of the Bonferroni correction: Is the Bonferroni correction really so bad? Am. J. Epidemiol. 2019, 188, 617–618. [Google Scholar] [CrossRef]
- Mehta, C.R.; Patel, N.R. A network algorithm for performing Fisher’s exact test in r× c contingency tables. J. Am. Stat. Assoc. 1983, 78, 427–434. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Abingdon, UK, 2013. [Google Scholar]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis; Springer: Berlin/Heidelberg, Germany, 2001; Volume 608. [Google Scholar]
- Takahashi, M.; Bussaka, H.; Nakagawa, N. Evaluation of the cerebral vasculature by intraarterial DSA-with emphasis on in vivo resolution. Neuroradiology 1984, 26, 253–259. [Google Scholar] [CrossRef]
- Neulen, A.; Meyer, S.; Kramer, A.; Pantel, T.; Kosterhon, M.; Kunzelmann, S.; Goetz, H.; Thal, S.C. Large vessel vasospasm is not associated with cerebral cortical hypoperfusion in a murine model of subarachnoid hemorrhage. Transl. Stroke Res. 2019, 10, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Pearl, J.; Macdonald, R. Vasospasm after aneurysmal subarachnoid hemorrhage: Need for further study. Acta Neurochir. Suppl. 2008, 105, 207–210. [Google Scholar] [PubMed]
- Etminan, N.; Vergouwen, M.D.; Ilodigwe, D.; Macdonald, R.L. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2011, 31, 1443–1451. [Google Scholar] [CrossRef] [Green Version]
- Struffert, T.; Deuerling-Zheng, Y.; Engelhorn, T.; Kloska, S.; Gölitz, P.; Bozzato, A.; Kapsreiter, M.; Strother, C.M.; Doerfler, A. Monitoring of Balloon Test Occlusion of the Internal Carotid Artery by Parametric Color Coding and Perfusion Imaging within the Angio Suite: First Results. Clin. Neuroradiol. 2013, 23, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Gölitz, P.; Kaschka, I.; Lang, S.; Roessler, K.; Knossalla, F.; Doerfler, A. Real-Time, In Vivo Monitoring, and Quantitative Assessment of Intra-Arterial Vasospasm Therapy. Neurocrit. Care 2016, 25, 40–46. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Aneurysmal SAH with relevant vasospasm | Traumatic SAH |
| DSA data available for each of the three time points: initial DSA with symptomatic SAH (T0), DSA before (T1), and after endovascular treatment (T2) | Hemorrhage from arteriovenous malformation |
| Period of time between January 2013 and December 2019 | Tumor-associated hemorrhage |
| Motion artifacts | |
| Right/Left anterior oblique DSA projections <80° and/or >100° | |
| Patients without DSA data before vasospasms |
| Subgroups | Study Population | Group 1 | Group 2 | p-Value | |
|---|---|---|---|---|---|
| Number of cases | 26 (100%) | 10 (38.5%) | 16 (61.5%) | ||
| Age (years) | 50.26 ± 11.4 | 45.9 ± 15.2 | 53 ± 7.7 | 0.23 | |
| Sex | Female | 20 (70%) | 7 (70%) | 13 (81%) | 0.6 |
| Male | 6 (30%) | 3 (30%) | 3 (19%) | ||
| Fisher Score (I–IV) | 4 (IQR 1) | 4 (IQR 1) | 4 (IQR 1) | 0.9 | |
| Hunt and Hess (0–5) | 2 (IQR 3) | 2 (IQR 2.75) | 2 (IQR 3) | 0.6 | |
| Localization of Aneurysm | ICA | 1 (3.8%) | 0 | 1 (6.3%) | 0.6 |
| ACA | 13 (50%) | 5 (50%) | 8 (50%) | ||
| MCA | 4 (15.4%) | 1 (10%) | 3 (18.8%) | ||
| BA | 7 (26.9%) | 4 (40%) | 3 (18.8%) | ||
| MCA + BA | 1 (3.8%) | 0 | 1 (6.3%) | ||
| Treatment modality | Clip | 8 (30.8%) | 2 (20%) | 6 (37.5%) | 0.4 |
| Coil | 18 (69.2%) | 8 (80%) | 10 (62.5%) | ||
| Days until symptomatic vasospasm | 7 ± 2.2 | 6.8 ± 2.5 | 7 ± 3 | 0.8 | |
| Localization of vasospasm | ACA | 6 (23.1%) | 3 (30%) | 3 (18.8%) | 0.07 |
