Utility of the Shortness of Breath in Daily Activities Questionnaire (SOBDA-Q) to Detect Sedentary Behavior in Patients with Chronic Obstructive Pulmonary Disease (COPD)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Evaluation of Physical Activity
2.3. Assessment of Questionnaire Patient-Reported Outcome Measures (PROMs)
2.4. Assessment of Pulmonary Function
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparison of the Physical Activity among Patients
3.3. Comparison of PROMs among Patients
3.4. Correlation between PROMs and Physical Activity
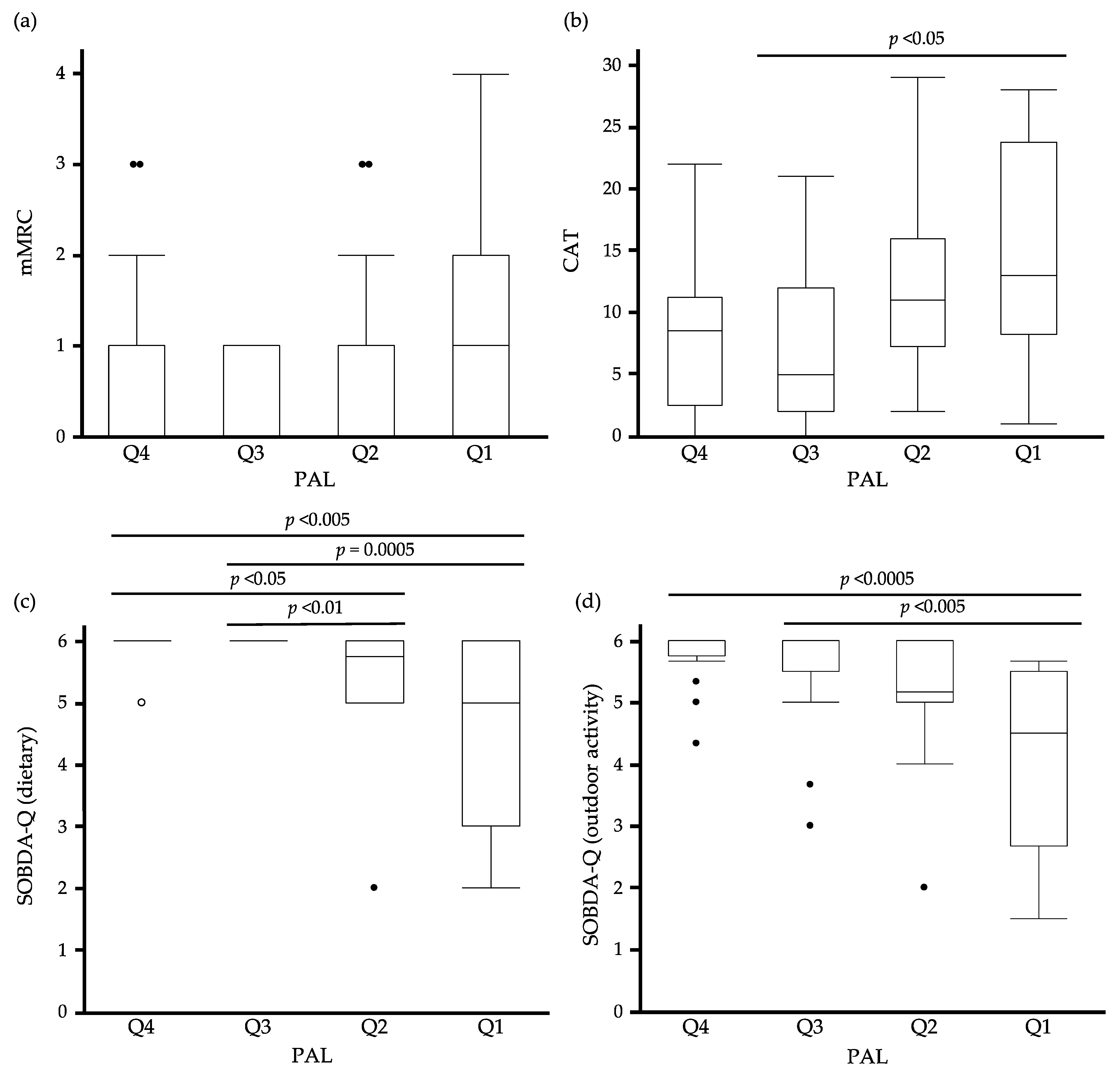
3.5. The Relationship between Quartiles of PAL and PROMs
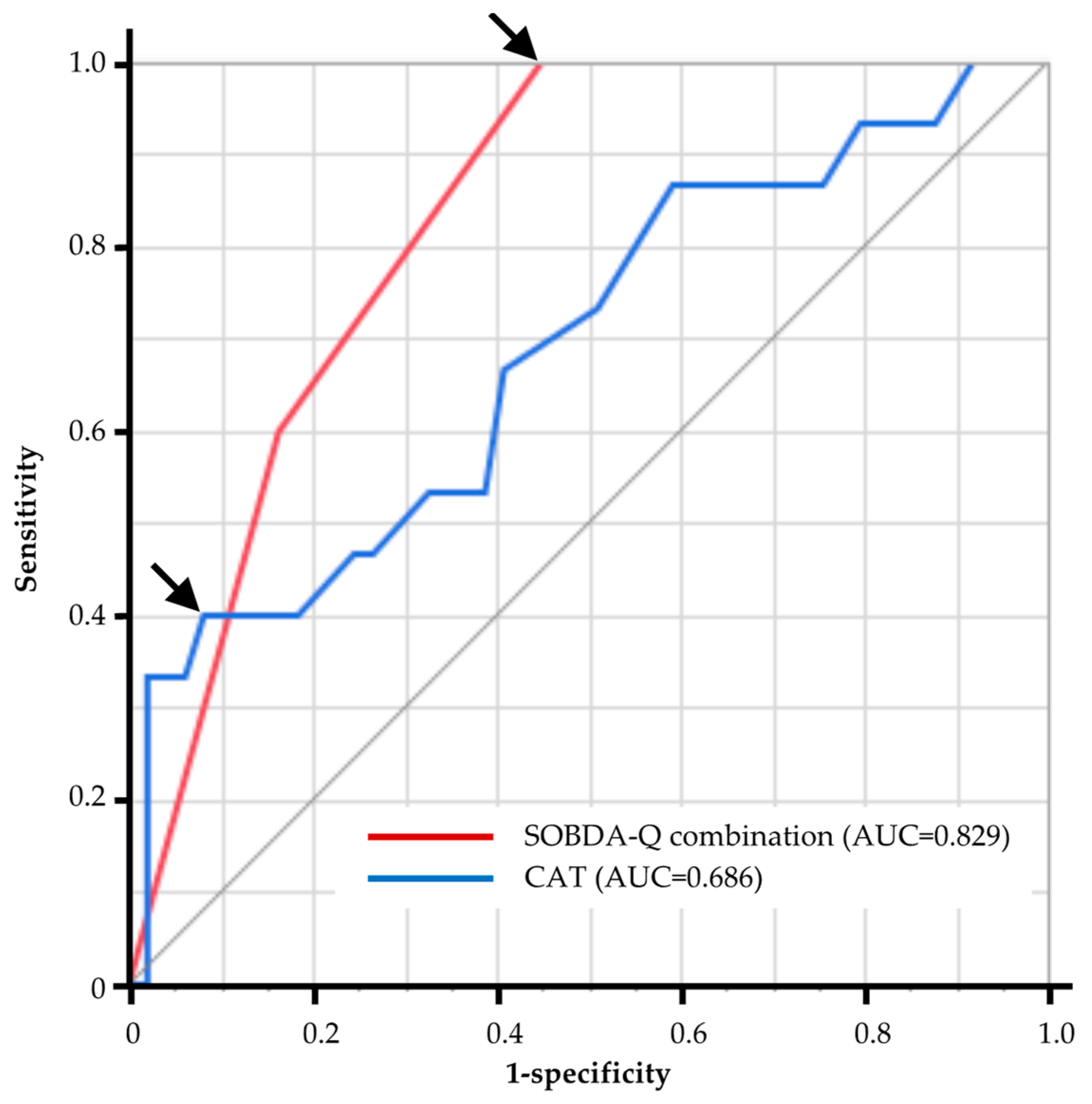
3.6. The Diagnostic Ability of PROMs to Predict Sedentary Patients with COPD
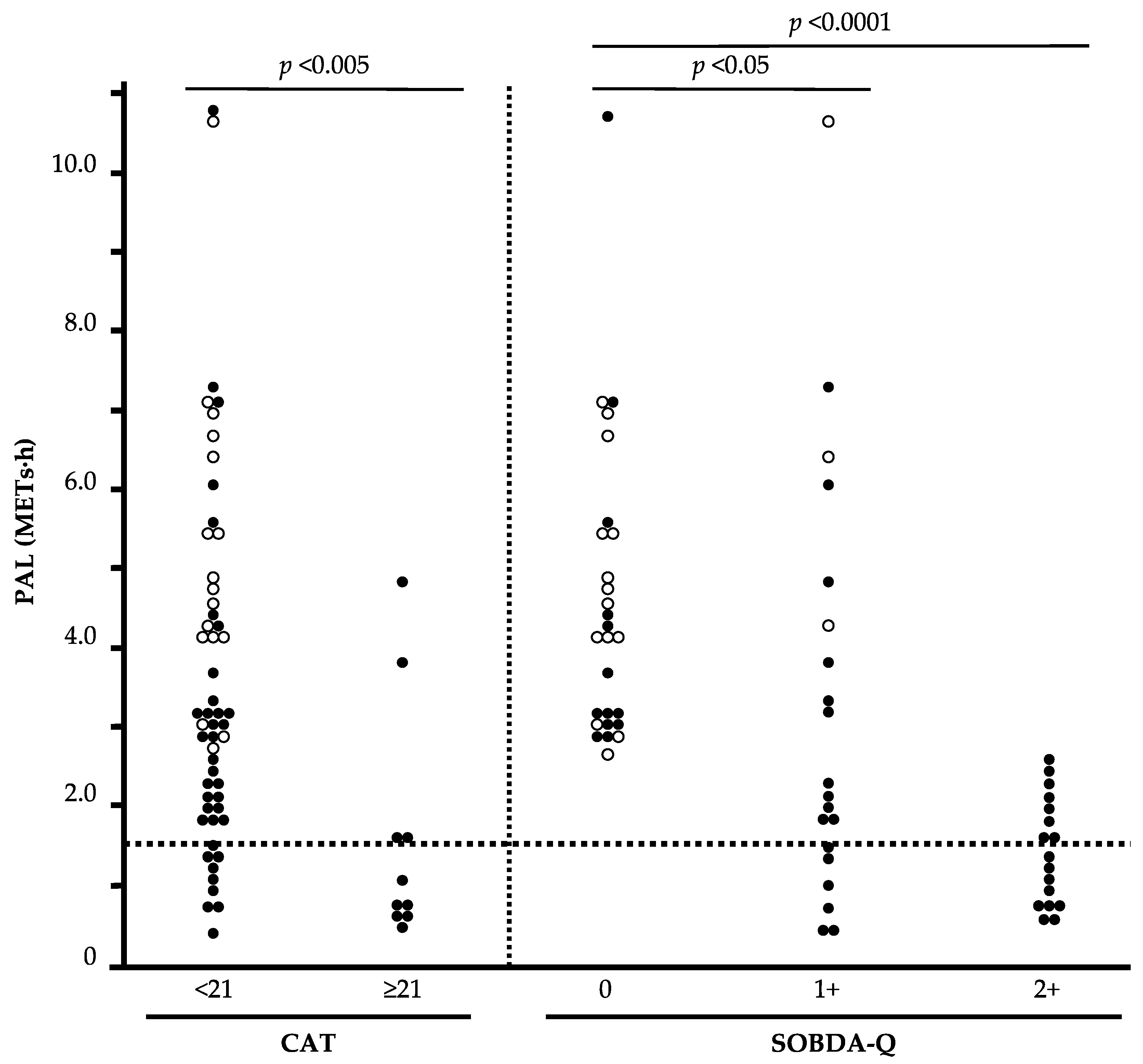
3.7. The Relationship between Stratified CAT, SOBDA-Q Combination, and PAL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2023 report). Available online: http://www.goldcopd.org/ (accessed on 10 February 2023).
- Giacomini, M.; DeJean, D.; Simeonov, D.; Smith, A. Experiences of living and dying with COPD: A systematic review and synthesis of the qualitative empirical literature. Ont. Health Technol. Assess. Ser. 2012, 12, 1–47. [Google Scholar]
- Pitta, F.; Troosters, T.; Spruit, M.A.; Probst, V.S.; Decramer, M.; Gosselink, R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 171, 972–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlanetto, K.C.; Donaria, L.; Schneider, L.P.; Lopes, J.R.; Ribeiro, M.; Fernandes, K.B.; Hernandes, N.A.; Pitta, F. Sedentary Behavior Is an Independent Predictor of Mortality in Subjects With COPD. Respir. Care 2017, 62, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Waschki, B.; Kirsten, A.; Holz, O.; Muller, K.C.; Meyer, T.; Watz, H.; Magnussen, H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest 2011, 140, 331–342. [Google Scholar] [CrossRef]
- Pitta, F.; Troosters, T.; Probst, V.S.; Spruit, M.A.; Decramer, M.; Gosselink, R. Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. Eur. Respir. J. 2006, 27, 1040–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eakin, E.G.; Resnikoff, P.M.; Prewitt, L.M.; Ries, A.L.; Kaplan, R.M. Validation of a new dyspnea measure: The UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest 1998, 113, 619–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugino, A.; Minakata, Y.; Kanda, M.; Akamatsu, K.; Koarai, A.; Hirano, T.; Sugiura, H.; Matsunaga, K.; Ichinose, M. Validation of a compact motion sensor for the measurement of physical activity in patients with chronic obstructive pulmonary disease. Respiration 2012, 83, 300–307. [Google Scholar] [CrossRef]
- Matthews, C.E.; Ainsworth, B.E.; Thompson, R.W.; Bassett, D.R., Jr. Sources of variance in daily physical activity levels as measured by an accelerometer. Med. Sci. Sports Exerc. 2002, 34, 1376–1381. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [Green Version]
- Pate, R.R.; O’Neill, J.R.; Lobelo, F. The evolving definition of “sedentary”. Exerc. Sport. Sci. Rev. 2008, 36, 173–178. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Washington, T.L.; Ainsworth, B.E.; Troiano, R.P. Linking the American Time Use Survey (ATUS) and the Compendium of Physical Activities: Methods and rationale. J. Phys. Act. Health 2009, 6, 347–353. [Google Scholar] [CrossRef] [Green Version]
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med. Sci. Sports Exerc. 2007, 39, 1423–1434. [Google Scholar] [CrossRef] [Green Version]
- Hirano, T.; Doi, K.; Matsunaga, K.; Takahashi, S.; Donishi, T.; Suga, K.; Oishi, K.; Yasuda, K.; Mimura, Y.; Harada, M.; et al. A Novel Role of Growth Differentiation Factor (GDF)-15 in Overlap with Sedentary Lifestyle and Cognitive Risk in COPD. J. Clin. Med. 2020, 9, 2737. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bull, F.C.; Maslin, T.S.; Armstrong, T. Global physical activity questionnaire (GPAQ): Nine country reliability and validity study. J. Phys. Act. Health 2009, 6, 790–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa-Takata, K.; Tabata, I.; Sasaki, S.; Rafamantanantsoa, H.H.; Okazaki, H.; Okubo, H.; Tanaka, S.; Yamamoto, S.; Shirota, T.; Uchida, K.; et al. Physical activity level in healthy free-living Japanese estimated by doubly labelled water method and International Physical Activity Questionnaire. Eur. J. Clin. Nutr. 2008, 62, 885–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, H.L.; Jacobs, D.R., Jr.; Schucker, B.; Knudsen, J.; Leon, A.S.; Debacker, G. A questionnaire for the assessment of leisure time physical activities. J. Chronic. Dis. 1978, 31, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Baecke, J.A.; Burema, J.; Frijters, J.E. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am. J. Clin. Nutr. 1982, 36, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Washburn, R.A.; Smith, K.W.; Jette, A.M.; Janney, C.A. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J. Clin. Epidemiol. 1993, 46, 153–162. [Google Scholar] [CrossRef]
- Ries, A.L. Impact of chronic obstructive pulmonary disease on quality of life: The role of dyspnea. Am. J. Med. 2006, 119, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Mahler, D.A.; Wells, C.K. Evaluation of clinical methods for rating dyspnea. Chest 1988, 93, 580–586. [Google Scholar] [CrossRef] [Green Version]
- Bestall, J.C.; Paul, E.A.; Garrod, R.; Garnham, R.; Jones, P.W.; Wedzicha, J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999, 54, 581–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watz, H.; Waschki, B.; Meyer, T.; Magnussen, H. Physical activity in patients with COPD. Eur. Respir. J. 2009, 33, 262–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Kline Leidy, N. Development and first validation of the COPD Assessment Test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef] [Green Version]
- Bradley, M.M.; Lang, P.J. Measuring emotion: The Self-Assessment Manikin and the Semantic Differential. J. Behav. Ther. Exp. Psychiatry 1994, 25, 49–59. [Google Scholar] [CrossRef]
- Wolkove, N.; Fu, L.Y.; Purohit, A.; Colacone, A.; Kreisman, H. Meal Induced Oxygen Desaturation and Dyspnea in Chronic Obstructive Pulmonary Disease. Can. Respir. J. 1998, 5, 347020. [Google Scholar] [CrossRef] [Green Version]
- Tangri, S.; Woolf, C.R. The breathing pattern in chronic obstructive lung disease during the performance of some common daily activities. Chest 1973, 63, 126–127. [Google Scholar] [CrossRef]
- Smith, J.; Wolkove, N.; Colacone, A.; Kreisman, H. Coordination of eating, drinking and breathing in adults. Chest 1989, 96, 578–582. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.J.; Couser, J.I.; Celli, B.R. Respiratory response to arm elevation in patients with chronic airflow obstruction. Am. Rev. Respir. Dis. 1991, 143, 476–480. [Google Scholar] [CrossRef]
- Celli, B.R.; Rassulo, J.; Make, B.J. Dyssynchronous breathing during arm but not leg exercise in patients with chronic airflow obstruction. N. Engl. J. Med. 1986, 314, 1485–1490. [Google Scholar] [CrossRef]
- Lee, L.; Friesen, M.; Lambert, I.R.; Loudon, R.G. Evaluation of dyspnea during physical and speech activities in patients with pulmonary diseases. Chest 1998, 113, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binazzi, B.; Lanini, B.; Romagnoli, I.; Garuglieri, S.; Stendardi, L.; Bianchi, R.; Gigliotti, F.; Scano, G. Dyspnea during speech in chronic obstructive pulmonary disease patients: Effects of pulmonary rehabilitation. Respiration 2011, 81, 379–385. [Google Scholar] [CrossRef]
- O’Donnell, D.E.; Revill, S.M.; Webb, K.A. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001, 164, 770–777. [Google Scholar] [CrossRef] [Green Version]
- Hirano, T.; Matsunaga, K.; Hamada, K.; Uehara, S.; Suetake, R.; Yamaji, Y.; Oishi, K.; Asami, M.; Edakuni, N.; Ogawa, H.; et al. Combination of assist use of short-acting beta-2 agonists inhalation and guidance based on patient-specific restrictions in daily behavior: Impact on physical activity of Japanese patients with chronic obstructive pulmonary disease. Respir. Investig. 2019, 57, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Shioya, T.; Sato, S.; Iwakura, M.; Takahashi, H.; Terui, Y.; Uemura, S.; Satake, M. Improvement of physical activity in chronic obstructive pulmonary disease by pulmonary rehabilitation and pharmacological treatment. Respir. Investig. 2018, 56, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Perez, T.A.; Castillo, E.G.; Ancochea, J.; Pastor Sanz, M.T.; Almagro, P.; Martinez-Camblor, P.; Miravitlles, M.; Rodriguez-Carballeira, M.; Navarro, A.; Lamprecht, B.; et al. Sex differences between women and men with COPD: A new analysis of the 3CIA study. Respir. Med. 2020, 171, 106105. [Google Scholar] [CrossRef]
- Katsura, H.; Yamada, K.; Wakabayashi, R.; Kida, K. Gender-associated differences in dyspnoea and health-related quality of life in patients with chronic obstructive pulmonary disease. Respirology 2007, 12, 427–432. [Google Scholar] [CrossRef]
- Pitta, F.; Takaki, M.Y.; Oliveira, N.H.; Sant’anna, T.J.; Fontana, A.D.; Kovelis, D.; Camillo, C.A.; Probst, V.S.; Brunetto, A.F. Relationship between pulmonary function and physical activity in daily life in patients with COPD. Respir. Med. 2008, 102, 1203–1207. [Google Scholar] [CrossRef] [Green Version]
| HP (N = 17) | Non-Sedentary COPD (N = 32) | Sedentary COPD (N = 15) | p Value | |
|---|---|---|---|---|
| Number, n (M/F) | 4/13 | 32/0 | 14/1 | <0.0001 |
| Age (years) | 61.1 ± 10.6 | 70.0 ± 8.0 | 70.9 ± 10.2 | <0.05 |
| BMI (kg/m2) | 21.8 ± 3.5 | 22.6 ± 2.8 | 24.9 ± 4.1 | n.s. |
| Smoking status (non/ex/cu) | 15/2/0 | 0/24/8 | 0/9/6 | <0.0001 |
| Smoking history (pack years) | 3.6 ± 11.0 | 45.2 ± 26.7 | 51.8 ± 22.0 | <0.0001 |
| GOLD (1/2/3/4) | - | 16/13/3/0 | 5/9/1/0 | - |
| CAT (<10/≥10) | 17/0 | 12/20 | 5/10 | <0.0001 |
| mMRC(0/1/2/3/4) | 13/4/0/0/0 | 16/9/2/5/0 | 5/5/4/0/1 | <0.05 |
| FEV1 | 2.38 ± 0.42 | 2.21 ± 0.56 | 1.90 ± 0.51 | <0.05 |
| %FEV1 | 106.8 ± 12.6 | 77.7 ± 18.7 | 70.4 ± 16.0 | <0.0001 |
| HP (N = 17) | Non-Sedentary COPD (N = 32) | Sedentary COPD (N = 15) | p Value | |
|---|---|---|---|---|
| PAL (METs·h) | 5.18 ± 1.95 a | 3.50 ± 1.99 a | 0.87 ± 0.33 | <0.0001 |
| ≥1METs (min) | 776.1 ± 151.4 b | 609.4 ± 180.9 c | 495.5 ± 222.3 | <0.0005 |
| ≥2METs (min) | 259.8 ± 79.8 a | 208.5 ± 87.2 a | 93.8 ± 50.8 | <0.0001 |
| ≥3METs (min) | 83.9 ± 33.4 a | 55.2 ± 31.3 a | 14.7 ± 5.6 | <0.0001 |
| ≥4METs (min) | 20.2 ± 10.9 a | 12.2 ± 14.6 a | 2.7 ± 2.0 | <0.0001 |
| Total number of steps | 6315.5 ± 2284.8 a | 5224.7 ± 2860.0 a | 1714.6 ± 1755.8 | <0.0001 |
| HP (N = 17) | Non-Sedentary COPD (N = 32) | Sedentary COPD (N = 15) | p Value | |
