Current Smoking Determines the Levels of Circulating MPO and MMP-9 in Adults with Coronary Artery Disease and Obstructive Sleep Apnea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Home Sleep Apnea Test
2.3. Epworth Sleepiness Scale
2.4. Comorbidities and Medications
2.5. MPO and MMP-9 Levels
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K.; et al. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, M.; Shi, J. Influence of Obstructive Sleep Apnoea on Coronary Artery Disease in a Chinese Population. J. Int. Med. Res. 2022, 50, 03000605221115389. [Google Scholar] [CrossRef] [PubMed]
- Gać, P.; Urbanik, D.; Macek, P.; Martynowicz, H.; Mazur, G.; Poręba, R. Coexistence of Cardiovascular Risk Factors and Obstructive Sleep Apnoea in Polysomnography. Respir. Physiol. Neurobiol. 2022, 295, 103782. [Google Scholar] [CrossRef] [PubMed]
- Urbanik, D.; Gać, P.; Martynowicz, H.; Podgórski, M.; Poręba, M.; Mazur, G.; Poręba, R. Obstructive Sleep Apnea as a Predictor of Arrhythmias in 24-h ECG Holter Monitoring. Brain Sci. 2021, 11, 486. [Google Scholar] [CrossRef]
- Urbanik, D.; Gać, P.; Martynowicz, H.; Poręba, M.; Podgórski, M.; Negrusz-Kawecka, M.; Mazur, G.; Sobieszczańska, M.; Poręba, R. Obstructive Sleep Apnea as a Predictor of Reduced Heart Rate Variability. Sleep Med. 2019, 54, 8–15. [Google Scholar] [CrossRef]
- Macek, P.; Poręba, M.; Stachurska, A.; Martynowicz, H.; Mazur, G.; Gać, P.; Poręba, R. Obstructive Sleep Apnea and Sleep Structure Assessed in Polysomnography and Right Ventricular Strain Parameters. Brain Sci. 2022, 12, 331. [Google Scholar] [CrossRef]
- Hernando del Portillo, J.; Miguel, H.B.; Angélica, B.M.; Dario, E.; Jaime, C. High Frequency of Coronary Artery Ectasia in Obstructive Sleep Apnea. J. Clin. Sleep Med. 2023, 18, 433–438. [Google Scholar] [CrossRef]
- Peker, Y.; Hedner, J.; Kraiczi, H.; Löth, S. Respiratory disturbance index: An independent predictor of mortality in coronary artery disease. Am. J. Respir. Crit. Care Med. 2000, 162, 81–86. [Google Scholar] [CrossRef]
- Libby, P.; Theroux, P. Pathophysiology of Coronary Artery Disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [Green Version]
- Van der Veen, B.S.; de Winther, M.P.J.; Heeringa, P. Myeloperoxidase: Molecular Mechanisms of Action and Their Relevance to Human Health and Disease. Antioxid. Redox Signal. 2009, 11, 2899–2937. [Google Scholar] [CrossRef]
