Pseudohyponatremia: Mechanism, Diagnosis, Clinical Associations and Management
Abstract
:1. Introduction
2. Methods for Measuring Serum Sodium Concentration
3. Mechanisms of Pseudohyponatremia
3.1. Sodium Concentration Lowering by the Electrolyte Exclusion Effect
3.2. Sodium Concentration Lowering by the Dilution Effect
3.3. Sodium Concentration Lowering by the Hyperviscosity Effect
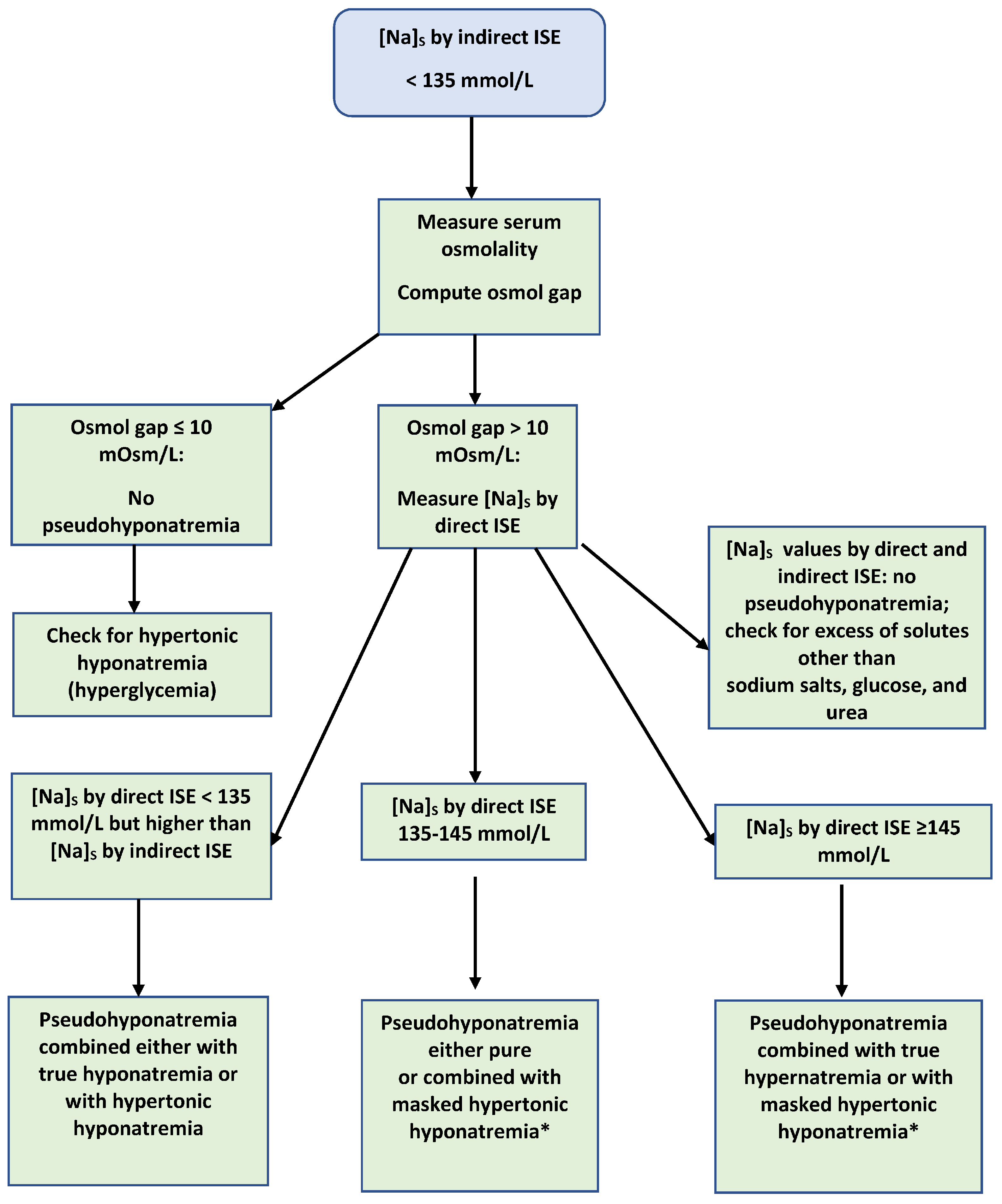
4. Diagnosis of Pseudohyponatremia
5. Clinical Conditions Associated with Pseudohyponatremia
5.1. Hyperproteinemia
5.2. Hyperlipidemia
5.3. Diabetic Ketoacidosis
5.4. Enzyme Mutations Causing Hypertriglyceridemia
5.5. Hypercholesterolemia Caused by Cholestasis
5.6. Pseudohyponatremia in the Absence of Elevated Serum Solids Content
5.7. Differences in [Na]S Values Measured by Different Direct ISE Apparatuses
5.8. Clinical Conditions Associated with Elevated Serum Solids Content
6. Frequency of Spurious Serum Sodium Measurements
7. Management of Pseudohyponatremia—Future Developments
8. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fortgens, P.; Pillay, T.S. Pseudohyponatremia revisited: A modern-day pitfall. Arch. Pathol. Lab. Med. 2011, 135, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, G.L. Serum sodium. In Clinical Methods: The History, Physical, and Laboratory Examination, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; pp. 879–883. [Google Scholar]
- Palmer, B.F.; Gates, J.R.; Lader, M. Causes and management of hyponatremia. Ann. Pharmacother. 2003, 37, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Jaber, B.L.; Madias, N.E. Incidence and prevalence of hyponatremia. Am. J. Med. 2006, 119 (Suppl. S1), S30–S35. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Morley, J.E.; Rubenstein, L.Z. Hyponatremia in a nursing home population. J. Am. Geriatr. Soc. 1995, 43, 1410–1413. [Google Scholar] [CrossRef]
- Sterns, R.H. Severe symptomatic hyponatremia: Treatment and outcome. A study of 64 cases. Ann. Intern. Med. 1987, 107, 656–664. [Google Scholar] [CrossRef]
- Asadollahi, K.; Beeching, N.; Gill, G. Hyponatraemia as a risk factor for hospital mortality. QJM 2006, 99, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Sterns, R.H. .; Nigwekar, S.U.; Cappuccio, J.D. Mortality and serum sodium: Do patients die from or with hyponatremia? Clin. J. Am. Soc. Nephrol. 2011, 6, 960–965. [Google Scholar] [CrossRef] [Green Version]
- Lu, D.Y.; Cheng, H.M.; Cheng, Y.L.; Hsu, P.F.; Huang, W.M.; Guo, C.Y.; Yu, Y.C.; Chen, C.H.; Sung, S.H. Hyponatremia and worsening sodium levels are associated with long-term outcome in patients hospitalized for acute heart failure. J. Am. Heart Assoc. 2016, 5, e002668. [Google Scholar] [CrossRef] [Green Version]
- Ayus, J.C.; Negri, A.L.; Moritz, M.L.; Lee, K.M.; Caputo, D.; Borda, M.E.; Go, A.S.; Eghi, C. Hyponatremia, inflammation at admission, and mortality in hospitalized COVID-19 patients: A prospective cohort study. Front. Med. 2021, 8, 748364. [Google Scholar] [CrossRef]
- Seay, N.W.; Lehrich, R.W.; Greenberg, A. Diagnosis and management of disorders of body tonicity—Hyponatremia and hypernatremia: Core curriculum 2020. Am. J. Kidney Dis. 2020, 75, 272–286. [Google Scholar] [CrossRef] [Green Version]
- Waugh, W.H. Utility of expressing serum sodium per unit of water in assessing hyponatremia. Metabolism 1969, 18, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.H.; Siggaard-Andersen, O.; Weisberg, H.F.; Zijlstra, W.G. Ion-selective electrodes for sodium and potassium: A new problem of what is measured and what should be reported. Clin. Chem. 1985, 31, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, R.; Morgan, D.B.; Madsen, P.E.R. Pseudohyponatraemia. Lancet 1981, 1, 96. [Google Scholar] [CrossRef]
- Theis, S.R.; Khandahar, P.B. Pseudohyponatremia. In StatPearls (Internet); Stat Pearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sam, R.; Ing, T.S. Sodium and water disturbances. In A Practical Manual of Renal Medicine; Lai, K.N., Ed.; World Scientific Publishing Company: Singapore, 2009; pp. 45–80. [Google Scholar]
- Liamis, G.; Liberopoulos, E.; Barkas, F.; Elisaf, M. Spurious electrolyte disorders: A diagnostic challenge for clinicians. Am. J. Nephrol. 2013, 38, 50–57. [Google Scholar] [CrossRef]
- Raimann, J.G.; Tzamaloukas, A.H.; Levin, N.W.; Ing, T.S. Osmotic pressure in clinical medicine with an emphasis on dialysis. Semin. Dial. 2017, 30, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.M.; Reed, J. Pseudohyponatremia in acute hyperlipemic pancreatitis. A potential pitfall in therapy. Arch. Surg. 1985, 120, 1053–1055. [Google Scholar] [CrossRef]
- Hald, P.M. The flame photometer for the measurement of sodium and potassium in biological materials. J. Biol. Chem. 1947, 167, 499–510. [Google Scholar] [CrossRef]
- Levy, G.B. Determination of sodium with ion-selective electrodes. Clin. Chem. 1981, 27, 1435–1438. [Google Scholar] [CrossRef]
- Worth, H.G. Plasma sodium concentration: Bearer of false prophecies? Br. Med. J. 1983, 287, 567–568. [Google Scholar] [CrossRef] [Green Version]
- Worth, H.G. A comparison of the measurement of sodium and potassium by flame photometry and ion-selective electrode. Ann. Clin. Biochem. 1985, 22, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Langhoff, E.; Ladefoged, J. Sodium activity, sodium concentration, and osmolality in plasma in acute and chronic renal failure. Clin. Chem. 1985, 31, 1811–1814. [Google Scholar] [CrossRef] [PubMed]
- Malandrini, S.; Lava, S.A.G.; BIanchetti, M.G.; Meani, F.; Faré, P.B.; Camozzi, P.; Cugliari, M.; Agostoni, C.; Milani, G.P. Which laborarory technique is used for the blood sodium analysis in clinical research? A systematic review. Clin. Chem. Lab. Med. 2021, 59, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- College of American Pathologists. Chemistry/Therapeutic Drug Monitoring C-A:CAP Surveys; American College of Pathologists: Chicago, IL, USA, 2015. [Google Scholar]
- Schindler, E.I.; Brown, S.M.; Scott, M.G. Electrolytes and blood gases. In Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 6th ed.; Rifai, N., Horvath, A.R., Wittner, C.T., Eds.; Elsevier: St. Louis, MO, USA, 2018; pp. 604–625. [Google Scholar]
- Cowell, D.C.; Browning, D.M.; Clarke, S.; Kilshaw, D.; Randell, J.; Singer, R. Sodium and potassium ion selective electrodes: A review of theory and calibration. Med. Lab. Sci. 1985, 42, 252–261. [Google Scholar] [PubMed]
- Külpmann, W.R.; Höbbel, T. International consensus on the standardization of sodium and potassium measurements by ion-selective electrodes in undiluted samples. Scand. J. Clin. Lab. Investig. Suppl. 1996, 224, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Koch, D.D.; Graves, S.; Ladenson, J.H. Relationship between direct-potentiometric and flame-photometric measurement of sodium in blood. Clin. Chem. 1982, 28, 1931–1935. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, L.S. Pseudohyponatremia: A reappraisal. Am. J. Med. 1989, 86, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Aw, T.C.; Kiechle, F.L. Pseudohyponatremia. Am. J. Emerg. Med. 1985, 3, 236–239. [Google Scholar] [CrossRef]
- Ladenson, J.H. Direct potentiometric analysis of sodium and potassium in human plasma: Evidence for electrolyte interaction with a nonprotein, protein-associated substance(s). J. Lab. Clin. Med. 1977, 90, 654–665. [Google Scholar] [PubMed]
- Ladenson, J.H.; Apple, F.S.; Koch, D.D. Misleading hyponatremia due to hyperlipidemia: A method-dependent error. Ann. Intern. Med. 1981, 95, 707–708. [Google Scholar] [CrossRef] [PubMed]
- Vader, H.L.; Vink, C.L. The influence of viscosity on dilution methods. Its problems in the determination of serum sodium. Clin. Chim. Acta. 1975, 65, 379–388. [Google Scholar] [CrossRef]
- Gertz, M.A. Waldenström macroglobulinemia: 2021 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2021, 96, 258–269. [Google Scholar] [CrossRef]
- Nanji, A.A.; Blank, D. Effect of temperature and methodology in spurious hyponatremia due to serum hyperviscosity. Clin. Chem. 1983, 29, 595. [Google Scholar] [CrossRef]
- McGowan, M.W.; Artiss, J.D.; Zak, B. Description of analytical problems arising from elevated serum solids. Anal. Biochem. 1984, 142, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Nanji, A.A.; Blank, D.W. Pseudohyponatremia and hyperviscosity. J. Clin. Pathol. 1983, 36, 834–835. [Google Scholar] [CrossRef] [Green Version]
- Sterns, R.H.; Schrier, R.W.; Narins, R.G. Hyponatremia: Pathophysiology, diagnosis, and therapy. In Clinical Disorders of Fluid and Electrolyte Metabolism, 5th ed; Narins, R.G., Ed.; Mc Graw-Hill: New York, NY, USA, 1994; pp. 591–593. [Google Scholar]
- Overlack, A.; Niederle, N.; Klautke, G.; Stumpe, K.O.; Krück, F. Pseudohyponatremia in multiple myeloma. Klin. Wochenschr. 1980, 58, 875–880. [Google Scholar] [CrossRef]
- Steinberger, B.A.; Ford, S.M.; Coleman, T.A. Intravenous immunoglobulin therapy results in post-infusional hyperproteinemia, increased serum viscocity, and pseudohyponatremia. Am. J. Hematol. 2003, 73, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Baker, A.L.; Chow, M.J.; Hay, R.V. Hyperviscosity syndrome in a hypercholesterolemic patient with primary biliary cirrhosis. Gastroenterology 1990, 98, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Edelman, I.S.; Leibman, J.; O’Meara, M.P.; Birkenfeld, L.W. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J. Clin. Investig. 1958, 37, 1236–1256. [Google Scholar] [CrossRef] [Green Version]
- Davis, F.E.; Kenyon, K.; Kirk, J. A rapid titrimetric method for determining the water content of human blood. Science 1953, 118, 276–277. [Google Scholar] [CrossRef]
- Albrink, M.J.; Hald, P.M.; Man, E.B.; Peters, J.P. The displacement of serum water by the lipids of hyperlipemic serum. A new method for the rapid determination of serum water. J. Clin. Investig. 1955, 34, 1483–1488. [Google Scholar] [CrossRef]
- Faye, S.; Payne, R.B. Rapid measurement of serum water to assess pseudohyponatremia. Clin. Chem. 1986, 32, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Steffes, M.W.; Freier, E.F. A simple and precise method of determining true sodium, potassium, and chloride concentrations in hyperlipemia. J. Lab. Clin. Med. 1976, 88, 683–688. [Google Scholar] [PubMed]
- Dimeski, G.; Mollee, P.; Carter, A. Effects of hyperlipidemia on plasma sodium, potassium and chloride measurements by an indirect ion-selective electrode measuring system. Clin. Chem. 2006, 52, 155–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Story, D.A.; Morimatsu, H.; Egi, M.; Bellomo, R. The effect of albumin concentration on plasma sodium and chloride measurements in critically ill patients. Anesth. Analg. 2007, 104, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Goldwasser, P.; Ayoub, I.; Barth, R.H. Pseudohypernatremia and pseudohyponatremia: A linear correction. Nephrol. Dial. Transplant. 2015, 30, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.K.; Ornekian, V.; Butch, A.W.; Kurtz. I. A new method for determining plasma water content: Application in pseudohyponatremia. Am. J. Physiol. Renal. Physiol. 2007, 292, F1652–F1656. [Google Scholar] [CrossRef]
- Lang, T.; Prinsloo, P.; Broughton, A.F.; Lawson, N.; Marenah, C.B. Effect of low protein concentration on serum sodium measurement: Pseudohypernatraemia and pseudohyponatraemia. Ann. Clin. Biochem. 2002, 39, 66–67. [Google Scholar] [CrossRef]
- Dimeski, G.; Morgan, T.J.; Presneil, J.J.; Venkatesh, B. Disagreement between ion selective electrode direct and indirect sodium measurements: Estimation of the problem in a tertiary referral hospital. J. Crit. Care. 2012, 27, 326.e9–326.e16. [Google Scholar] [CrossRef]
- Musso, C.G.; Bargman, J.M. Asymptomatic hyponatremia in peritoneal dialysis patients: An algorithmic approach. Int. Urol. Nephrol. 2014, 46, 2239–2241. [Google Scholar] [CrossRef]
- Dhatt, G.; Talor, Z.; Kazory, A. Direct ion-selective method is useful in diagnosis of pseudohyponatremia. J. Emerg. Med. 2012, 43, 348–349. [Google Scholar] [CrossRef]
- Rondon-Berrios, H.; Agaba, E.I.; Tzamaloukas, A.H. Hyponatremia: Pathophysiology, classification, manifestations and management. Int. Urol. Nephrol. 2014, 46, 2153–2165. [Google Scholar] [CrossRef]
- Arzhan, S.; Lew, S.Q.; Ing, T.S.; Tzamaloukas, A.H.; Unruh, M.L. Dysnatremias in chronic kidney disease: Pathophysiology, manifestations, and treatment. Front. Med. 2021, 8, 769287. [Google Scholar] [CrossRef]
- Liamis, G.; Filippatos, T.D.; Liontos, A.; Elisaf, M.S. Serum osmolal gap in clinical practice: Usefulness and limitations. Postgrad. Med. 2017, 129, 456–459. [Google Scholar] [CrossRef]
- Rohrscheib, M.; Rondon-Berrios, H.; Argyropoulos, C.; Glew, R.H.; Murata, G.H.; Tzamaloukas, A.H. Indices of serum tonicity in clinical practice. Am. J. Med. Sci. 2015, 349, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Sklar, A.H.; Linas, S.L. The osmolal gap in renal failure. Ann. Intern. Med. 1983, 98, 481–482. [Google Scholar] [CrossRef] [PubMed]
- Guglielminotti, J.; Pernet, P.; Maury, E.; Alzieu, M.; Vaubourdolle, M.; Guidet, B.; Offenstadt, G. Osmolar gap hyponatremia in critically ill patients: Evidence for the sick cell syndrome? Crit. Care Med. 2002, 30, 1051–1055. [Google Scholar] [CrossRef]
- Tzamaloukas, A.H.; Jackson, J.E.; Long, D.A.; Gallegos, J.C. Serum ethyl alcohol levels and osmolal gaps. J. Toxicol. Clin. Toxicol. 1982, 19, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, M.; Zhou, J.X. Correlation of measured and calculated serum osmolality during mannitol or hypertonic saline infusion in patients after craniotomy: A study protocol and statistical analysis plan for a randomized controlled trial. BMJ Open 2014, 4, e004921. [Google Scholar] [CrossRef] [PubMed]
- Ing, T.S.; Ganta, K.; Bhave, G.; Lew, S.Q.; Agaba, E.I.; Argyropoulos, C.; Tzamaloukas, A.H. The corrected sodium concentration in hyperglycemic crises: Computation and clinical applications. Front. Med. 2020, 7, 477. [Google Scholar] [CrossRef] [PubMed]
- Tarail, R.; Buchwald, K.W.; Holland, J.F.; Selawry, O.S. Misleading reductions of serum sodium and chloride associated with hyperproteinemia in patients with multiple myeloma. Proc. Soc. Exp. Biol. Med. 1962, 110, 145–148. [Google Scholar] [CrossRef]
- Frick, P.G.; Schmid, J.R.; Kistler, H.J.; Hitzig, W.H. Hyponatremia associated with hyperproteinemia in multiple myeloma. Helv. Med. Acta 1967, 33, 317–329. [Google Scholar]
- Pain, R.W. Test and teach. Number forty-one. Diagnosis: Hypertriglyceridemia with pseudohyponatremia in acute or chronic alcoholism; multiple myeloma with pseudohyponatremia, decreased anion gap and hypercalcemia. Pathology 1983, 15, 233–331. [Google Scholar] [CrossRef] [PubMed]
- Vaswani, S.K.; Sprague, R. Pseudohyponatremia in multiple myeloma. South. Med. J. 1993, 86, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Olivero, J.J. Case in point. Pseudohyponatremia due to hyperproteinemia in multiple myeloma. Hosp. Pract. 1994, 29, 61. [Google Scholar] [CrossRef] [PubMed]
- Giri, P.; George, J.; Gupta, A.K.; Gupta, R. Pseudohyponatremia in multiple myeloma. J. Assoc. Physicians India 2010, 58, 519–520. [Google Scholar]
- Jelinek, A.G.; Bachmann, L.M. Unexpected test results in a patient with multiple myeloma. Clin. Chem. 2014, 60, 1375–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tho, Z.L.B.; Charles, J.S.; Teo, D.B. Less is more: Pseudohyponatremia in multiple myeloma. Am. J. Med. 2022, 135, e44–e46. [Google Scholar] [CrossRef]
- Nanji, A.A.; Halstead, A.C. Changes in serum anion gap and sodium level in monoclonal gammopathies. Can. Med. Assoc. J. 1982, 127, 32–35. [Google Scholar]
- Zelmat, M.S. Direct and indirect ion selective electrodes methods: The differences specified through a case of Waldenström’s macroglobulinemia. Ann. Biol. Clin. 2015, 73, 345–352. [Google Scholar] [CrossRef]
- Grateau, G.; Bachmeyer, C.; Tauléra, O.; Sarfati, G.; Cremer, G.; Séréni, D. Pseudohyponatremia and pseudohyperphosphatemia in a patient with human immunodeficiency virus infection. Nephron 1993, 64, 640. [Google Scholar] [CrossRef]
- Garibaldi, B.T.; Cameron, S.J.; Choi, M. Pseudohyponatremia in a patient with HIV and hepatitis C coinfection. J. Gen. Intern. Med. 2008, 23, 202–205. [Google Scholar] [CrossRef] [Green Version]
- Lawn, N.; Wijdicks, E.F.; Burritt, M.F. Intravenous immune globulin and pseudohyponatremia. N. Engl. J. Med. 1998, 339, 632. [Google Scholar] [CrossRef]
- Ng, S.K. Intravenous immunoglobulin infusion causing pseudohyponatremia. Lupus 1999, 8, 488–490. [Google Scholar] [CrossRef]
- Tarcan, A.; Gökmen, Z.; Dikmenoğlu, N.; Gürakan, B. Pseudohyponatremia and hyperviscosity due to IVIG therapy in a term newborn. Acta Pediatr. 2005, 94, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Mayan, H.; Gurevitz, O.; Muallem, M.; Farfel, Z. Multiple spurious laboratory results in a patient with hyperlipemic pancreatitis treated by plasmapheresis. Isr. J. Med. Sci. 1996, 32, 762–766. [Google Scholar] [PubMed]
- Melnick, S.; Nazir, S.; Gish, D.; Aryal, M.R. Hypertriglyceridemic pancreatitis associated with confounding laboratory abnormalities. J. Community Hosp. Intern. Med. Perpsect. 2016, 6, 31808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Attar, B.M.; Omar, A.Y.; Agrawal, R.; Demetria, M.V. Pseudohyponatremia in hypertriglyceridemia-induced acute pancreatitis: A tool for diagnosis rather than merely a laboratory error? Pancreas 2019, 48, 126–130. [Google Scholar] [CrossRef]
- Hansen, R.S.; Revsholm, J.; Motawea, M.; Folkestad, L. Pseudohyponatraemia caused by acute pancreatitis-derived hypertriglyceridaemia. BMJ Case Rep. 2021, 14, e241806. [Google Scholar] [CrossRef]
- Hinson, A.; Newbern, D.; Linardic, C.M. Asparaginase-induced hypertriglyceridemia presenting as pseudohyponatremia during leukemia treatment. Case Rep. Pediatr. 2014, 2014, 635740. [Google Scholar] [CrossRef] [Green Version]
- Kothari, J.; Thomas, A.; Goldstone, A. Pseudohyponatraemia due to L-asparaginase-associated dyslipidaemia in T-cell lymphoblastic lymphoma. BMJ Case Rep. 2014, 2014, bcr2013202829. [Google Scholar] [CrossRef] [Green Version]
- Morand, A.; Barlogis, V.; Rouby, F.; Reynaud, R.; Marrec, C.; Michel, G. Hypertriglyceridemia, discovered on a pseudohyponatremia, induced by l-asparaginase in the treatment of B acute lymphoblastic leukemia in child. Therapie 2016. online ahead of print. [Google Scholar]
- Zawitkowska, J.; Lejman, M.; Zaucha-Prażmo, A.; Sekula, N.; Greczkowska-Chmiel, T.; Drabko, K. Severe drug-induced hypertriglyceridemia treated with plasmapheresis in children with acute lymphoblastic leukemia. Transfus. Apher. Sci. 2019, 58, 634–637. [Google Scholar] [CrossRef]
- Bell, J.A.; Hilton, P.J.; Walker, G. Severe hyponatraemia in hyperlipaemic diabetic ketosis. Br. Med. J. 1972, 4, 709–710. [Google Scholar] [CrossRef] [Green Version]
- Dangerous pseudohyponatraemia. Lancet 1980, 2, 1121.
- Frier, B.M.; Steer, C.R.; Baird, J.D.; Bloomfield, S. Misleading plasma electrolytes in diabetic children with severe hypertriglyceridaemia. Arch. Dis. Child. 1980, 55, 771–775. [Google Scholar] [CrossRef] [Green Version]
- Goldman, M.H.; Kashani, M. Spurious hyponatremia in diabetic ketoacidosis with massive lipid elevations. J. Med. Soc. N. J. 1982, 79, 591–592. [Google Scholar]
- Kaminska, E.S.; Pourmotabbed, G. Spurious laboratory values in diabetic ketoacidosis and hypelipidemia. Am. J. Emerg. Med. 1993, 11, 77–80. [Google Scholar] [CrossRef]
- Twomey, P.J.; Cordle, J.; Pledger, D.R.; Miao, Y. An unusual case of hyponatraemia in diabetic ketoacidosis. J. Clin. Pathol. 2005, 58, 1219–1220. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, R.; Salih, M.; Elmokdad, C.; Sidhu, A. Diabetic ketoacidosis, very severe hypertriglyceridemia, and pseudohyponatremia successfully managed with insulin infusion. Cureus 2020, 12, e9306. [Google Scholar] [CrossRef]
- Lai, M.Y.; Lin, C.C.; Chung, S.L.; Wu, C.H.; Yang, W.C.; Tseng, Y.T. Milky plasma, diabetes, and severe hyponatremia. Kidney Int. 2009, 75, 996. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, A.P.; Hurst, A.C.E.; Garg, A. Extreme hypertriglyceridemia, pseudohyponatremia, and pseudoacidosis in a neonate with lipoprotein lipase deficiency due to segmental uniparental disomy. J. Clin. Lipidol. 2017, 11, 757–762. [Google Scholar] [CrossRef] [PubMed]
- le Riche, M.; Burgess, L.J.; Marais, A.D. Pseudohyponatraemia in a patient with obstructive jaundice. Clin. Chim. Acta. 2006, 366, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Ravella, S.; Lefavour, G.S.; Carayannopoulos, M.O.; Parikh, A. Hyponatremia in a patient with obstructive jaundice. Kidney Int. 2015, 58, 921–922. [Google Scholar] [CrossRef] [Green Version]
- Igbinedion, S.O.; Pandit, S.; Manuram, M.S.; Boktor, M. Pseudohyponatraemia secondary to hyperlipidaemia in obstructive jaundice. BMJ Case Rep. 2017, 2017, bcr2017221984. [Google Scholar] [CrossRef]
- Sivakumar, T.; Chaidarum, S.; Lee, H.K.; Cervinski, M.; Comi, R. Multiple lipoprotein and electrolyte laboratory artifacts caused by lipoprotein X in obstructive biliary cholestasis secondary to pancreatic cancer. J. Clin. Lipidol. 2011, 5, 324–328. [Google Scholar] [CrossRef]
- Adashek, M.L.; Clark, B.W.; Sperati, C.J.; Massey, C.J. The hyperlipidemia effect: Pseudohyponatremia in pancreatic cancer. Am. J. Med. 2017, 130, 1372–1375. [Google Scholar] [CrossRef] [Green Version]
- Hickman, P.E.; Dwyer, K.P.; Masarei, J.R. Pseudohyponatraemia, hypercholesterolaemia, and primary biliary cirrhosis. J. Clin. Pathol. 1989, 42, 167–171. [Google Scholar] [CrossRef]
- Ko, G.T.; Yeung, V.T.; Chow, C.C.; Mak, T.W.; Cockram, C.S. Pseudohyponatraemia secondary to hypercholesterolaemia. Ann. Clin. Biochem. 1997, 34, 324–325. [Google Scholar] [CrossRef] [Green Version]
- Hussain, I.; Ahmad, Z.; Garg, A. Extreme hypecholesterolemia presenting with pseudohyponatremia–A case report and review of the literature. J. Clin. Lipidol. 2015, 9, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Klinke, J.A.; Shapira, S.C.; Akbari, E.; Holmes, D.T. Quetiapine-associated cholestasis causing lipoprotein-X and pseudohyponatraemia. J. Clin. Pathol. 2010, 63, 741–743. [Google Scholar] [CrossRef]
- El Haage, L.; Reineks, E.; Nasr, C. Pseudohyponatremia in a setting of hypercholesterolemia. AACE Case Rep. 2018, 5, e172–e174. [Google Scholar] [CrossRef] [Green Version]
- Coakley, J.C.; Vervaart, P.P.; McKay, M.R. Factitious hyponatremia in a patient with cholestatic jaundice following bone marrow transplantation. Pathology 1986, 18, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Turchin, A.; Seifter, J.L.; Seely, E.W. Clinical problem-solving. Mind the gap. N. Engl. J. Med. 2003, 349, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, Y.; Teramoto, T.; Shirai, K.; Tsukamoto, H.; Sanda, T.; Miyamura, K.; Yamamori, I.; Hirabayashi, N.; Kodera, Y. Severe hypercholesterolemia associated with decreased hepatic triglyceride lipase activity and pseudohyponatremia in patients after allogeneic stem cell transplantation. Int. J. Hematol. 2005, 82, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Turchin, A.; Wiebe, D.A.; Seely, E.W.; Graham, T.; Longo, W.; Soiffer, R. Severe hypercholesterolemia mediated by lipoprotein X in patients with chronic graft-versus-host disease of the liver. Bone Marrow Transplant. 2005, 35, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Hanna, R.M.; Nguyen, M.K.; Kurtz, I.; Wilson, J. A novel case of pseudohyponatremia caused by hypercholesterolemia. Kidney Int. Rep. 2018, 4, 491–493. [Google Scholar] [CrossRef] [Green Version]
- Vo, H.; Gosmanov, A.R.; Garcia-Rosell, M.; Wall, B.M. Pseudohyponatremia in acute liver disease. Am. J. Med. Sci. 2013, 345, 62–64. [Google Scholar] [CrossRef]
- Girot, H.; Déhais, M.; Fraissinet, F.; Wils, J.; Brunel, V. Atypical pseudohyponatremia. Clin. Chem. 2018, 64, 414–415. [Google Scholar] [CrossRef]
- Yavasoglu, I.; Tombuloglu, M.; Kadikoylu, G.; Donmez, A.; Cagirgan, S.; Bolaman, Z. Cholesterol levels in patients with multiple myeloma. Am. J. Hematol. 2008, 87, 223–228. [Google Scholar] [CrossRef]
- Burnside, N.J.; Alberta, L.; Robinson-Bostom, L.; Bostom, A. Type III hyperlipoproteinemia with xanthomas and multiple myeloma. J. Am. Acad. Dermatol. 2005, 53 (Suppl. S1), S281–S284. [Google Scholar] [CrossRef]
- Nanji, A.A. Multiple myeloma and syndrome of inappropriate secretion of antidiuretic hormone. South. Med. J. 1983, 76, 270. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Shafi, F.; Iqbal, M.; Kollipara, R.; Rouf, E. Syndrome of inappropriate antidiuretic hormone due to multiple myeloma. Mo. Med. 2011, 108, 377–379. [Google Scholar]
- Nakayama-Ichiyama, S.; Yokote, T.; Iwaki, K.; Takubo, T.; Tsuji, M.; Hanafusa, T. Syndrome of inappropriate antidiuretic hormone secretion associated with plasma cell myeloma. Br. J. Haematol. 2011, 152, 125. [Google Scholar] [CrossRef] [PubMed]
- Bloth, B.; Christensson, T.; Mellstedt, H. Extreme hyponatremia in patients with myelomatosis: An effect of cationic paraproteins. Acta Med. Scand. 1978, 203, 273–275. [Google Scholar] [CrossRef]
- Decaux, O.; Laurat, E.; Perlat, A.; Cazalets, C.; Jego, P.; Grosbois, B. Systemic manifestations of monoclonal gammopathy. Eur. J. Intern. Med. 2009, 20, 457–461. [Google Scholar] [CrossRef]
- Liamis, G.; Milionis, H.; Elisaf, M. A review of drug-induced hyponatremia. Am. J. Kidney Dis. 2008, 52, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Silbernagl, S. The role of brush border enzymes in tubular reabsorption of disaccharides: A microperfusion study in rat kidney. Pflugers Arch. 1977, 371, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Palevsky, P.M.; Rendulic, D.; Diven, W.F. Maltose-induced hyponatremia. Ann. Intern. Med. 1993, 118, 526–528. [Google Scholar] [CrossRef]
- Daphnis, E.; Stylianou, K.; Alexandrakis, M.; Xylouri, I.; Vardaki, E.; Stratigis, S.; Kyriazis, J. Acute renal failure, translocational hyponatremia and hyperkalemia following intravenous immunoglobulin therapy. Nephron. Clin. Pract. 2007, 106, c143–c148. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Rastogi, A.; Kurtz, I. True hyponatremia secondary to intravenous immunoglobulin. Clin. Exp. Nephrol. 2006, 10, 124–126. [Google Scholar] [CrossRef]
- Hoogerbrugge, N.; Jansen, H.; Hoogerbrugge, P.M. Transient hyperlipidemia induced by L-asparaginase therapy of ALL with L-asparaginase is related to decreased lipoprotein lipase activity. Leukemia 1997, 11, 1377–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremer, P.; Lakomek, M.; Beck, W.; Prindull, G. The effect of L-asparaginase on lipid metabolism during induction chemotherapy of childhood lymphoblastic leukaemia. Eur. J. Pediatr. 1988, 147, 64–67. [Google Scholar] [CrossRef]
- Tzamaloukas, A.H.; Khitan, Z.J.; Glew, R.H.; Roumelioti, M.E.; Rondon-Berrios, H.; Elisaf, M.S.; Raj, D.S.; Owen, J.; Sun, Y.; Siamopoulos, K.C.; Rohrscheib, M.; et al. Serum sodium concentration and tonicity in hyperglycemic crises: Major influences and treatment implications. J. Am. Heart Assoc. 2019, 8, e011786. [Google Scholar] [CrossRef] [PubMed]
- Al-Musheifri, A.; Jones, G.R.D. Glucose interference in direct ion-sensitive electrode sodium measurements. Ann. Clin. Biochem. 2008, 45, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Goyal, B.; Data, K.S.; Mir, A.A.; Ikkurthi, S.; Prasad, R.; Pal, A. Increasing glucose concentrations interfere with estimates of electrolytes by indirect ion selective methods. Indian. J. Clin. Biochem. 2016, 31, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Viljoen, A.; Wierzbicki, A.S. Diagnosis and treatment of severe hypertriglyceridemia. Expert. Rev. Cardiovasc. Ther. 2012, 10, 505–514. [Google Scholar] [CrossRef]
- Rahalkar, A.R.; Giffen, F.; Har, B.; Ho, J.; Morrison, K.M.; Hill, J.; Wang, J.; Hegele, R.A.; Joy, T. Novel LP mutations associated with lipoprotein lipase deficiency: Two case reports and a literature review. Can. J. Physiol. Pharmacol. 2009, 87, 151–160. [Google Scholar] [CrossRef]
- Chen, T.Z.; Xie, S.L.; Jin, R.; Huang, Z.M. A novel lipoprotein lipase gene missense mutation in Chinese patients with severe hypetriglyceridemia and pancreatitis. Lipids Health Dis. 2014, 13, 52. [Google Scholar] [CrossRef] [Green Version]
- Buonuomo, P.S.; Bartuli, A.; Rabacchi, C.; Bertolini, S.; Calandra, S. A 3-day-old neonate with severe hypertriglyceridemia from novel mutations of the GPIHBP1 gene. J. Clin. Lipidol. 2015, 9, 265–270. [Google Scholar] [CrossRef]
- Kassner, U.; Salewsky, B.; Wühle-Demuth, M.; Szijarto, I.A.; Grenkowitz, T.; Binner, P.; März, W.; Steinhagen-Thiessen, E.; Demuth, I. Severe hypertriglyceridemia in a patient heterozygous for lipoprotein lipase gene allele with two novel missense variants. Eur. J. Hum. Genet. 2015, 23, 1259–1261. [Google Scholar] [CrossRef] [Green Version]
- Soto, A.G.; McIntyre, A.; Agrawal, S.; Bialo, S.R.; Hegele, R.A.; Boney, C.M. Severe hypertriglyceridemia due to a novel p.Q240H mutation in the lipoprotein lipase gene. Lipids Health Dis. 2015, 14, 102. [Google Scholar] [CrossRef] [Green Version]
- Lun, Y.; Sun, X.; Wang, P.; Chi, J.; Hou, X.; Wang, Y. Severe hypertriglyceridemia due to two novel loss-of-function lipoprotein lipase gene mutations (C310R/E396V) in a Chinese family associated with recurrent acute pancreatitis. Oncotarget 2017, 8, 47741–47754. [Google Scholar] [CrossRef]
- Dron, J.S.; Wang, J.; McIntyre, A.D.; Iacocca, M.A.; Robinson, J.F.; Ban, M.R.; Cao, H.; Hegele, R.A. Six years’ experience with LipSeqq: Clinical and research learnings from a hybrid, targeted sequencing panel for dyslipidemias. BMC Med. Genom. 2020, 13, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidel, D.; Alaupovic, P.; Furman, R.H. A lipoprotein characterizing obstructive jaundice. I. Method for quantitative separation and identification of lipoproteins in jaundiced patients. J. Clin. Investig. 1969, 48, 1211–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sörös, P.; Böttcher, J.; Maschek, H.; Selberg, O.; Müller, M.J. Lipoprotein-X in patients with cirrhosis: Its relationship to cholestasis and hypercholesterolemia. Hepatology 1998, 28, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Guarda, L.A.; Allen, L.G. Liver injury associated with quetiapine: An illustrative case report. J. Clin. Psychopharmacol. 2017, 37, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Thies, P.W.; Dull, W.L. Trimethoprim-sulfamethoxazole-induced cholestatic hepatitis. Inadvertent rechallenge. Arch. Intern. Med. 1984, 144, 1691–1692. [Google Scholar] [CrossRef]
- Renkes, P.; Trechot, P.; Blain, H. Valacyclovir-induced hepatitis. Acta Clin. Belg. 1999, 54, 17–18. [Google Scholar] [CrossRef]
- Mitchell, E.; Gilbert, M.; Loomes, K.M. Alagille syndrome. Clin. Liver Dis. 2018, 22, 625–641. [Google Scholar] [CrossRef]
- Moreno, G.; Gunsolus, I.L. Reverse pseudohyperkalemia and pseudohyponatremia in a patient with C-cell non-Hodgkin lyphoma. Clin. Biochem. 2020, 78, 63–65. [Google Scholar] [CrossRef]
- Ghersin, Z.; Fernandes, N.D.; Winkler, A.; Yager, P. Pseudohyperkalemia and pseudohyponatremia in two children with T-cell acute lymphoblastic leukemia. J. Pediatr. 2021, 232, 294–298. [Google Scholar] [CrossRef]
- Oh, M.S. Pathogenesis and diagnosis of hyponatremia. Nephron 2002, 92 (Suppl. S1), 2–8. [Google Scholar] [CrossRef]
- Kilpatrick, E.S.; Burton, I.D. Pseudohyperkalemia, pseudohyponatraemia and pseudohypoglycaemia in a patient with hereditary stomatocytosis. Ann. Clin. Biochem. 1997, 34, 561–563. [Google Scholar] [CrossRef] [Green Version]
- Weld, B.A.; Morgan, T.J.; Presneill, J.J.; Weier, S.; Cowley, D. Plasma sodium measurements by direct ion selective methods in laboratory and point of care may not be clinically interchangeable. J. Clin. Monit. Comput. 2017, 31, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Goldwasser, P.; Roche-Recinos, A.; Barth, R.H. Graded interference with the direct potentiometric measurement of sodium by hemoglobin. Clin. Biochem. 2017, 50, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Boeyckens, A.; Schots, J.; Vandenplas, H.; Senesael, F.; Goedhuys, W.; Gorus, F.K. Ektachem slides for direct potentiometric determination of sodium in plasma: Effect of natremia, blood pH, and type of electrolyte reference fluid on concordance with flame photometry and other potentiometric methods. Clin. Chem. 1992, 38, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Liamis, G.; Filippatos, T.D.; Liontos, A.; Elisaf, M.S. Hyponatremia in patients with liver diseases: Not just a cirrhosis-induced hemodynamic compromise. Hepatol. Int. 2016, 10, 762–772. [Google Scholar] [CrossRef]
- Koumpis, E.; Florentin, M.; Hatzimichael, E.; Liamis, G. Hyponatremia in patients with hematologic diseases. J. Clin. Med. 2020, 9, 3721. [Google Scholar] [CrossRef]
- Chow, E.; Fox, N.; Gama, R. Effect of low serum protein on sodium and potassium measurement by ion-selective electrodes in critically ill patients. Br. J. Biomed. Sci. 2008, 65, 128–131. [Google Scholar] [CrossRef]
- Lava, S.A.G.; Bianchetti, M.G.; Milani, G.P. Testing of Na+ in blood. Clin. Kidney J. 2017, 10, 147–148. [Google Scholar] [CrossRef] [Green Version]
- Katrangi, W.; Baumann, N.A.; Nett, R.C.; Karon, B.S.; Block, D.R. Prevelance of clinically significant differences in sodium measurements due to abnormal protein concentrations using an indirect ion-selective electrode method. J. Appl. Lab. Med. 2019, 4, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Liamis, G.L.; Milionis, H.J.; Rizos, E.; Siamopoulos, K.C.; Elisaf, M.S. Mechanisms of hyponatraemia in alcohol patients. Alcohol Alcohol. 2000, 35, 612–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langelaan, M.L.P.; Kamp, L.; Zardijk, E.; Raijmakers, M.T.M. Prevalence of pseudonatremia in a clinical laboratory-role of the water content. Clin. Chem. Lab. Med. 2017, 55, 546–553. [Google Scholar] [CrossRef]
- Dawson, A.; Kanukuntla, A.; Kata, P.; Ali, R.; Cheriyath, P. Pseudohyponatremia leading to a fatal outcome in a patient with familial hypertriglyceridemia. Cureus 2021, 13, e17066. [Google Scholar] [CrossRef]
- Adrogué, H.J.; Madias, N.E. Hyponatremia. N. Engl. J. Med. 2000, 342, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Rohrscheib, M.; Sam, R.; Raj, D.S.; Argyropoulos, C.P.; Unruh, M.L.; Lew, S.Q.; Ing, T.S.; Levin, N.W.; Tzamaloukas, A.H. Edelman revisited: Concepts, achievements, and challenges. Front. Med. 2022, 8, 808865. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, C.; Rondon-Berrios, H.; Raj, D.S.; Malhotra, D.; Agaba, E.I.; Rohrscheib, M.; Khitan, Z.; Murata, G.H.; Shapiro, J.I.; Tzamaloukas, A.H. Hypertonicity: Pathophysiologic concept and experimental studies. Cureus 2016, 8, e596. [Google Scholar] [CrossRef] [Green Version]
- Kramer, U.; Kress, M.; Reinauer, H.; Spannagl, M.; Kaiser, P. Candidate reference measurement procedures for chloride, potassium, sodium, calcium, magnesium, and lithium by inductively coupled plasma (isotope dilution) sector field mass spectometry (ICP-(ID) SFMS) in serum. Clin. Lab. 2013, 59, 1017–1029. [Google Scholar] [CrossRef]
- Fan, X.; Li, Q.; Jin, Z.; Yu, X.; Ding, M.; Ju, Y. Establishment and application of a new serum sodium candidate reference method. Clin. Chim. Acta. 2020, 508, 249–253. [Google Scholar] [CrossRef]
- Quiles, R.; Fernández-Romero, J.M.; Fernández, E.; Luque de Castro, M.D.; Varcárcel, M. Automated enzymatic determination of sodium in serum. Clin. Chem. 1993, 39, 500–503. [Google Scholar] [CrossRef]
- Hill, C.E.; Burd, E.M.; Kraft, C.S.; Ryan, E.L.; Duncan, A.; Winkler, A.M.; Cardella, J.C.; Ritchie, J.C.; Parslow, T.G. Laboratory test support for Ebola patients within a high-containment facility. Lab. Med. 2014, 45, e109–e111. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A. POEMS syndrome: 2019 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2019, 94, 812–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.Y.; Nabel, C.S.; Finkelman, B.S.; Ruth, J.; Kurzrock, R.; van Rhee, F.; Krymskaya, V.P.; Kelleher, D.; Rubenstein, A.H.; Fajgenbaum, D.C. Idiopathic multicentric Castleman's disease: A systematic literature review. Lancet Haematol. 2016, 3, e163–e175. [Google Scholar] [CrossRef]
- Stirneman, J.; Belmatoug, N.; Camou, F.; Serratrice, C.; Froissart, R.; Caillaud, C.; Levade, T.; Astudillo, L.; Serratrice, J.; Brassier, A.; et al. A review of Gaucher disease pathophysiology, clinical presentation and treatments. Int. J. Mol. Sci. 2017, 18, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klop, B.; do Rego, A.T.; Cabezas, M.C. Alcohol and plasma triglycerides. Curr. Opin. Lipidol. 2013, 24, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Soued, M.; Serio, M.K.; Moser, A.H.; Dinarello, C.A.; Grunfeld, C. Multiple cytokines stimulate hepatic lipid synthesis in vivo. Endocrinology 1989, 125, 267–274. [Google Scholar] [CrossRef]
- Graessle, D.; Bonacini, M.; Chen, S. Alpha-inteferon and reversible hypertriglyceridemia. Ann. Intern. Med. 1993, 118, 316–317. [Google Scholar] [CrossRef]
- Harris, L.V.D.; Albrink, M.J.; Van Eck, W.F.; Man, E.B.; Peters, J.P. Serum lipids in diabetic acidosis. Metabolism 1953, 2, 120–132. [Google Scholar]
- Hamwi, G.J.; Garcia, O.; Kruger, F.A.; Gwinup, G.; Cornwell, D.G. (1962) Hyperlipidemia in uncontrolled diabetes. Metabolism 1962, 11, 850–862. [Google Scholar]
- Sterky, G.; Larsson, Y.; Persson, B. Blood lipids in diabetic and non-diabetic schoolchildren. Acta Paediatr. 1963, 52, 11–21. [Google Scholar] [CrossRef]
- Bagdade, J.D.; Porte, D., Jr.; Bierman, E.L. Diabetic lipidemia. A form of acquired fat-induced lipemia. N. Engl. J. Med. 1967, 276, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Chance, G.W.; Albutt, E.C.; Edkins, S.M. Serum lipids and lipoproteins in untreated diabetic children. Lancet 1969, 1, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.E.; Schreibman, P.H.; Day, V.C.; Arky, R.A. Hyperlipidemia in an adult diabetic population. J. Chron. Dis. 1970, 23, 501–506. [Google Scholar] [CrossRef]
- Hayes, T.M. Plasma lipoproteins in adult diabetes. Clin. Endocrinol. 1972, 1, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Billimoria, J.D.; Isaacs, A.J.; Melki, K. A lipid and liporotein profile of treated and untreated diabetics. Ann. Clin. Biochem. 1976, 13, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Chace, H.P.; Glasgow, A.M. Juvenile diabetes mellitus and serum lipids and lipoprotein levels. Am. J. Dis. Child. 1976, 130, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Chait, A. Hypertriglyceridemia secondary to obesity and diabetes. Biochim. Biophys. Acta. 2012, 1821, 819–825. [Google Scholar] [CrossRef]
- Conley, B.A.; Egorin, M.J.; Sridhara, R.; Finley, R.; Hemady, R.; Wu, S.; Tait, N.S.; Van Echo, D.A. Phase I clinical trial of all-trans-retinoic acid with correlation of its pharmacokinetics and pharmacodynamics. Cancer Chemother. Pharmacol. 1997, 39, 291–299. [Google Scholar] [CrossRef]
- Joukhadar, R.; Chiu, K. Severe hypercholesterolemia in patients with graft-vs-host disease affecting the liver after stem cell transplantation. Endocr. Pract. 2012, 18, 90–97. [Google Scholar] [CrossRef]
- Chudy-Onwugaje, K.; Anyadike, N.; Tsirlin, Y.; Mayer, I.; Rahmani, R. Severe hypercholesterolemia: A unique presentation of non-Hodgkin’s lymphoma in a patient with neurofibromatosis type Case Rep. Gastrointest. Med. 2014, 2014, 579352. [Google Scholar] [CrossRef]
- Vaziri, N.D. Disorders of lipid metabolism in nephrotic syndrome: Mechanisms and consequences. Kidney Int. 2016, 90, 41–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joven, J.; Villabona, C.; Vilella, E.; Masana, L.; Albertí, R.; Vallés, M. Abnormalities of lipoprotein metabolism in patients with the nephrotic syndrome. N. Engl. J. Med. 1990, 323, 579–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, D.C.; Bernard, D.B. Lipid abnormalities in the nephrotic syndrome: Causes, consequences, and treatment. Am. J. Kidney Dis. 1994, 23, 331–346. [Google Scholar] [CrossRef]
- Sar, F.; Taylan, I.; Kutlu, C.; Cayma, M.S.; Tatli, E.; Kazancioglu, R. Amyloidosis in a patient with autosomal dominant polycystic kidney disease and tuberculosis: A case report. Int. Urol. Nephrol. 2007, 39, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.B.; Allen, C.E.; Weitzman, S.; Filipovich, A.H.; McLain, K.L. How I treat hemophagocytic lymphohistiocytosis. Blood 2011, 118, 4041–4052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, Q.; Zheng, W.; Ma, J.; Zhang, W.; Wang, W.; Tian, X. Hemophagocytic lymphohistiocytosis: Clinical analysis of 103 adult patients. Medicine 2014, 93, 100–105. [Google Scholar] [CrossRef]
- Hojsak, I.; Kolaček, S. Fat overload syndrome after the rapid infusion of SMOFlipid emulsion. JPEN J. Parent. Enteral Nutr. 2014, 38, 119–121. [Google Scholar] [CrossRef]
- Waitzberg, D.L.; Torrinhas, R.S. The complexity of prescribing intravenous lipid emulsions. World Rev. Nutr. Diet. 2015, 112, 150–162. [Google Scholar]
- Li, X.X.; Cheng, Y.C.; Zhai, S.D.; Yao, P.; Zhan, S.Y.; Shi, L.W. Risk of liver injury associated with intravenous lipid emulsions: A prescription sequence symmetry analysis. Front. Pharmacol. 2021, 12, 589091. [Google Scholar] [CrossRef]
- Villa López, G.; Valero Zanuy, M.A.; González Barrios, I.; Maíz Jiménez, M.; Gomis Muñóz, P.; León Sanz, M. Acute hypertriglyceridemia in patients with COVID-19 receiving parenteral nutrition. Nutrients 2021, 13, 2287. [Google Scholar] [CrossRef]
- Koch, C.D.; Vera, M.A.; Messina, J.; Price, N.; Durant, T.J.S.; El-Khoury, J.M. Preventing pseudohyponatremia: IntralipidR-based lipemia cutoffs for sodium are inappropriate. Clin. Chim. Acta. 2021, 520, 63–66. [Google Scholar] [CrossRef] [PubMed]
| Serum Solids Content | Serum Water Content | [Na]S (mmol/L) |
|---|---|---|
| 0.07 | 0.93 | 0.93 × 151 = 140.4 |
| 0.14 | 0.86 | 0.86 × 151 = 129.9 |
| 0.21 | 0.79 | 0.79 × 151 = 119.3 |
| Component | SSC = 0.07 SWC = 0.93 | SSC = 0.14 SWC = 0.86 | SSC = 0.21 SWC = 0.79 |
|---|---|---|---|
| Diluent, L | 0.3 | 0.3 | 0.3 |
| Serum, L | 0.01 | 0.01 | 0.01 |
| Serum sample water, L | 0.0093 | 0.0086 | 0.0079 |
| Total sample water, L | 0.3093 (0.3 + 0.0093) | 0.3086 (0.3 + 0.0086) | 0.3079 (0.3 + 0.0079) |
| Dilution factor serum water (38) | 33.2581 (0.3093/0.0093) | 35.8837 (0.3086/0.0086) | 38.9747 (0.3079/0.0079) |
| Sodium content of sample, mmoL | 1.4043 (0.0093 × 151) | 1.2986 (0.0086 × 151) | 1.1929 (0.0079 × 151) |
| [Na]DSW, mmol/L | 4.540 (1.4043/0.3093) | 4.2080 (1.2986/0.3086) | 3.8743 (1.1929/0.3079) |
| [Na]SW 1, mmol/L | 151.0 (4.540 × 33.2581) | 140.0 (4.2080 × 33.2581) | 128.9 (3.8743 × 33.2581) |
| [Na]S 1, mmol/L | 140.4 (151 × 0.93) | 130.2 (140 × 0.93) | 119.8 (128.9 × 0.93) |
| High Serum Solids Component | Clinical Condition | References |
|---|---|---|
| Hyperproteinemia | Multiple myeloma | [39,62,63,64,65,66,67,68,69] |
| Monoclonal gammopathies | [70] | |
| Waldenström’s macroglobulinemia | [71] | |
| HIV disease (hypergammaglobulinemia) | [72,73] | |
| Immunoglobulin infusion | [40,74,75,76] | |
| Hypertriglyceridemia | Pancreatitis | [18,77,78,79,80] |
| Acute or chronic alcoholism | [64] | |
| Asparaginase treatment | [81,82,83,84] | |
| Diabetic ketoacidosis | [85,86,87,88,89,90,91] | |
| Type 2 diabetes poorly controlled | [92] | |
| Genetic defects (lipoprotein lipase) | [93] | |
| Lipoproteinemia, types I and V | [31] | |
| Hypercholesterolemia | Obstructive/cholestatic jaundice | [94,95,96] |
| Pancreatic cancer with biliary obstruction | [97,98] | |
| Primary biliary cirrhosis | [41,99,100,101] | |
| Drug-induced cholestatic hepatitis | [102,103] | |
| Graft-versus-host liver disease | [104,105,106,107,108] | |
| Hepatitis | [109] | |
| Genetic defects (Alagille syndrome) | [110] |
| High Serum Solids Component | Clinical Condition |
|---|---|
| Hypergammaglobulinemia | Cirrhosis, Autoimmune hepatitis |
| Alcoholic liver disease, Hepatitis C | |
| Interferon infusion | |
| POEMS syndrome | |
| Castleman’s disease | |
| Post-transplant monoclonal gammopathies | |
| Chronic lymphocytic leukemia | |
| Cryoglobulinemia, Cold agglutinin disease | |
| Gaucher’s disease | |
| Hypertriglyceridemia | Alcoholism |
| Interferon infusion | |
| Diabetes mellitus, Obesity | |
| All-trans-retinoic acid (ATRA) | |
| Hypercholesterolemia | Diabetes mellitus |
| Stem cell transplantation | |
| Non-Hodgkin’s lymphoma | |
| Mixed hyperlipidemia | Diabetes mellitus |
| Nephrotic syndrome from various causes | |
| Hemophagocytic lymphohistiocytosis | |
| Intravenous lipid emulsions | |
| Parenteral nutrition in COVID-19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, F.; Sam, R.; Lew, S.Q.; Massie, L.; Misra, M.; Roumelioti, M.-E.; Argyropoulos, C.P.; Ing, T.S.; Tzamaloukas, A.H. Pseudohyponatremia: Mechanism, Diagnosis, Clinical Associations and Management. J. Clin. Med. 2023, 12, 4076. https://doi.org/10.3390/jcm12124076
Aziz F, Sam R, Lew SQ, Massie L, Misra M, Roumelioti M-E, Argyropoulos CP, Ing TS, Tzamaloukas AH. Pseudohyponatremia: Mechanism, Diagnosis, Clinical Associations and Management. Journal of Clinical Medicine. 2023; 12(12):4076. https://doi.org/10.3390/jcm12124076
Chicago/Turabian StyleAziz, Fahad, Ramin Sam, Susie Q. Lew, Larry Massie, Madhukar Misra, Maria-Eleni Roumelioti, Christos P. Argyropoulos, Todd S. Ing, and Antonios H. Tzamaloukas. 2023. "Pseudohyponatremia: Mechanism, Diagnosis, Clinical Associations and Management" Journal of Clinical Medicine 12, no. 12: 4076. https://doi.org/10.3390/jcm12124076
APA StyleAziz, F., Sam, R., Lew, S. Q., Massie, L., Misra, M., Roumelioti, M.-E., Argyropoulos, C. P., Ing, T. S., & Tzamaloukas, A. H. (2023). Pseudohyponatremia: Mechanism, Diagnosis, Clinical Associations and Management. Journal of Clinical Medicine, 12(12), 4076. https://doi.org/10.3390/jcm12124076