| MCA | 11 (42.3%) | 1 (10%) | 10 (62.5%) | ||
| ACA + MCA | 6 (23.1%) | 4 (40%) | 2 (12.5%) | ||
| PCA | 1 (3.8%) | 1 (10%) | 0 | ||
| PCA + MCA | 1 (3.8%) | 1 (10%) | 0 | ||
| Global | 1 (3.8%) | 0 | 1 (6.3%) | ||
| Treatment of Vasospasm | PTA | 4 (15.4%) | 2 (20%) | 2 (12.5%) | 0.6 |
| IN | 17 (65.4%) | 7 (70%) | 10 (62.5%) | ||
| PTA + IN | 5 (19.2%) | 1 (10%) | 4 (25%) | ||
| mRS at discharge | 5 (IQR 2) | 2.5 (IQR 3.5) | 5 (IQR 0.75) | 0.001 | |
| mRS at follow up | 1 (IQR 3) | 1 (IQR 0) | 4 (IQR 4) | 0.012 | |
| Years until follow up | 3.1 ± 1.9 | 3.3 ± 2.2 | 2.9 ± 1.8 | 0.8 | |
| Model 1 | |||
| Predicted Variable | Parameters | Coefficient 1 | Coefficient 2 |
| Age | MST1 | ||
| mRS at discharge | R | 0.45 | |
| R2 | 0.2 | ||
| Sig. (Model) | 0.07 | ||
| Constant (Model) | 0.59 | ||
| Regressions coefficient B | 0.07 | −4.39 | |
| β (standardized) | 0.45 | −0.038 | |
| Sig. (Coefficients) | 0.025 | 0.84 | |
| Power (1 − β) | 0.56 | ||
| Model 2 | |||
| Predicted Variable | Parameters | Coefficient 1 | Coefficient 2 |
| Age | MST2 | ||
| mRS at discharge | R | 0.62 | |
| R2 | 0.39 | ||
| Sig. (Model) | 0.004 | ||
| Constant (Model) | 2.1 | ||
| Regression coefficient B | 0.072 | −30.43 | |
| β (standardized) | 0.46 | −0.43 | |
| Sig. (Coefficients) | 0.01 | 0.014 | |
| Power (1 − β) | 0.94 | ||
| Model 3 | |||
| Predicted Variable | Parameters | Coefficient 1 | Coefficient 2 |
| Age | ΔMS = MST1 − MST2 | ||
| mRS at discharge | R | 0.579 | |
| R2 | 0.335 | ||
| Sig. (Model) | 0.009 | ||
| Constant (Model) | 0.859 | ||
| Regression coefficient B | 0.07 | 23.35 | |
| β (standardized) | 0.45 | 0.37 | |
| Sig. (Coefficients) | 0.015 | 0.04 | |
| Power (1 − β) | 0.87 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reder, S.R.; Lückerath, S.; Neulen, A.; Beiser, K.U.; Grauhan, N.F.; Othman, A.E.; Brockmann, M.A.; Brockmann, C.; Kronfeld, A. DSA-Based 2D Perfusion Measurements in Delayed Cerebral Ischemia to Estimate the Clinical Outcome in Patients with Aneurysmal Subarachnoid Hemorrhage: A Technical Feasibility Study. J. Clin. Med. 2023, 12, 4135. https://doi.org/10.3390/jcm12124135
Reder SR, Lückerath S, Neulen A, Beiser KU, Grauhan NF, Othman AE, Brockmann MA, Brockmann C, Kronfeld A. DSA-Based 2D Perfusion Measurements in Delayed Cerebral Ischemia to Estimate the Clinical Outcome in Patients with Aneurysmal Subarachnoid Hemorrhage: A Technical Feasibility Study. Journal of Clinical Medicine. 2023; 12(12):4135. https://doi.org/10.3390/jcm12124135
Chicago/Turabian StyleReder, Sebastian R., Steffen Lückerath, Axel Neulen, Katja U. Beiser, Nils F. Grauhan, Ahmed E. Othman, Marc A. Brockmann, Carolin Brockmann, and Andrea Kronfeld. 2023. "DSA-Based 2D Perfusion Measurements in Delayed Cerebral Ischemia to Estimate the Clinical Outcome in Patients with Aneurysmal Subarachnoid Hemorrhage: A Technical Feasibility Study" Journal of Clinical Medicine 12, no. 12: 4135. https://doi.org/10.3390/jcm12124135
APA StyleReder, S. R., Lückerath, S., Neulen, A., Beiser, K. U., Grauhan, N. F., Othman, A. E., Brockmann, M. A., Brockmann, C., & Kronfeld, A. (2023). DSA-Based 2D Perfusion Measurements in Delayed Cerebral Ischemia to Estimate the Clinical Outcome in Patients with Aneurysmal Subarachnoid Hemorrhage: A Technical Feasibility Study. Journal of Clinical Medicine, 12(12), 4135. https://doi.org/10.3390/jcm12124135







