Time Spent with Saturation below 80% versus 90% in Patients with Obstructive Sleep Apnoea
Abstract
1. Introduction
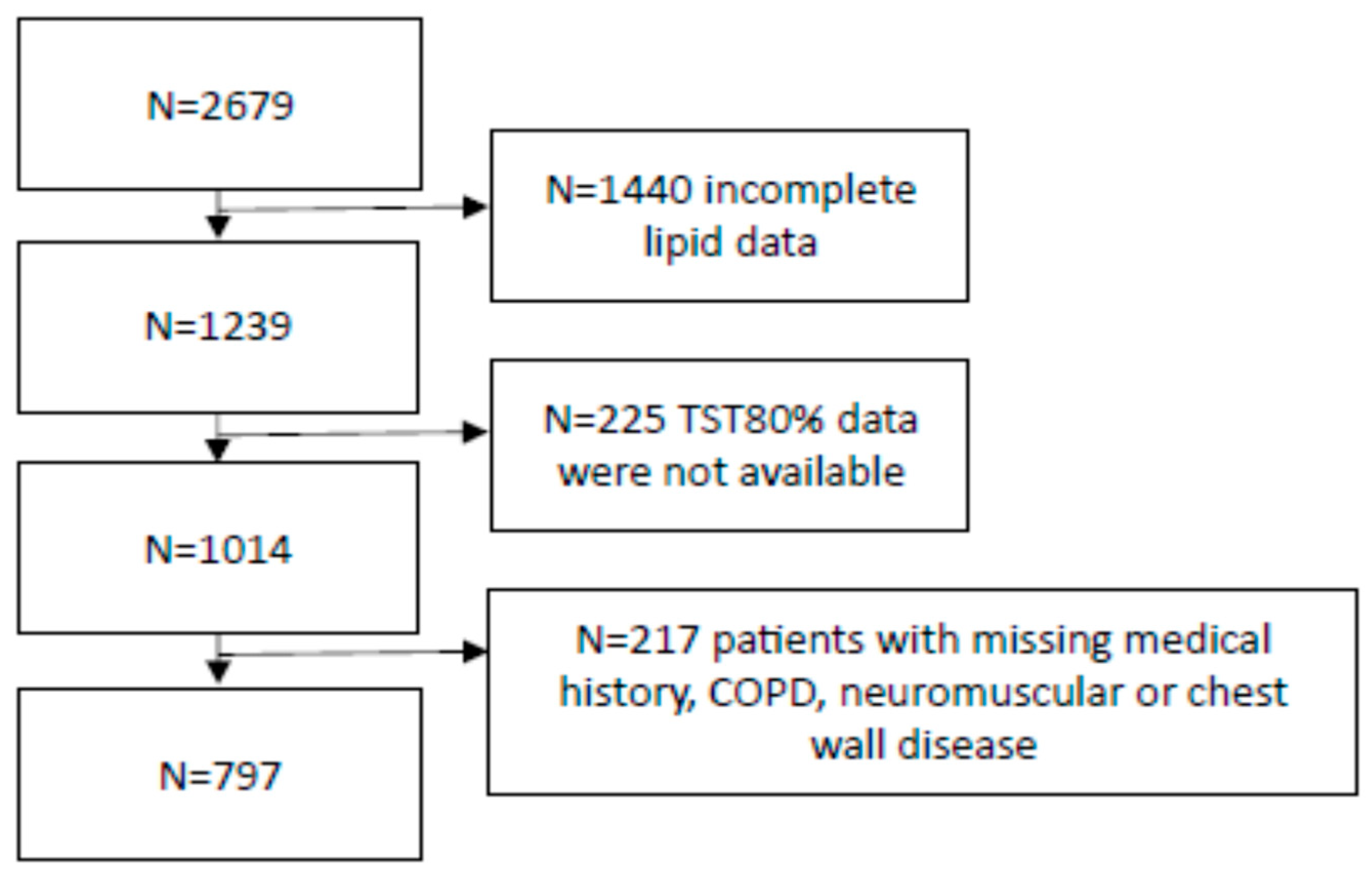
2. Methods
2.1. Design and Subjects
2.2. Sleep Studies
2.3. Statistical Analyses
3. Results
3.1. Subjects’ Characteristics
3.2. Correlation Analyses
3.3. Comparison of Diagnostic Performance of TST90% and TST80% to Detect Comorbidities
3.4. Adjusted Comparison of Groups 4 and 5
3.5. Comparison of Patients with Increased and Normal Cardiovascular Risk
3.6. Sensitivity Analyses in Patients Who Had Polysomnography as a Diagnostic Test
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Barbé, F.; Durán-Cantolla, J.; Sánchez-de-la-Torre, M.; Martínez-Alonso, M.; Carmona, C.; Barceló, A.; Chiner, E.; Masa, J.F.; Gonzalez, M.; Marín, J.M.; et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: A randomized controlled trial. JAMA 2012, 307, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Glantz, H.; Eulenburg, C.; Wegscheider, K.; Herlitz, J.; Thunström, E. Effect of Positive Airway Pressure on Cardiovascular Outcomes in Coronary Artery Disease Patients with Nonsleepy Obstructive Sleep Apnea. The RICCADSA Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2016, 194, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-de-la-Torre, M.; Sánchez-de-la-Torre, A.; Bertran, S.; Abad, J.; Duran-Cantolla, J.; Cabriada, V.; Mediano, O.; Masdeu, M.J.; Alonso, M.L.; Masa, J.F.; et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): A randomised controlled trial. Lancet. Respir. Med. 2020, 8, 359–367. [Google Scholar] [CrossRef]
- Meszaros, M.; Bikov, A. Obstructive Sleep Apnoea and Lipid Metabolism: The Summary of Evidence and Future Perspectives in the Pathophysiology of OSA-Associated Dyslipidaemia. Biomedicines 2022, 10, 2754. [Google Scholar] [CrossRef]
- Gonzaga, C.; Bertolami, A.; Bertolami, M.; Amodeo, C.; Calhoun, D. Obstructive sleep apnea, hypertension and cardiovascular diseases. J. Hum. Hypertens. 2015, 29, 705–712. [Google Scholar] [CrossRef]
- Reutrakul, S.; Mokhlesi, B. Obstructive Sleep Apnea and Diabetes: A State of the Art Review. Chest 2017, 152, 1070–1086. [Google Scholar] [CrossRef]
- May, A.M.; Mehra, R. Obstructive sleep apnea: Role of intermittent hypoxia and inflammation. Semin. Respir. Crit. Care Med. 2014, 35, 531–544. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef]
- Oldenburg, O.; Wellmann, B.; Buchholz, A.; Bitter, T.; Fox, H.; Thiem, U.; Horstkotte, D.; Wegscheider, K. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur. Heart J. 2016, 37, 1695–1703. [Google Scholar] [CrossRef]
- Smagula, S.F.; Stone, K.L.; Redline, S.; Ancoli-Israel, S.; Barrett-Connor, E.; Lane, N.E.; Orwoll, E.S.; Cauley, J.A. Actigraphy- and Polysomnography-Measured Sleep Disturbances, Inflammation, and Mortality Among Older Men. Psychosom. Med. 2016, 78, 686–696. [Google Scholar] [CrossRef]
- Xu, P.H.; Fong, D.Y.T.; Lui, M.M.S.; Lam, D.C.L.; Ip, M.S.M. Cardiovascular outcomes in obstructive sleep apnoea and implications of clinical phenotyping on effect of CPAP treatment. Thorax 2023, 78, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Gershon, A.S.; Hawker, G.; Leung, R.S.; Tomlinson, G. Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: A decade-long historical cohort study. PLoS Med. 2014, 11, e1001599. [Google Scholar] [CrossRef]
- Wang, L.; Wei, D.H.; Zhang, J.; Cao, J. Time Under 90% Oxygen Saturation and Systemic Hypertension in Patients with Obstructive Sleep Apnea Syndrome. Nat. Sci. Sleep 2022, 14, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J. Biol. Med. 2007, 80, 51–60. [Google Scholar] [PubMed]
- Catrina, S.B.; Zheng, X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia 2021, 64, 709–716. [Google Scholar] [CrossRef]
- Nanduri, J.; Peng, Y.J.; Yuan, G.; Kumar, G.K.; Prabhakar, N.R. Hypoxia-inducible factors and hypertension: Lessons from sleep apnea syndrome. J. Mol. Med. 2015, 93, 473–480. [Google Scholar] [CrossRef]
- Nguyen, L.K.; Cavadas, M.A.; Scholz, C.C.; Fitzpatrick, S.F.; Bruning, U.; Cummins, E.P.; Tambuwala, M.M.; Manresa, M.C.; Kholodenko, B.N.; Taylor, C.T.; et al. A dynamic model of the hypoxia-inducible factor 1α (HIF-1α) network. J. Cell Sci. 2013, 126, 1454–1463. [Google Scholar] [CrossRef]
- Li, J.; Savransky, V.; Nanayakkara, A.; Smith, P.L.; O’Donnell, C.P.; Polotsky, V.Y. Hyperlipidemia and lipid peroxidation are dependent on the severity of chronic intermittent hypoxia. J. Appl. Physiol. 2007, 102, 557–563. [Google Scholar] [CrossRef]
- Jun, J.C.; Shin, M.K.; Yao, Q.; Bevans-Fonti, S.; Poole, J.; Drager, L.F.; Polotsky, V.Y. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am. J. Physiol. -Endocrinol. Metab. 2012, 303, E377–E388. [Google Scholar] [CrossRef]
- Del Rio, R.; Moya, E.A.; Parga, M.J.; Madrid, C.; Iturriaga, R. Carotid body inflammation and cardiorespiratory alterations in intermittent hypoxia. Eur. Respir. J. 2012, 39, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.C.; Shin, M.K.; Devera, R.; Yao, Q.; Mesarwi, O.; Bevans-Fonti, S.; Polotsky, V.Y. Intermittent hypoxia-induced glucose intolerance is abolished by α-adrenergic blockade or adrenal medullectomy. Am. J. Physiol. -Endocrinol. Metab. 2014, 307, E1073–E1083. [Google Scholar] [CrossRef] [PubMed]
- Azarbarzin, A.; Sands, S.A.; Stone, K.L.; Taranto-Montemurro, L.; Messineo, L.; Terrill, P.I.; Ancoli-Israel, S.; Ensrud, K.; Purcell, S.; White, D.P.; et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur. Heart J. 2019, 40, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Azarbarzin, A.; Sands, S.A.; Taranto-Montemurro, L.; Vena, D.; Sofer, T.; Kim, S.W.; Stone, K.L.; White, D.P.; Wellman, A.; Redline, S. The Sleep Apnea-Specific Hypoxic Burden Predicts Incident Heart Failure. Chest 2020, 158, 739–750. [Google Scholar] [CrossRef]
- Sigurdardottir, F.D.; Øverby, C.T.; Nikkonen, S.; Karhu, T.; Dammen, T.; Nordhus, I.H.; Thorshov, T.; Einvik, G.; Kainulainen, S.; Leppänen, T.; et al. Novel oxygen desaturation parameters are associated with cardiac troponin I: Data from the Akershus Sleep Apnea Project. J. Sleep Res. 2022, 31, e13581. [Google Scholar] [CrossRef]
- Kulkas, A.; Tiihonen, P.; Eskola, K.; Julkunen, P.; Mervaala, E.; Töyräs, J. Novel parameters for evaluating severity of sleep disordered breathing and for supporting diagnosis of sleep apnea-hypopnea syndrome. J. Med. Eng. Technol. 2013, 37, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K.; Sadr, N.; Bin, Y.S.; Cook, K.; Dissanayake, H.U.; Cistulli, P.A.; de Chazal, P. Comparative associations of oximetry patterns in Obstructive Sleep Apnea with incident cardiovascular disease. Sleep 2022, 45, zsac179. [Google Scholar] [CrossRef]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Kushida, C.A.; Littner, M.R.; Morgenthaler, T.; Alessi, C.A.; Bailey, D.; Coleman, J., Jr.; Friedman, L.; Hirshkowitz, M.; Kapen, S.; Kramer, M.; et al. Practice parameters for the indications for polysomnography and related procedures: An update for 2005. Sleep 2005, 28, 499–521. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Farré, R.; Montserrat, J.M.; Gozal, D.; Almendros, I.; Navajas, D. Intermittent Hypoxia Severity in Animal Models of Sleep Apnea. Front. Physiol. 2018, 9, 1556. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.C.; Brady, D.C.; Soans, R.; Kim, E.Y.; Valverde, L.; Keenan, B.T.; Guo, X.; Kim, W.Y.; Park, M.J.; Galante, R.; et al. Different cyclical intermittent hypoxia severities have different effects on hippocampal microvasculature. J. Appl. Physiol. 2016, 121, 78–88. [Google Scholar] [CrossRef]
- Arnardottir, E.S.; Maislin, G.; Schwab, R.J.; Staley, B.; Benediktsdottir, B.; Olafsson, I.; Juliusson, S.; Romer, M.; Gislason, T.; Pack, A.I. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: The Icelandic Sleep Apnea Cohort. Sleep 2012, 35, 921–932. [Google Scholar] [CrossRef] [PubMed]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef]
- Bryant, P.A.; Trinder, J.; Curtis, N. Sick and tired: Does sleep have a vital role in the immune system? Nat. Rev. Immunol. 2004, 4, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Keogh, C.E.; Scholz, C.C.; Rodriguez, J.; Selfridge, A.C.; von Kriegsheim, A.; Cummins, E.P. Carbon dioxide-dependent regulation of NF-κB family members RelB and p100 gives molecular insight into CO(2)-dependent immune regulation. J. Biol. Chem. 2017, 292, 11561–11571. [Google Scholar] [CrossRef]
- Meszaros, M.; Horvath, P.; Kis, A.; Kunos, L.; Tarnoki, A.D.; Tarnoki, D.L.; Lazar, Z.; Bikov, A. Circulating levels of clusterin and complement factor H in patients with obstructive sleep apnea. Biomark. Med. 2021, 15, 323–330. [Google Scholar] [CrossRef]
- Pevernagie, D.A.; Gnidovec-Strazisar, B.; Grote, L.; Heinzer, R.; McNicholas, W.T.; Penzel, T.; Randerath, W.; Schiza, S.; Verbraecken, J.; Arnardottir, E.S. On the rise and fall of the apnea-hypopnea index: A historical review and critical appraisal. J. Sleep Res. 2020, 29, e13066. [Google Scholar] [CrossRef]
- Baumert, M.; Immanuel, S.A.; Stone, K.L.; Litwack Harrison, S.; Redline, S.; Mariani, S.; Sanders, P.; McEvoy, R.D.; Linz, D. Composition of nocturnal hypoxaemic burden and its prognostic value for cardiovascular mortality in older community-dwelling men. Eur. Heart J. 2020, 41, 533–541. [Google Scholar] [CrossRef]


| Group 1 (n = 92) | Group 2 (n = 79) | Group 3 (n = 396) | Group 4 (n = 194) | Group 5 (n = 36) | p Value | |
|---|---|---|---|---|---|---|
| Age (years) | 46 ± 15 | 49 ± 12 | 55 ± 12 #¶ | 56 ± 11 #¶ | 49 ± 10 µ | <0.001 |
| Gender (males%) | 27 | 62 | 67 | 74 | 83 | <0.001 |
| BMI (kg/m2) | 25 ± 5 | 29 ± 5 # | 32 ± 6 #¶ | 36 ± 7 #¶@ | 39 ± 7 #¶@ | <0.001 |
| Current smokers (%) | 5 | 23 | 19 | 15 | 33 | 0.001 |
| Hypertension (%) | 38 | 53 | 71 | 82 | 75 | <0.001 |
| Diabetes (%) | 10 | 6 | 19 | 24 | 28 | <0.001 |
| Dyslipidaemia (%) | 42 | 77 | 77 | 85 | 92 | <0.001 |
| Cardiovascular disease (%) | 5 | 4 | 16 | 23 | 22 | <0.001 |
| Lipid-lowering medication (%) | 5 | 25 | 37 | 43 | 25 | <0.001 |
| SBP (mmHg) | 124 ± 17 | 130 ± 13 | 135 ± 14 # | 138 ± 14 #¶@ | 137 ± 15 # | <0.001 |
| DBP (mmHg) | 77 ± 10 | 80 ± 9 | 82 ± 10 # | 85 ± 11 #¶@ | 83 ± 10 # | <0.001 |
| Total cholesterol (mmol/L) | 5.4 ± 1.0 | 5.3 ± 1.1 | 5.3 ± 1.2 | 5.3 ± 1.1 | 5.7 ± 1.2 | 0.27 |
| LDL-C (mmol/L) | 3.1 ± 1.0 | 3.3 ± 1 | 3.3 ± 1.0 | 3.4 ± 1.0 | 3.7 ± 1.0 # | 0.04 |
| HDL-C (mmol/L) | 1.7 ± 0.6 | 1.2 ± 0.3 # | 1.2 ± 0.4 # | 1.2 ± 0.3 # | 1.1 ± 0.2 # | <0.001 |
| Triglycerides (mmol/L) | 1.3 ± 0.7 | 1.7 ± 0.7 | 1.8 ± 0.9 # | 2.1 ± 1.2 #¶@ | 2.0 ± 0.6 # | <0.001 |
| CRP (mg/L) | 4.5 ± 12.6 | 2.2 ± 7.1 | 2.1 ± 3.8 # | 2.9 ± 5.7 | 2.0 ± 2.6 | 0.03 |
| Framingham risk score | 3.3 ± 5.4 | 7.0 ± 7.6 # | 9.7 ± 7.9 #¶ | 11.4 ± 7.3 #¶ | 11.3 ± 8.6 #¶ | <0.001 |
| Patients at >10% cardiovascular risk (%) | 12 | 31 | 45 | 56 | 52 | <0.001 |
| AHI (1/h) | 2.4 ± 1.4 | 18.7 ± 9.8 # | 28.9 ± 17.9 #¶ | 48.7 ± 23.0 #¶@ | 76.7 ± 16.4 #¶@µ | <0.001 |
| ODI (1/h) | 1.2 ± 1.1 | 14.1 ± 9.1 # | 24.1 ± 17.4 #¶ | 45.0 ± 24.4 #¶@ | 78.5 ± 16.9 #¶@µ | <0.001 |
| TST90% | 0 ± 0 | 0 ± 0 | 2.8 ± 2.8 | 28.5 ± 17.3 #¶@ | 63.6 ± 16.6 #¶@µ | <0.001 |
| TST80% | 0 ± 0 | 0 ± 0 | 0.1 ± 0.3 | 1.6 ± 2.1 #¶@ | 27.3 ± 17.5 #¶@µ | <0.001 |
| ESS score | 6.1 ± 3.4 | 6.7 ± 4.1 | 7.9 ± 4.0 # | 9.8 ± 4.1 #¶@ | 12.3 ± 3.4 #¶@µ | <0.001 |
| Hypertension | Diabetes | Dyslipidaemia | Cardiovascular Disease | |
|---|---|---|---|---|
| TST90% | 0.67/0.63–0.71/ | 0.62/0.58–0.67/ | 0.63/0.58–0.67/ | 0.65/0.61–0.70/ |
| TST80% | 0.60/0.56–0.64/ | 0.58/0.53–0.63/ | 0.59/0.55–0.64/ | 0.61/0.55–0.66/ |
| AHI | 0.67/0.63–0.71/ | 0.60/0.55–0.65/ | 0.67/0.63–0.72/ | 0.61/0.56–0.66/ |
| Normal Cardiovascular Risk (n = 440) | Increased Cardiovascular Risk (n = 337) | p Value | |
|---|---|---|---|
| Age (years) | 49 ± 12 | 60 ± 10 | <0.001 |
| Gender (males%) | 48 | 88 | <0.001 |
| BMI (kg/m2) | 32 ± 7 | 33 ± 6 | <0.001 |
| Current Smoker (%) | 10 | 28 | <0.001 |
| Hypertension (%) | 56 | 87 | <0.001 |
| Diabetes (%) | 13 | 26 | <0.001 |
| Dyslipidaemia (%) | 70 | 83 | <0.001 |
| Cardiovascular disease (%) | 10 | 24 | <0.001 |
| SBP (mmHg) | 131 ± 14 | 139 ± 14 | <0.001 |
| DBP (mmHg) | 81 ± 11 | 82 ± 9 | 0.131 |
| Total cholesterol (mmol/L) | 5.2 ± 1.1 | 5.5 ± 1.2 | <0.001 |
| LDL-C (mmol/L) | 3.2 ± 1.0 | 3.6 ± 1.1 | <0.001 |
| HDL-C (mmol/L) | 1.3 ± 0.5 | 1.1 ± 0.3 | <0.001 |
| Triglycerides (mmol/L) | 1.7 ± 1.0 | 2.0 ± 1.0 | <0.001 |
| CRP (mg/L) | 2.4 ± 5.8 | 2.7 ± 6.4 | 0.463 |
| AHI (1/h) | 27.8 ± 23.9 | 38.4 ± 23.5 | <0.001 |
| ODI (1/h) | 24.1 ± 23.9 | 34.4 ± 24.7 | <0.001 |
| TST90% | 8.9 ± 16.6 | 14.8 ± 20.9 | <0.001 |
| TST80% | 1.3 ± 5.2 | 2.2 ± 8.6 | 0.061 |
| ESS score | 7.9 ± 4.2 | 8.9 ± 4.2 | 0.001 |
| Group 1 (n = 67) | Group 2 (n = 51) | Group 3 (n = 295) | Group 4 (n = 155) | Group 5 (n = 30) | p Value | |
|---|---|---|---|---|---|---|
| Age (years) | 45 ± 16 | 48 ± 12 | 54 ± 12 #¶ | 56 ± 11 #¶ | 49 ± 9 µ | <0.001 |
| Gender (males%) | 24 | 65 | 68 | 76 | 83 | <0.001 |
| BMI (kg/m2) | 25 ± 4 | 29 ± 6 # | 32 ± 6 #¶ | 36 ± 6 #¶@ | 40 ± 7 #¶@µ | <0.001 |
| Current smokers (%) | 6 | 22 | 18 | 15 | 37 | 0.001 |
| Hypertension (%) | 34 | 47 | 68 | 83 | 73 | <0.001 |
| Diabetes (%) | 7 | 6 | 17 | 26 | 27 | <0.001 |
| Dyslipidaemia (%) | 46 | 73 | 76 | 85 | 93 | <0.001 |
| Cardiovascular disease (%) | 4 | 2 | 15 | 22 | 23 | <0.001 |
| Lipid-lowering medication (%) | 7 | 22 | 38 | 47 | 23 | <0.001 |
| SBP (mmHg) | 124 ± 14 | 129 ± 13 | 135 ± 14 # | 138 ± 14 #¶ | 137 ± 15 # | <0.001 |
| DBP (mmHg) | 77 ± 10 | 80 ± 9 | 81 ± 10 # | 85 ± 11 #¶@ | 82 ± 10 | <0.001 |
| Total cholesterol (mmol/L) | 5.3 ± 1.0 | 5.3 ± 1.2 | 5.2 ± 1.1 | 5.3 ± 1.1 | 5.8 ± 1.2 | 0.125 |
| LDL-C (mmol/L) | 3.0 ± 1.0 | 3.2 ± 1.1 | 3.3 ± 1.0 | 3.5 ± 1.0 # | 3.8 ± 0.9 #@ | <0.001 |
| HDL-C (mmol/L) | 1.7 ± 0.6 | 1.3 ± 0.4 # | 1.2 ± 0.5 # | 1.2 ± 0.2 # | 1.1 ± 0.2 # | <0.001 |
| Triglycerides (mmol/L) | 1.3 ± 0.8 | 1.6 ± 0.5 | 1.8 ± 0.9 # | 2.1 ± 1.2 #¶ | 2.0 ± 0.6 # | <0.001 |
| CRP (mg/L) | 4.9 ± 14.0 | 2.4 ± 8.5 | 2.0 ± 4.1 # | 2.2 ± 3.7 | 1.7 ± 2.7 | 0.044 |
| Framingham risk score | 2.3 ± 3.9 | 6.5 ± 7.7 # | 9.3 ± 7.8 # | 11.4 ± 7.1 #¶@ | 10.6 ± 8.5 # | <0.001 |
| Patients at >10% cardiovascular risk (%) | 5 | 28 | 43 | 56 | 47 | <0.001 |
| AHI (1/h) | 2.5 ± 1.4 | 19.1 ± 10.6 # | 28.0 ± 17.5 #¶ | 48.2 ± 22.6 #¶@ | 77.0 ± 15.4 #¶@µ | <0.001 |
| ODI (1/h) | 1.1 ± 1.0 | 13.4 ± 10.0 # | 22.3 ± 15.8 #¶ | 44.7 ± 24.7 #¶@ | 79.2 ± 15.6 #¶@µ | <0.001 |
| TST90% | 0 ± 0 | 0 ± 0 | 3.0 ± 2.8 | 30.6 ± 18.1 #¶@ | 65.8 ± 16.8 #¶@µ | <0.001 |
| TST80% | 0 ± 0 | 0 ± 0 | 0.1 ± 0.3 | 1.6 ± 2.1 #¶@ | 28.7 ± 18.7 #¶@µ | <0.001 |
| ESS score | 6.1 ± 3.4 | 6.7 ± 4.1 | 7.9 ± 4.0 # | 9.8 ± 4.1 #¶@ | 12.3 ± 3.4 #¶@µ | <0.001 |
| Hypertension | Diabetes | Dyslipidaemia | Cardiovascular Disease | 10 Year Cardiovascular Risk | |
|---|---|---|---|---|---|
| TST90% | 0.68/0.63–0.73/ | 0.64/0.59–0.70/ | 0.67/0.62–0.72/ | 0.67/0.61–0.72/ | 0.64/0.60–0.69/ |
| TST80% | 0.61/0.57–0.66/ | 0.61/0.53–0.67/ | 0.60/0.55–0.66/ | 0.63/0.57–0.70/ | 0.59/0.54–0.64/ |
| AHI | 0.67/0.62–0.72/ | 0.62/0.55–0.65/ | 0.67/0.62–0.72/ | 0.62/0.56–0.68/ | 0.66/0.62–0.71/ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikov, A.; Frent, S.; Deleanu, O.; Meszaros, M.; Birza, M.R.; Popa, A.M.; Manzur, A.R.; Gligor, L.; Mihaicuta, S. Time Spent with Saturation below 80% versus 90% in Patients with Obstructive Sleep Apnoea. J. Clin. Med. 2023, 12, 4205. https://doi.org/10.3390/jcm12134205
Bikov A, Frent S, Deleanu O, Meszaros M, Birza MR, Popa AM, Manzur AR, Gligor L, Mihaicuta S. Time Spent with Saturation below 80% versus 90% in Patients with Obstructive Sleep Apnoea. Journal of Clinical Medicine. 2023; 12(13):4205. https://doi.org/10.3390/jcm12134205
Chicago/Turabian StyleBikov, András, Stefan Frent, Oana Deleanu, Martina Meszaros, Mariela Romina Birza, Alina Mirela Popa, Andrei Raul Manzur, Loredana Gligor, and Stefan Mihaicuta. 2023. "Time Spent with Saturation below 80% versus 90% in Patients with Obstructive Sleep Apnoea" Journal of Clinical Medicine 12, no. 13: 4205. https://doi.org/10.3390/jcm12134205
APA StyleBikov, A., Frent, S., Deleanu, O., Meszaros, M., Birza, M. R., Popa, A. M., Manzur, A. R., Gligor, L., & Mihaicuta, S. (2023). Time Spent with Saturation below 80% versus 90% in Patients with Obstructive Sleep Apnoea. Journal of Clinical Medicine, 12(13), 4205. https://doi.org/10.3390/jcm12134205