- Karakas, M.; Koenig, W.; Zierer, A.; Herder, C.; Rottbauer, W.; Baumert, J.; Meisinger, C.; Thorand, B. Myeloperoxidase Is Associated with Incident Coronary Heart Disease Independently of Traditional Risk Factors: Results from the MONICA/KORA Augsburg Study. J. Intern. Med. 2012, 271, 43–50. [Google Scholar] [CrossRef]
- Zhang, R.; Brennan, M.L.; Fu, X.; Aviles, R.J.; Pearce, G.L.; Penn, M.S.; Topol, E.J.; Sprecher, D.L.; Hazen, S.L. Association between Myeloperoxidase Levels and Risk of Coronary Artery Disease. JAMA 2001, 286, 2136–2142. [Google Scholar] [CrossRef] [Green Version]
- Kolodziej, A.R.; Abo-Aly, M.; Elsawalhy, E.; Campbell, C.; Ziada, K.M.; Abdel-Latif, A. Prognostic Role of Elevated Myeloperoxidase in Patients with Acute Coronary Syndrome: A Systemic Review and Meta-Analysis. Mediat. Inflamm. 2019, 2019, 2872607. [Google Scholar] [CrossRef]
- Meuwese, M.C.; Stroes, E.S.G.; Hazen, S.L.; van Miert, J.N.; Kuivenhoven, J.A.; Schaub, R.G.; Wareham, N.J.; Luben, R.; Kastelein, J.J.P.; Khaw, K.-T.; et al. Serum Myeloperoxidase Levels Are Associated with the Future Risk of Coronary Artery Disease in Apparently Healthy Individuals: The EPIC-Norfolk Prospective Population Study. J. Am. Coll Cardiol. 2007, 50, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases: Structure, Function, and Biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [Green Version]
- Szmitko, P.E.; Wang, C.-H.; Weisel, R.D.; Jeffries, G.A.; Anderson, T.J.; Verma, S. Biomarkers of Vascular Disease Linking Inflammation to Endothelial Activation: Part II. Circulation 2003, 108, 2041–2048. [Google Scholar] [CrossRef]
- Welsh, P.; Whincup, P.H.; Papacosta, O.; Wannamethee, S.G.; Lennon, L.; Thomson, A.; Rumley, A.; Lowe, G.D.O. Serum Matrix Metalloproteinase-9 and Coronary Heart Disease: A Prospective Study in Middle-Aged Men. QJM 2008, 101, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Jefferis, B.J.; Whincup, P.; Welsh, P.; Wannamethee, G.; Rumley, A.; Lennon, L.; Thomson, A.; Lawlor, D.; Carson, C.; Ebrahim, S.; et al. Prospective Study of Matrix Metalloproteinase-9 and Risk of Myocardial Infarction and Stroke in Older Men and Women. Atherosclerosis 2010, 208, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Hlatky, M.A.; Ashley, E.; Quertermous, T.; Boothroyd, D.B.; Ridker, P.; Southwick, A.; Myers, R.M.; Iribarren, C.; Fortmann, S.P.; Go, A.S. Matrix Metalloproteinase Circulating Levels, Genetic Polymorphisms, and Susceptibility to Acute Myocardial Infarction among Patients with Coronary Artery Disease. Am. Heart J. 2007, 154, 1043–1051. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Zhao, Q.; Qu, H.-J.; Li, X.-M.; Chen, Q.-J.; Liu, F.; Chen, B.-D.; Yang, Y.-N. Usefulness of Plasma Matrix Metalloproteinase-9 Levels in Prediction of in-Hospital Mortality in Patients Who Received Emergent Percutaneous Coronary Artery Intervention Following Myocardial Infarction. Oncotarget 2017, 8, 105809–105818. [Google Scholar] [CrossRef]
- Akpinar, M.E.; Yigit, O.; Altundag, A.; Demirel, G.Y.; Kocak, I. Salivary and Serum Myeloperoxidase in Obstructive Sleep Apnea. J. Otolaryngol. Head Neck Surg. 2012, 41, 215–221. [Google Scholar] [PubMed]
- Hanikoglu, F.; Huseyinoglu, N.; Ozben, S.; Cort, A.; Ozdem, S.; Ozben, T. Increased Plasma Soluble Tumor Necrosis Factor Receptor-1 and Myeloperoxidase Activity in Patients with Obstructive Sleep Apnea Syndrome. Int. J. Neurosci. 2015, 125, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Arısoy, A.; Ekin, S.; Sertogullarindan, B.; Gunbatar, H.; Sunnetcioglu, A.; Aksoy, N.; Sezen, H.; Asker, S.; Turan, M.; Yildiz, H. The Relationship Among Oxidative and Anti-Oxidative Parameters and Myeloperoxidase in Subjects With Obstructive Sleep Apnea Syndrome. Respir. Care 2016, 61, 200–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tazaki, T.; Minoguchi, K.; Yokoe, T.; Samson, K.T.R.; Minoguchi, H.; Tanaka, A.; Watanabe, Y.; Adachi, M. Increased Levels and Activity of Matrix Metalloproteinase-9 in Obstructive Sleep Apnea Syndrome. Am. J. Respir. Crit. Care Med. 2004, 170, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Kaditis, A.G.; Alexopoulos, E.I.; Karathanasi, A.; Ntamagka, G.; Oikonomidi, S.; Kiropoulos, T.S.; Zintzaras, E.; Gourgoulianis, K. Adiposity and Low-Grade Systemic Inflammation Modulate Matrix Metalloproteinase-9 Levels in Greek Children with Sleep Apnea. Pediatr. Pulmonol. 2010, 45, 693–699. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Liu, H.; Li, Y.; Liu, X.; Zhu, J. Increased Serum Levels of C-Reactive Protein and Matrix Metalloproteinase-9 in Obstructive Sleep Apnea Syndrome. Chin. Med. J. 2007, 120, 1482–1486. [Google Scholar] [CrossRef]