|---|---|---|---|---|
| mMRC | 0.2 ± 0.4 b | 0.9 ± 1.1 | 1.1 ± 1.1 | <0.05 |
| CAT | 4.1 ± 3.2 a | 11.7 ± 6.7 | 15.4 ± 9.1 | <0.0001 |
| SOBDA-Q | ||||
| Morning | 5.9 ± 0.2 a | 5.5 ± 0.7 c | 4.4 ± 1.5 | <0.001 |
| Dietary | 5.9 ± 0.2 a | 5.6 ± 0.8 b | 4.3 ± 1.6 | <0.0005 |
| Indoor activity | 5.9 ± 0.3 b | 5.2 ± 1.0 | 4.3 ± 1.8 | <0.05 |
| Outdoor activity | 6.0 ± 0.1 a | 5.1 ± 1.1 c | 3.8 ± 1.7 | <0.0001 |
| Recreation | 5.6 ± 0.4 a | 4.8 ± 1.1 c | 3.7 ± 1.7 | <0.0005 |
| Night-time activity | 5.9 ± 0.3 a | 5.4 ± 0.9 c | 4.3 ± 1.5 | <0.001 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Correlation Coefficient (ρ) | p Value | Correlation Coefficient (F) | p Value | |
| mMRC | −0.27 | <0.05 | 0.25 | n.s. |
| CAT | −0.42 | <0.001 | 4.72 | <0.05 |
| SOBDA-Q | ||||
| Morning | 0.48 | <0.0001 | 5.46 | <0.05 |
| Dietary | 0.6 | <0.0001 | 5.5 | <0.05 |
| Indoor activity | 0.44 | <0.001 | 5.33 | <0.05 |
| Outdoor activity | 0.62 | <0.0001 | 6.28 | <0.05 |
| Recreation | 0.44 | <0.0005 | 4.23 | <0.05 |
| Night-time activity | 0.44 | <0.0005 | 5.04 | <0.05 |
| Variables | AUC | Cut Off | Sensitivity | Specificity |
|---|---|---|---|---|
| mMRC | 0.641 | 1 | 0.67 | 0.59 |
| CAT | 0.686 | 21 | 0.4 | 0.92 |
| SOBDA-Q | ||||
| Morning | 0.733 | 5 | 0.67 | 0.76 |
| Dietary | 0.759 | 5.5 | 0.67 | 0.8 |
| Indoor activity | 0.679 | 5 | 0.54 | 0.76 |
| Outdoor activity | 0.817 | 5.667 | 1 | 0.58 |
| Recreation | 0.755 | 4.667 | 0.67 | 0.76 |
| Night-time activity | 0.756 | 5.667 | 0.73 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaji, Y.; Hirano, T.; Ogawa, H.; Fukatsu-Chikumoto, A.; Matsuda, K.; Hamada, K.; Ohata, S.; Suetake, R.; Murata, Y.; Oishi, K.; et al. Utility of the Shortness of Breath in Daily Activities Questionnaire (SOBDA-Q) to Detect Sedentary Behavior in Patients with Chronic Obstructive Pulmonary Disease (COPD). J. Clin. Med. 2023, 12, 4105. https://doi.org/10.3390/jcm12124105
Yamaji Y, Hirano T, Ogawa H, Fukatsu-Chikumoto A, Matsuda K, Hamada K, Ohata S, Suetake R, Murata Y, Oishi K, et al. Utility of the Shortness of Breath in Daily Activities Questionnaire (SOBDA-Q) to Detect Sedentary Behavior in Patients with Chronic Obstructive Pulmonary Disease (COPD). Journal of Clinical Medicine. 2023; 12(12):4105. https://doi.org/10.3390/jcm12124105
Chicago/Turabian StyleYamaji, Yoshikazu, Tsunahiko Hirano, Hiromasa Ogawa, Ayumi Fukatsu-Chikumoto, Kazuki Matsuda, Kazuki Hamada, Shuichiro Ohata, Ryo Suetake, Yoriyuki Murata, Keiji Oishi, and et al. 2023. "Utility of the Shortness of Breath in Daily Activities Questionnaire (SOBDA-Q) to Detect Sedentary Behavior in Patients with Chronic Obstructive Pulmonary Disease (COPD)" Journal of Clinical Medicine 12, no. 12: 4105. https://doi.org/10.3390/jcm12124105
APA StyleYamaji, Y., Hirano, T., Ogawa, H., Fukatsu-Chikumoto, A., Matsuda, K., Hamada, K., Ohata, S., Suetake, R., Murata, Y., Oishi, K., Asami-Noyama, M., Edakuni, N., Kakugawa, T., & Matsunaga, K. (2023). Utility of the Shortness of Breath in Daily Activities Questionnaire (SOBDA-Q) to Detect Sedentary Behavior in Patients with Chronic Obstructive Pulmonary Disease (COPD). Journal of Clinical Medicine, 12(12), 4105. https://doi.org/10.3390/jcm12124105






