Comparison of Orbital Atherectomy and Rotational Atherectomy in Calcified Left Main Disease: Short-Term Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. A Study Design and Population
2.2. PCI Procedures
2.3. Study Endpoints
2.4. Statistical Analysis
3. Results
4. Discussion
- −
- In the high-risk study cohort, orbital atherectomy demonstrated a good short-term safety profile that was comparable to that of conventional rotational atherectomy.
- −
- There were no significant differences in primary or secondary outcomes between the two debulking devices.
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gemici, G.; Guneysu, T.; Eroğlu, E.; Bayrak, F.; Sevinc, D.; Aytaclar, S.; Kaya, Z.; Mutlu, B.; Degertekin, M. Prevalence of left main coronary artery disease among patients referred to multislice computed tomography coronary examinations. Int. J. Cardiovasc. Imaging 2008, 25, 433–438. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzo, F.; Presutti, D.G.; Picardi, E.; Moretti, C.; Omedè, P.; Sciuto, F.; Novara, M.; Yan, A.T.; Goodman, S.; Mahajan, N.; et al. Prevalence and non-invasive predictors of left main or three-vessel coronary disease: Evidence from a collaborative international meta-analysis including 22,740 patients. Heart 2012, 98, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Copeland-Halperin, R.S.; Baber, U.; Aquino, M.; Rajamanickam, A.; Roy, S.; Hasan, C.; Barman, N.; Kovacic, J.C.; Moreno, P.; Krishnan, P.; et al. Prevalence, correlates, and impact of coronary calcification on adverse events following PCI with newer-generation DES: Findings from a large multiethnic registry. Catheter. Cardiovasc. Interv. 2017, 91, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Ba, R.W.B.; Patel, M.R.; Martinsen, B.J.; Azemi, T.; Giugliano, G.; Resar, J.R.; Mehran, R.; Cohen, D.J.; Popma, J.J.; et al. Impact of calcification on percutaneous coronary intervention: MACE-Trial 1-year results. Catheter. Cardiovasc. Interv. 2019, 94, 187–194. [Google Scholar] [CrossRef]
- Shlofmitz, E. Lesion preparation: An essential component of percutaneous coronary intervention in calcified lesions. Kardiol. Pol. 2019, 77, 820–821. [Google Scholar] [CrossRef]
- Tomey, M.I.; Kini, A.S.; Sharma, S.K. Current Status of Rotational Atherectomy. JACC Cardiovasc. Interv. 2014, 7, 345–353. [Google Scholar] [CrossRef]
- Sharma, S.K.; Tomey, M.I.; Teirstein, P.S.; Kini, A.S.; Reitman, A.B.; Lee, A.C.; Généreux, P.; Chambers, J.W.; Grines, C.L.; Himmelstein, S.I.; et al. North American Expert Review of Rotational Atherectomy. Circ. Cardiovasc. Interv. 2019, 12, e007448. [Google Scholar] [CrossRef]
- Bouisset, F.; Ribichini, F.; Bataille, V.; Reczuch, K.; Lhermusier, T.; Dobrzycki, S.; Meyer-Gessner, M.; Bressollette, E.; Zajdel, W.; Faurie, B.; et al. Clinical Outcomes of Left Main Coronary Artery PCI With Rotational Atherectomy. Am. J. Cardiol. 2022, 186, 36–42. [Google Scholar] [CrossRef]
- Dahdouh, Z.; Roule, V.; Dugué, A.E.; Sabatier, R.; Lognoné, T.; Grollier, G. Rotational Atherectomy for Left Main Coronary Artery Disease in Octogenarians: Transradial Approach in a Tertiary Center and Literature Review. J. Interv. Cardiol. 2013, 26, 173–182. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Martinsen, B.J.; Lee, M.; Rao, S.V.; Généreux, P.; Higgins, J.; Chambers, J.W.; Kirtane, A.J.; Brilakis, E.; Kandzari, D.E.; et al. Orbital atherectomy for the treatment of severely calcified coronary lesions: Evidence, technique, and best practices. Expert Rev. Med. Devices 2017, 14, 867–879. [Google Scholar] [CrossRef]
- Rola, P.; Włodarczak, S.; Furtan, Ł.; Doroszko, A.; Lesiak, M.; Włodarczak, A. First experience with orbital atherectomy in calcified unprotected left main percutaneous coronary intervention. Adv. Interv. Cardiol./Postępy W Kardiol. Interwencyjnej 2022, 18, 64–66. [Google Scholar] [CrossRef]
- Khan, A.A.; Murtaza, G.; Khalid, M.F.; White, C.J.; Mamas, M.A.; Mukherjee, D.; Jneid, H.; Shanmugasundaram, M.; Nagarajarao, H.S.; Paul, T.K. Outcomes of rotational atherectomy versus orbital atherectomy for the treatment of heavily calcified coronary stenosis: A systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2020, 98, 884–892. [Google Scholar] [CrossRef]
- Doshi, R.; Thakkar, S.; Patel, K.; Majmundar, M.; Shlofmitz, E.; Kumar, A.; Gupta, N.; