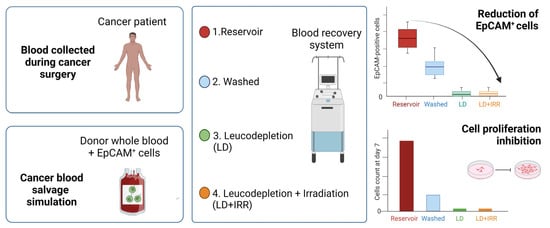
Reduction of EpCAM-Positive Cells from a Cell Salvage Product Is Achieved by Leucocyte Depletion Filters Alone
Abstract
:1. Introduction
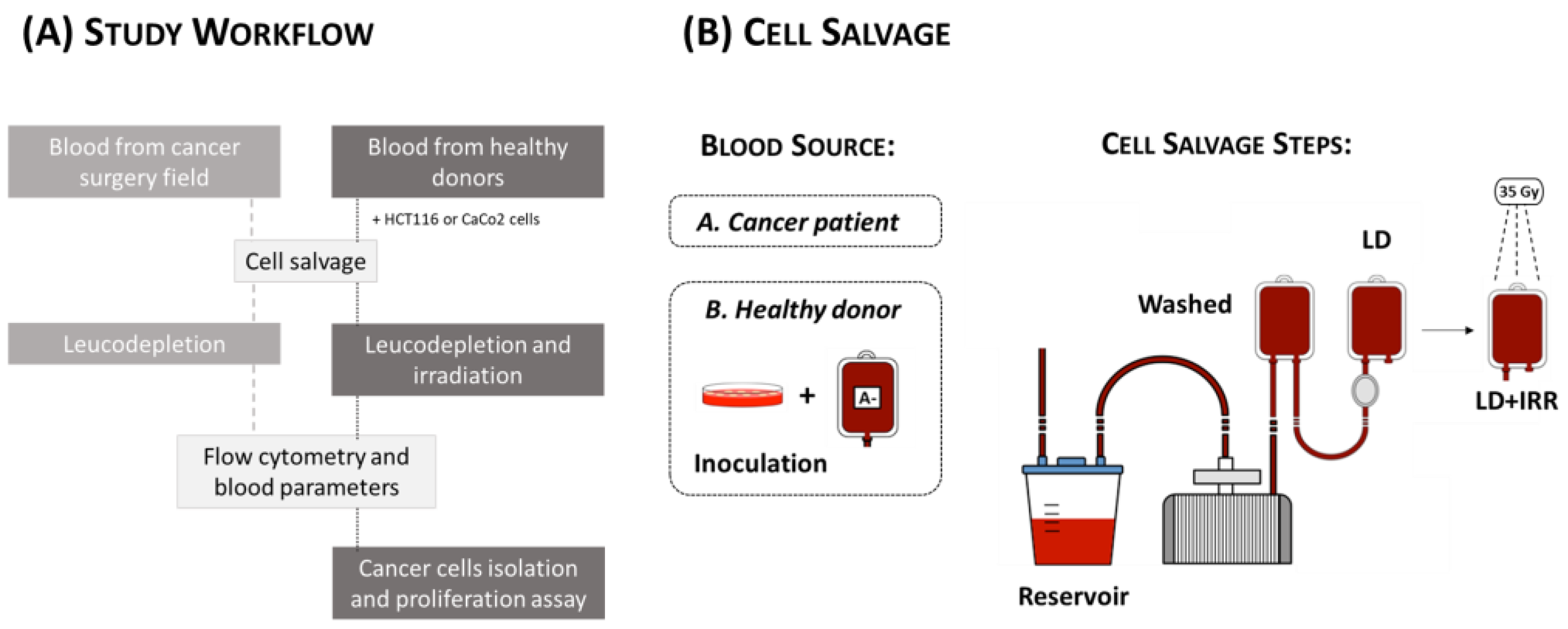
2. Materials and Methods
2.1. Study Population
2.2. Cell Salvage on Cancer Patients
2.3. Cell Culture
2.4. Cell Salvage In Vitro Simulation
2.5. Flow Cytometric Assessment of Circulating Tumor Cells (CTCs)
2.6. Cancer Cell Isolation and Proliferation Assay
2.7. Blood Count, Haemolysis and Erythrocyte Osmotic Resistance
2.8. Data Management and Statistical Analysis
3. Results
3.1. Cell Salvage in Cancer Patients
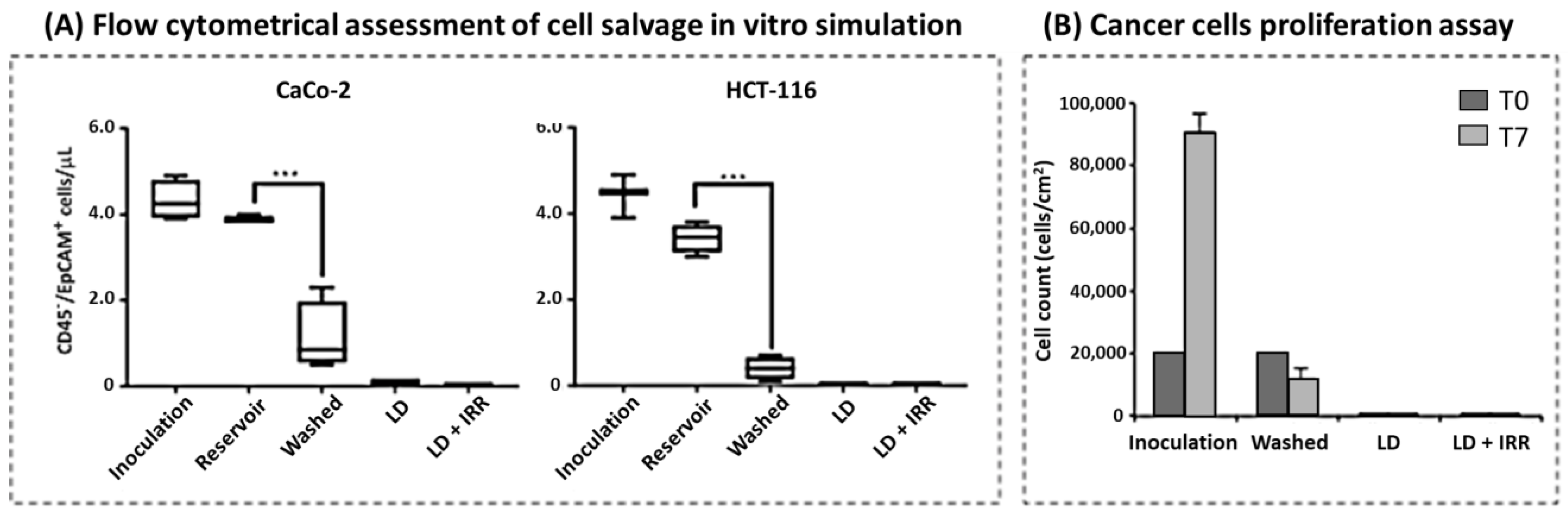
3.2. Cell Salvage In Vitro Simulation
3.3. Cancer Cell Isolation and Proliferation Assay
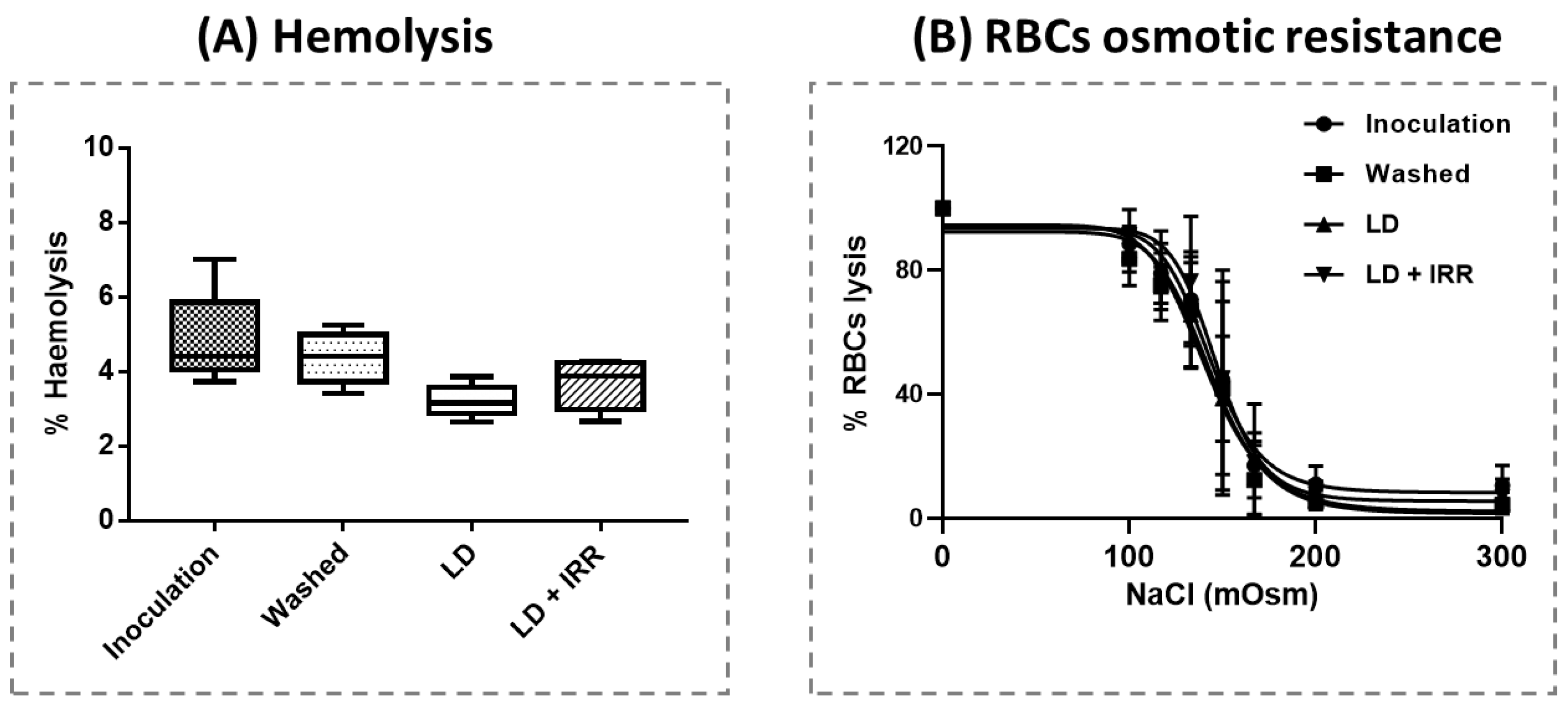
3.4. Blood Count, Haemolysis and Erythrocyte Osmotic Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liumbruno, G.; Bennardello, F.; Lattanzio, A.; Piccoli, P.; Rossetti, G. Recommendations for the transfusion of red blood cells. Blood Transfus. 2009, 7, 49–64. [Google Scholar] [PubMed]
- Kumar, N.; Chen, Y.; Nath, C.; Liu, E.H. What is the role of autologous blood transfusion in major spine surgery? Am. J. Orthop. 2012, 41, E89–E95. [Google Scholar]
- Meybohm, P.; Richards, T.; Isbister, J.; Hofmann, A.; Shander, A.; Goodnough, L.T.; Muñoz, M.; Gombotz, H.; Weber, C.F.; Choorapoikayil, S.; et al. Patient Blood Management Bundles to Facilitate Implementation. Transfus. Med. Rev. 2017, 31, 62–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaw, P.B.; Sentany, M.; Link, W.J.; Wahle, W.M.; Glover, J.L. Tumor cells carried through autotransfusion. Contraindication to intraoperative blood recovery? JAMA 1975, 231, 490–491. [Google Scholar] [CrossRef]
- Dale, R.F.; Kipling, R.M.; Smith, M.F.; Collier, D.S.J.; Smith, P.J. Separation of malignant cells during autotransfusion. Br. J. Surg. 1988, 75, 581. [Google Scholar] [CrossRef]
- Hansen, E.; Wolff, N.; Knuechel, R.; Ruschoff, J.; Hofstaedter, F.; Taeger, K. Tumor cells in blood shed from the surgical field. Arch. Surg. 1995, 130, 387–393. [Google Scholar] [CrossRef]
- Waters, J.H. Indications and contraindications of cell salvage. Transfusion 2004, 44, 40S–44S. [Google Scholar] [CrossRef]
- Miller, G.V.; Ramsden, C.W.; Primrose, J.N. Autologous transfusion: An alternative to transfusion with banked blood during surgery for cancer. Br. J. Surg. 1991, 78, 713–715. [Google Scholar] [CrossRef]
- Waters, J.H.; Yazer, M.; Chen, Y.F.; Kloke, J. Blood salvage and cancer surgery: A meta-analysis of available studies. Transfusion 2012, 52, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Nieder, A.M.; Carmack, A.J.; Sved, P.D.; Kim, S.S.; Manoharan, M.; Soloway, M.S. Intraoperative cell salvage during radical prostatectomy is not associated with greater biochemical recurrence rate. Urology 2005, 65, 730–734. [Google Scholar] [CrossRef]
- Hirano, T.; Yamanaka, J.; Iimuro, Y.; Fujimoto, J. Long-term safety of autotransfusion during hepatectomy for hepatocellular carcinoma. Surg. Today 2005, 35, 1042–1046. [Google Scholar] [CrossRef]
- Bower, M.R.; Ellis, S.F.; Scoggins, C.R.; McMasters, K.M.; Martin, R.C. Phase II comparison study of intraoperative autotransfusion for major oncologic procedures. Ann. Surg. Oncol. 2011, 18, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.P.; Morris, P.C.; Alagoz, T.; Anderson, B.; Bottles, K.; Buller, R.E. Intraoperative autologous blood collection and autotransfusion in the surgical management of early cancers of the uterine cervix. Obs. Obstet. Gynecol. 1995, 86, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, G.S.; Joh, J.W.; Suh, K.S.; Park, J.B.; Ko, J.S.; Kwon, C.H.; Yi, N.J.; Gwak, M.S.; Lee, K.W.; et al. Long-term results for living donor liver transplantecipients with hepatocellular carcinoma using intraoperative blood salvage with leukocyte depletion filter. Transpl. Int. 2013, 26, 84–89. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicine and Healthcare. Guide to the Preparation, Use and Quality Assurance of Blood Components, 20th ed.; The Council of Europe: Strasbourg, France, 2020; Available online: https://www.edqm.eu/en/blood-guide (accessed on 17 March 2023).
- Liumbruno, G.M.; Bennardello, F.; Lattanzio, A.; Piccoli, P.; Rossetti, G. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party. Recommendations for the transfusion management of patients in the peri-operative period. II. The intra-operative period. Blood Transfus. 2011, 9, 189–217. [Google Scholar] [PubMed]
- Zong, Y.-N.; Xu, C.-Y.; Gong, Y.-Q.; Zhang, X.-Q.; Zeng, H.; Liu, C.; Bin Zhang, B.; Xue, L.-X.; Guo, X.-Y.; Wei, F.; et al. Effectiveness of intraoperative cell salvage combined with a modified leucocyte depletion filter in metastatic spine tumour surgery. BMC Anesth. Anesthesiol. 2022, 22, 217. [Google Scholar] [CrossRef]
- Mei, K.; Du, L.; Yan, M.; Zhang, Z.; Zhang, F.; Gong, L.; Sun, K.; Zhang, J.; Tang, Y.; Jiang, C.; et al. Modified Leukocyte Filter Removes Tumor Cells from the Salvaged Blood. PLoS ONE 2015, 10, e0130864. [Google Scholar] [CrossRef]
- Gwak, M.S.; Lee, K.W.; Kim, S.Y.; Lee, J.; Joh, J.W.; Kim, S.J.; Lee, H.H.; Park, J.W.; Kim, G.S.; Lee, S.K. Can a leukocyte depletion filter (LDF) reduce the risk of reintroduction of hepatocellular carcinoma cells? Liver Transpl. 2005, 11, 331–335. [Google Scholar] [CrossRef]
- Marraccini, C.; Merolle, L.; Berni, P.; Boito, K.; Tamagnini, I.; Kuhn, E.; Ragazzi, M.; Baricchi, R.; Pertinhez, T.A. Safety of leucodepleted salvaged blood in oncological surgery: An in vitro model. Vox Sang. 2017, 112, 803–805. [Google Scholar] [CrossRef]
- Catling, S.; Williams, S.; Freites, O.; Rees, M.; Davies, C.; Hopkins, L. Use of a leucocyte filter to remove tumour cells from intra-operative cell salvage blood. Anaesthesia 2008, 63, 1332–1338. [Google Scholar] [CrossRef]
- Schiroli, D.; Merolle, L.; Quartieri, E.; Chicchi, R.; Fasano, T.; De Luca, T.; Molinari, G.; Pulcini, S.; Pertinhez, T.A.; Di Bartolomeo, E.; et al. Comparison of Two Alternative Procedures to Obtain Packed Red Blood Cells for β-Thalassemia Major Transfusion Therapy. Biomolecules 2021, 11, 1638. [Google Scholar] [CrossRef] [PubMed]
- Detraglia, M.; Cook, F.B.; Stasiw, D.M.; Cerny, L.C. Erythrocyte fragility in aging. Biochim. Biophys. Acta 1974, 345, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.; Pallister, C.J. The impact of anaemia on outcome in cancer. Clin. Lab. Haematol. 2005, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Merolle, L.; Marraccini, C.; Di Bartolomeo, E.; Montella, M.T.; Pertinhez, T.A.; Baricchi, R.; Bonini, A. Postoperative patient blood management: Transfusion appropriateness in cancer patients. Blood Transfus. 2020, 18, 359–365. [Google Scholar] [PubMed]
- Aguilar-Nascimento, J.E.; Zampieri-Filho, J.P.; Bordin, J.O. Implications of perioperative allogeneic red blood cell transfusion on the immune-inflammatory response. Hematol. Transfus. Cell Ther. 2021, 43, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Frietsch, T.; Steinbicker, A.U.; Horn, A.; Metz, M.; Dietrich, G.; Weigand, M.A.; Waters, J.H.; Fischer, D. Safety of Intraoperative Cell Salvage in Cancer Surgery: An Updated Meta-Analysis of the Current Literature. Transfus. Med. Hemother 2022, 49, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, C.; Macchi, R.M.; Marschner, A.K.; Mellstedt, H. Epithelial cell adhesion molecule expression (CD326) in cancer: A short review. Cancer Treat. Rev. 2012, 38, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Shao, W.; Li, Z.; Zhao, R.; Ye, Z. Strategies for enrichment of circulating tumor cells. Transl. Cancer Res. 2020, 9, 2012–2025. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Zaw, A.S.; Khoo, B.L.; Nandi, S.; Lai, Z.; Singh, G.; Lim, C.T.; Thiery, J.P. Intraoperative cell salvage in metastatic spine tumour surgery reduces potential for reinfusion of viable cancer cells. Eur. Spine J. 2016, 25, 4008–4015. [Google Scholar] [CrossRef]
- Klein, A.A.; Bailey, C.R.; Charlton, A.J.; Evans, E.; Guckian-Fisher, M.; McCrossan, R.; Lim, C.T.; Thiery, J.P. Association of Anaesthetists guidelines: Cell salvage for peri-operative blood conservation. Anaesthesia 2018, 73, 1141–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, M.; Yang, J.T.; Liu, Y.Q.; Tang, L.H.; Wang, Y.; Wang, L.J.; Zhang, F.-J.; Yan, M. Irradiation Can Selectively Kill Tumor Cells while Preserving Erythrocyte Viability in a Co-Culture System. PLoS ONE 2015, 10, e0127181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Guo, X.; Zong, Y.; Xu, C.; Wang, J.; Zhang, B.; Liu, C.; Gong, Y.; Xue, L.; Ma, L.; et al. CTCs detection from intraoperative salvaged blood in RCC-IVC thrombus patients by negative enrichment and iFISH identification: A preliminary study. BMC Urol. 2021, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Lam, R.; Zaw, A.S.; Malhotra, R.; Tan, J.; Tan, G.; Setiobudi, T. Flow cytometric evaluation of the safety of intraoperative salvaged blood filtered with leucocyte depletion filter in spine tumour surgery. Ann. Surg. Oncol. 2014, 21, 4330–4335. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.H.; Menon, N.V.; Tan, P.S.; Pan, T.L.T.; Bonney, G.K.; Shridhar, I.G.; Madhavan, K.; Lim, C.T.; Kow, A.W.C. Presence of tumor cells in intra-operative blood salvage autotransfusion samples from hepatocellular carcinoma liver transplantation: Analysis using highly sensitive microfluidics technology. HPB 2021, 23, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Elmalky, M.; Yasin, N.; Rodrigues-Pinto, R.; Stephenson, J.; Carroll, C.; Smurthwaite, G.; Verma, R.; Mohammad, S.; Siddique, I. The safety, efficacy, and cost-effectiveness of intraoperative cell salvage in metastatic spine tumor surgery. Spine J. 2017, 17, 977–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, A.; Seyfried, T.; Ahrens, N.; Baier-Kleinhenz, L.; Schlitt, H.J.; Peschel, G.; Graf, B.M.; Sinner, B. Cell Salvage Cell Salvage During Liver Transplantation for Hepatocellular Carcinoma: A Retrospective Analysis of Tumor Recurrence Following Irradiation of the Salvaged Blood. Transpl. Transplant. Proc. 2021, 53, 1639–1644. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Blood Transfusion NICE Guideline [NG24]; NICE: London, UK, 2015. Available online: https://www.nice.org.uk/guidance/ng24/resources (accessed on 20 May 2023).
- Gakhar, H.; Bagouri, M.; Bommireddy, R.; Klezl, Z. Role of intraoperative red cell salvage and autologus transfusion in metastatic spine surgery: A pilot study and review of literature. Asian Spine J. 2013, 7, 167–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Kim, G.; Ko, J.S.; Sinn, D.H.; Yang, J.D.; Joh, J.W.; Lee, S.K.; Gwak, M.S. Safety of the Use of Blood Salvage and Autotransfusion During Liver Transplantation for Hepatocellular Carcinoma. Ann Surg. 2016, 264, 339–343. [Google Scholar] [CrossRef]
- Myrga, J.M.; Ayyash, O.M.; Bandari, J.; Fam, M.M.; Macleod, L.C.; Jacobs, B.L.; Davies, B.J. The Safety and Short-term Outcomes of Leukocyte Depleted Autologous Transfusions During Radical Cystectomy. Urology 2020, 135, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Rajasekaran, R.B.; Palmer, A.J.R.; Whitwell, D.; Cosker, T.D.A.; Pigott, D.; Zsolt, O.; Booth, R.; Gibbons, M.R.J.P.; Carr, A. The role of intraoperative cell salvage for musculoskeletal sarcoma surgery. J. Bone Oncol. 2021, 30, 100390. [Google Scholar] [CrossRef]
- Kumar, N.; Ahmed, Q.; Lee, V.K.M.; Chen, Y.; Zaw, A.S.; Goy, R.; Agrawal, R.V.; Dhewar, A.N.; Wong, H.K. Can There be a Place for Intraoperative Salvaged Blood in Spine Tumor Surgery? Ann. Surg. Oncol. 2014, 21, 2436–2443. [Google Scholar] [CrossRef]
- Kumar, N.; Ahmed, Q.; Lee, V.K.; Zaw, A.S.; Goy, R.; Wong, H.K. Are we ready for the use of intraoperative salvaged blood in metastatic spine tumour surgery? Eur. Spine J. 2016, 25, 3997–4007. [Google Scholar] [CrossRef]
- Winter, A.; Zacharowski, K.; Meybohm, P.; Schnitzbauer, A.; Ruf, P.; Kellermann, C.; Lindhofer, H. Removal of EpCAM-positive tumor cells from blood collected during major oncological surgery using the Catuvab device- a pilot study. BMC Anesthesiol. 2021, 21, 261. [Google Scholar] [CrossRef]
- Zhang, F.J.; Yang, J.T.; Tang, L.H.; Wang, W.N.; Sun, K.; Ming, Y.; Muhammad, K.G.; Zheng, Y.F.; Yan, M. Effect of X-ray irradiation on hepatocarcinoma cells and erythrocytes in salvaged blood. Sci. Rep. 2017, 7, 7995. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Yang, L.; He, C.; Tai, S.; Ma, C.; Yang, T.; Wang, D. Evaluation of riboflavin photochemical treatment for inactivation of HCT116 tumor cells mixed in simulative intraoperative salvage blood. Transfusion 2019, 59, 3205–3213. [Google Scholar] [CrossRef] [Green Version]
- Zhai, B.; Sun, X.Y. Controversy over the use of intraoperative blood salvage autotransfusion during liver transplantation for hepatocellular carcinoma patients. World J. Gastroenterol. 2013, 19, 3371–3374. [Google Scholar] [CrossRef]
- Kumar, N.; Chen, Y.; Zaw, A.S.; Nayak, D.; Ahmed, Q.; Soong, R.; Wong, H.K. Use of intraoperative cell-salvage for autologous blood transfusions in metastatic spine tumour surgery: A systematic review. Lancet Oncol. 2014, 15, e33–e41. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, M.C.; Correa, A.F.; Lyon, T.D.; Davies, B.J.; Ost, M.C. The use of intraoperative cell salvage in urologic oncology. Rev. Urol. 2017, 19, 89–96. [Google Scholar] [PubMed]
- Kumar, N.; Ravikumar, N.; Tan, J.Y.H.; Akbary, K.; Patel, R.S.; Kannan, R. Current Status of the Use of Salvaged Blood in Metastatic Spine Tumour Surgery. Neurospine 2018, 15, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Patient Population | |
| N (males, females) | 12 (4, 8) |
| Age (mean ± SD) | 70.4 ± 10.1 |
| Primary diagnosis | |
| Colorectal cancer (N) | 4 |
| Hepatocarcinoma (N) | 8 |
| Presence of diffused metastases (N/total) | 5/12 |
| Surgery | |
| Type of surgery | Partial hepatectomy |
| Duration (mean ± SD) hours | 2.2 ± 0.9 |
| Whole blood recovered (mean ± SD) mL | 1021 ± 519 |
| Washed RBCs (mean ± SD) mL | 115 ± 80 |
| Filtered RBCs (mean ± SD) mL | 70 ± 50 |
| Patient ID | Negative Control | Reservoir | Washed | LD | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| 1 | n.d. | n.d. | 10.51 | 2.19 | 0.53 | 0.2 | 0.28 | 0.04 |
| 2 | 0.91 | 0.1 | 26.35 | 0.35 | 4.87 | 0.01 | 1 | 0.01 |
| 3 | 2.47 | 0.13 | 25.76 | 4.33 | 12.49 | 1.22 | 0.91 | 0.11 |
| 4 | 3.62 | 0.12 | 4.4 | 0.11 | 2.12 | 0.46 | 1.41 | 0.03 |
| 5 | n.d. | n.d. | 7.27 | 1.1 | 16.35 | 4.67 | n.d. | n.d. |
| 6 | 0.33 | 0.11 | 3.29 | 0.71 | 1.79 | 0.16 | n.d. | n.d. |
| 7 | 21.3 | 2.11 | 63.75 | 11.58 | 32.4 | 10.95 | 1.58 | 0.58 |
| 8 | 9.16 | 0.71 | 12.25 | 0.1 | 7.02 | 0.34 | 3.68 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merolle, L.; Schiroli, D.; Farioli, D.; Razzoli, A.; Gavioli, G.; Iori, M.; Piccagli, V.; Lambertini, D.; Bassi, M.C.; Baricchi, R.; et al. Reduction of EpCAM-Positive Cells from a Cell Salvage Product Is Achieved by Leucocyte Depletion Filters Alone. J. Clin. Med. 2023, 12, 4088. https://doi.org/10.3390/jcm12124088
Merolle L, Schiroli D, Farioli D, Razzoli A, Gavioli G, Iori M, Piccagli V, Lambertini D, Bassi MC, Baricchi R, et al. Reduction of EpCAM-Positive Cells from a Cell Salvage Product Is Achieved by Leucocyte Depletion Filters Alone. Journal of Clinical Medicine. 2023; 12(12):4088. https://doi.org/10.3390/jcm12124088
Chicago/Turabian StyleMerolle, Lucia, Davide Schiroli, Daniela Farioli, Agnese Razzoli, Gaia Gavioli, Mauro Iori, Vando Piccagli, Daniele Lambertini, Maria Chiara Bassi, Roberto Baricchi, and et al. 2023. "Reduction of EpCAM-Positive Cells from a Cell Salvage Product Is Achieved by Leucocyte Depletion Filters Alone" Journal of Clinical Medicine 12, no. 12: 4088. https://doi.org/10.3390/jcm12124088
APA StyleMerolle, L., Schiroli, D., Farioli, D., Razzoli, A., Gavioli, G., Iori, M., Piccagli, V., Lambertini, D., Bassi, M. C., Baricchi, R., & Marraccini, C. (2023). Reduction of EpCAM-Positive Cells from a Cell Salvage Product Is Achieved by Leucocyte Depletion Filters Alone. Journal of Clinical Medicine, 12(12), 4088. https://doi.org/10.3390/jcm12124088