- Chuang, L.-P.; Chen, N.-H.; Lin, S.-W.; Chang, Y.-L.; Chao, I.-J.; Pang, J.-H.S. Increased Matrix Metalloproteinases-9 after Sleep in Plasma and in Monocytes of Obstructive Sleep Apnea Patients. Life Sci. 2013, 93, 220–225. [Google Scholar] [CrossRef]
- Fang, X.; Chen, J.; Wang, W.; Feng, G.; Li, X.; Zhang, X.; Zhang, Y.; Zhang, J.; Xu, Z.; Tai, J.; et al. Matrix Metalloproteinase 9 (MMP9) Level and MMP9 -1562C>T in Patients with Obstructive Sleep Apnea: A Systematic Review and Meta-Analysis of Case-Control Studies. Sleep Med. 2020, 67, 110–119. [Google Scholar] [CrossRef]
- Yüksel, M.; Kuzu-Okur, H.; Velioğlu-Öğünç, A.; Pelin, Z. Matrix Metalloproteinase-9 Level and Gene Polymorphism in Sleep Disordered Breathing Patients with or without Cardiovascular Disorders. Balk. Med. J. 2013, 30, 8–12. [Google Scholar] [CrossRef]
- Bauça, J.M.; Barcelo, A.; Fueyo, L.; Sanchís, P.; Pierola, J.; de la Peña, M.; Arqué, M.; Gómez, C.; Morell-Garcia, D.; Sánchez-de-la-Torre, A.; et al. Biomarker Panel in Sleep Apnea Patients after an Acute Coronary Event. Clin. Biochem. 2019, 68, 24–29. [Google Scholar] [CrossRef]
- Peker, Y.; Glantz, H.; Thunström, E.; Kallryd, A.; Herlitz, J.; Ejdebäck, J. Rationale and Design of the Randomized Intervention with CPAP in Coronary Artery Disease and Sleep Apnoea—RICCADSA Trial. Scand. Cardiovasc. J. 2009, 43, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Glantz, H.; Eulenburg, C.; Wegscheider, K.; Herlitz, J.; Thunström, E. Effect of Positive Airway Pressure on Cardiovascular Outcomes in Coronary Artery Disease Patients with Nonsleepy Obstructive Sleep Apnea. The RICCADSA Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2016, 194, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Thunström, E.; Glantz, H.; Eulenburg, C. Effect of Obstructive Sleep Apnea and CPAP Treatment on Cardiovascular Outcomes in Acute Coronary Syndrome in the RICCADSA Trial. J. Clin. Med. 2020, 9, 4051. [Google Scholar] [CrossRef] [PubMed]
- Sleep-Related Breathing Disorders in Adults: Recommendations for Syndrome Definition and Measurement Techniques in Clinical Research. Sleep 1999, 22, 667–689. [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [Green Version]
- Yongxin, S.; Wenjun, D.; Qiang, W.; Yunqing, S.; Liming, Z.; Chunsheng, W. Heavy Smoking Before Coronary Surgical Procedures Affects the Native Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 Gene Expression in Saphenous Vein Conduits. Ann. Thorac. Surg. 2013, 95, 55–61. [Google Scholar] [CrossRef]
- Nordskog, B.K.; Blixt, A.D.; Morgan, W.T.; Fields, W.R.; Hellmann, G.M. Matrix-Degrading and pro-Inflammatory Changes in Human Vascular Endothelial Cells Exposed to Cigarette Smoke Condensate. Cardiovasc. Toxicol. 2003, 3, 101–117. [Google Scholar] [CrossRef]
- Campbell, A.J.; Neill, A.M.; Scott, D.A.R. Clinical Reproducibility of the Epworth Sleepiness Scale for Patients With Suspected Sleep Apnea. J. Clin. Sleep Med. 2018, 14, 791–795. [Google Scholar] [CrossRef]


| Low MPO Group | High MPO Group | ||
|---|---|---|---|
| MPO < 116 ng/mL (n = 252) | MPO ≥ 116 ng/mL (n = 250) | p Values | |
| Age, years | 64.9 (58.9–70.8) | 63.5 (57.8–69.9) | 0.187 |
| Male sex, % | 82.1 | 85.6 | 0.292 |
| BMI, kg/m2 | 27.7 (25.5–30) | 27.9 (25.6–30.8) | 0.166 |
| Obesity, % | 24.2 | 30.0 | 0.144 |
| Current smokers *, % | 13.5 | 24.0 | 0.003 |
| OSA, % | 77.4 | 78.4 | 0.783 |
| Sleepiness, % | 33.3 | 30.8 | 0.543 |
| AHI, events/h | 20.8 (15.5–31.3) | 21.6 (15.4–36.1) | 0.823 |
| ODI, events/h | 10.1 (3.3–19.7) | 10.5 (3.9–21.7) | 0.607 |
| ESS score | 8.0 (4.0–10.0) | 7.0 (4.8.0–10.0) | 0.597 |
| Hypertension, % | 56.3 | 59.2 | 0.518 |
| Diabetes Mellitus, % | 24.6 | 19.2 | 0.143 |
| Pulmonary Disease, % | 9.9 | 8.0 | 0.451 |
| History of A.F., % | 17.9 | 15.6 | 0.498 |
| Baseline AMI *, % | 45.6 | 57.6 | 0.007 |
| Baseline ACS, % | 65.1 | 72.8 | 0.062 |
| Intervention-PCI *, % | 71.0 | 80.4 | 0.014 |
| Former PCI/Bypass, % | 20.2 | 18.4 | 0.602 |
| Stroke, % | 8.3 | 5.2 | 0.170 |
| Medications | |||
| Diuretics, % | 22.6 | 19.4 | 0.372 |
| Beta-blockers *, % | 82.7 | 90.3 | 0.013 |
| Acetylsalicylic acid, % | 90.5 | 90.4 | 0.948 |
| Warfarin, % | 7.4 | 7.3 | 0.960 |
| Clopidogrel, % | 53.7 | 61.4 | 0.080 |
| CAs, % | 16.9 | 21.8 | 0.169 |
| ACEIs, % | 42.0 | 46.4 | 0.327 |
| AT2As, % | 14.8 | 13.3 | 0.631 |
| LLDs, % | 93.4 | 94.8 | 0.528 |
| Low MMP-9 Group | High MMP-9 Group | ||
|---|---|---|---|
| MMP-9 < 269 ng/mL (n = 253) | MMP-9 ≥ 269 ng/mL (n = 249) | p Values | |
| Age *, years | 65.0 (59.2–71.1) | 63.1 (58.0–69.0) | 0.026 |
| Male sex *, % | 79.4 | 88.4 | 0.007 |
| BMI, kg/m2 | 27.6 (25.5–30.1) | 28.1 (25.6–30.5) | 0.279 |
| Obesity, % | 25.7 | 28.5 | 0.477 |
| Current smokers *, % | 11.1 | 26.5 | <0.001 |
| OSA, % | 80.2 | 75.5 | 0.201 |
| Sleepiness, % | 32.0 | 32.1 | 0.978 |
| AHI, events/h | 21.8 (15.9–32.8) | 20.4 (15–32.3) | 0.219 |
| ODI, events/h | 10.9 (4.1–20.7) | 9.9 (3.7–20.4) | 0.354 |
| ESS score | 7.0 (4.0–10.0) | 7.0 (5.0–10.0) | 0.676 |
| Hypertension, % | 55.7 | 59.8 | 0.351 |
| Diabetes Mellitus, % | 21.7 | 22.1 | 0.925 |
| Pulmonary Disease, % | 9.9 | 8.0 | 0.468 |
| History of A.F., % | 15.8 | 17.7 | 0.577 |
| Baseline AMI *, % | 47.0 | 56.2 | 0.039 |
| Baseline ACS, % | 66.4 | 71.5 | 0.219 |
| Intervention-PCI *, % | 71.5 | 79.9 | 0.029 |
| Former PCI/Bypass, % | 22.1 | 16.5 | 0.108 |
| Stroke, % | 6.7 | 6.9 | 0.942 |
| Medications | |||
| Diuretics *, % | 25.1 | 16.8 | 0.024 |
| Beta-blockers, % | 84.6 | 88.5 | 0.204 |
| Acetylsalicylic acid, % | 90.3 | 90.6 | 0.901 |
| Warfarin, % | 6.9 | 7.8 | 0.691 |
| Clopidogrel, % | 54.4 | 60.8 | 0.149 |
| CAs *, % | 15.8 | 23.0 | 0.045 |
| ACEIs, % | 43.7 | 44.7 | 0.833 |
| AT2As, % | 13.4 | 14.8 | 0.657 |
| LLDs, % | 93.1 | 95.1 | 0.356 |
| High MPO Levels | Bounds for 95% CI | |||
|---|---|---|---|---|
| OR | Lower | Upper | p Values | |
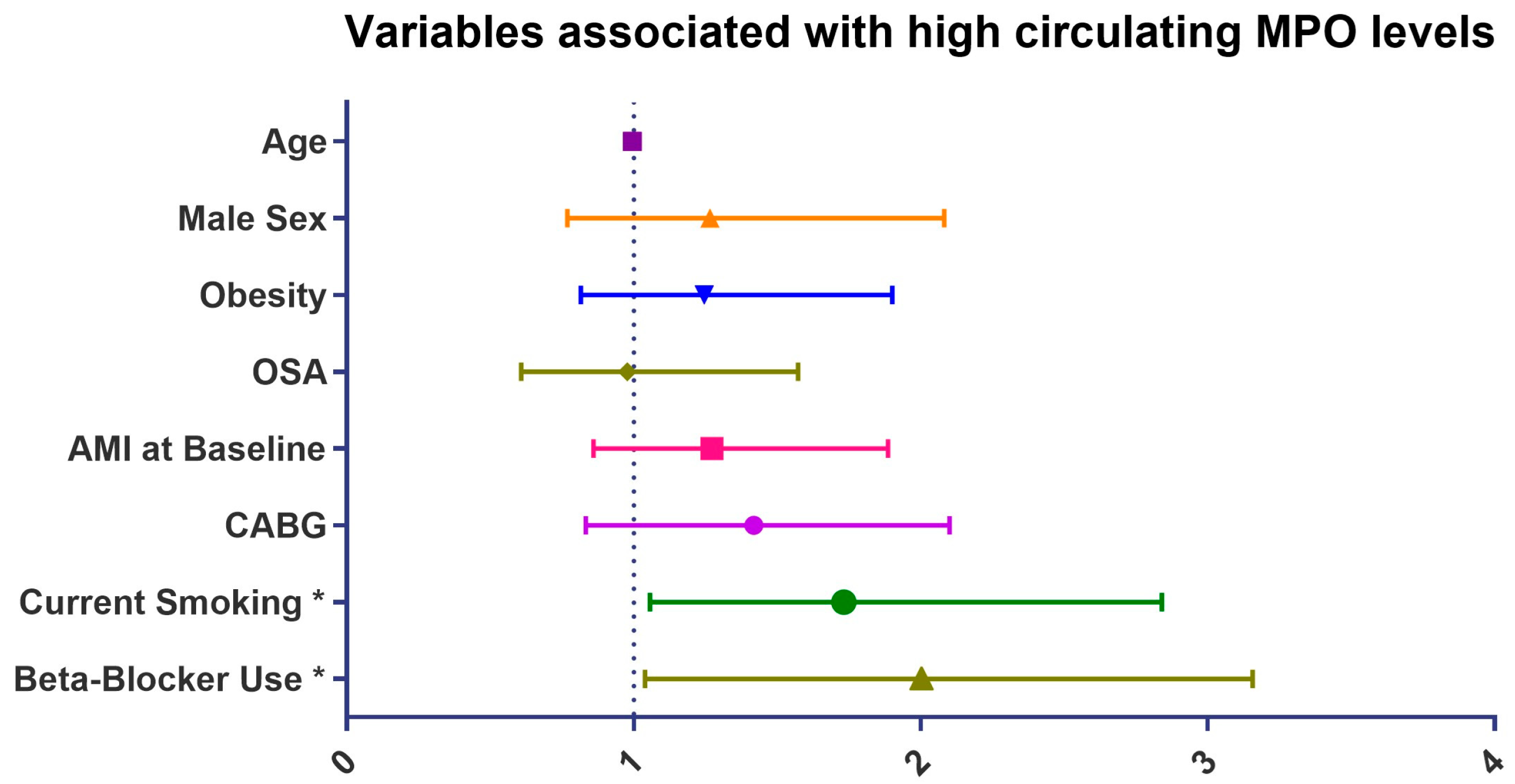
| Age | 0.99 | 0.97 | 1.01 | 0.166 |
| Male sex | 1.29 | 0.80 | 2.08 | 0.293 |
| BMI | 1.04 | 0.99 | 1.08 | 0.115 |
| Obesity | 1.34 | 0.90 | 1.99 | 0.145 |
| Current smoking * | 2.03 | 1.27 | 3.22 | 0.003 |
| OSA | 1.06 | 0.70 | 1.62 | 0.783 |
| AHI | 1.00 | 0.99 | 1.01 | 0.444 |
| ODI | 1.01 | 0.99 | 1.02 | 0.408 |
| ESS score | 0.99 | 0.94 | 1.03 | 0.490 |
| Hypertension | 1.12 | 0.79 | 1.60 | 0.518 |
| Diabetes Mellitus | 0.73 | 0.48 | 1.12 | 0.144 |
| Pulmonary disease | 0.79 | 0.43 | 1.46 | 0.452 |
| History of atrial fıbrillation | 0.85 | 0.53 | 1.36 | 0.498 |
| AMI at baseline * | 1.62 | 1.14 | 2.30 | 0.007 |
| ACS at baseline | 1.44 | 0.98 | 2.10 | 0.062 |
| CABG at baseline * | 1.67 | 1.11 | 2.53 | 0.015 |
| Former revascularization | 0.89 | 0.57 | 1.39 | 0.602 |
| Stroke | 0.61 | 0.30 | 1.24 | 0.173 |
| Medications | ||||
| Diuretics | 0.82 | 0.53 | 1.27 | 0.373 |
| Beta-blockers * | 1.95 | 1.14 | 3.34 | 0.015 |
| Acetylsalicylic acid | 0.98 | 0.54 | 1.79 | 0.948 |
| Warfarin | 0.98 | 0.50 | 1.94 | 0.960 |
| Clopidogrel | 1.38 | 0.96 | 1.97 | 0.080 |
| Calcium antagonist | 1.37 | 0.87 | 2.15 | 0.170 |
| ACEI | 1,20 | 0.84 | 1.71 | 0.327 |
| AT2As | 0.88 | 0.53 | 1.47 | 0.631 |
| LLDs | 1.27 | 0.60 | 2.71 | 0.529 |
| High MMP-9 Levels | Bounds for 95% CI | |||
|---|---|---|---|---|
| OR | Lower | Upper | p Values | |
| Age * | 0.97 | 0.95 | 0.99 | 0.009 |
| Male sex * | 1.96 | 1.20 | 3.21 | 0.007 |
| BMI | 1.02 | 0.98 | 1.07 | 0.312 |
| Obesity | 1.15 | 0.78 | 1.71 | 0.477 |
| Current smoking * | 2.90 | 1.79 | 4.70 | <0.001 |
| OSA | 0.76 | 0.50 | 1.16 | 0.202 |
| AHI | 1.00 | 0.99 | 1.01 | 0.361 |
| ODI | 1.00 | 0.98 | 1.01 | 0.504 |
| ESS score | 1.01 | 0.97 | 1.05 | 0.727 |
| Hypertension | 1.18 | 0.83 | 1.69 | 0.352 |
| Diabetes Mellitus | 1.02 | 0.67 | 1.56 | 0.925 |
| Pulmonary disease | 0.80 | 0.43 | 1.48 | 0.469 |
| History of atrial fibrillation | 1.14 | 0.72 | 1.83 | 0.577 |
| AMI at baseline * | 1.45 | 1.02 | 2.06 | 0.040 |
| ACS at baseline | 1.27 | 0.87 | 1.85 | 0.219 |
| CABG at baseline * | 1.58 | 1.05 | 2.39 | 0.029 |
| Former revascularization | 0.69 | 0.44 | 1.09 | 0.109 |
| Stroke | 1.03 | 0.51 | 2.06 | 0.942 |
| Medications | ||||
| Diuretics * | 0.60 | 0.39 | 0.94 | 0.025 |
| Beta-blockers | 1.40 | 0.83 | 2.37 | 0.206 |
| Acetylsalicylic acid | 1.04 | 0.57 | 1.90 | 0.901 |
| Warfarin | 1.15 | 0.58 | 2.26 | 0.692 |
| Clopidogrel | 1.30 | 0.91 | 1.86 | 0.149 |
| Calcium antagonist * | 1.59 | 1.01 | 2.50 | 0.046 |
| ACEIs | 1.04 | 0.73 | 1.48 | 0.833 |
| AT2As | 1.12 | 0.67 | 1.87 | 0.657 |
| LLDs | 1.43 | 0.67 | 3.06 | 0.358 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özkan, E.; Celik, Y.; Yucel-Lindberg, T.; Peker, Y. Current Smoking Determines the Levels of Circulating MPO and MMP-9 in Adults with Coronary Artery Disease and Obstructive Sleep Apnea. J. Clin. Med. 2023, 12, 4053. https://doi.org/10.3390/jcm12124053
Özkan E, Celik Y, Yucel-Lindberg T, Peker Y. Current Smoking Determines the Levels of Circulating MPO and MMP-9 in Adults with Coronary Artery Disease and Obstructive Sleep Apnea. Journal of Clinical Medicine. 2023; 12(12):4053. https://doi.org/10.3390/jcm12124053
Chicago/Turabian StyleÖzkan, Esra, Yeliz Celik, Tülay Yucel-Lindberg, and Yüksel Peker. 2023. "Current Smoking Determines the Levels of Circulating MPO and MMP-9 in Adults with Coronary Artery Disease and Obstructive Sleep Apnea" Journal of Clinical Medicine 12, no. 12: 4053. https://doi.org/10.3390/jcm12124053
APA StyleÖzkan, E., Celik, Y., Yucel-Lindberg, T., & Peker, Y. (2023). Current Smoking Determines the Levels of Circulating MPO and MMP-9 in Adults with Coronary Artery Disease and Obstructive Sleep Apnea. Journal of Clinical Medicine, 12(12), 4053. https://doi.org/10.3390/jcm12124053







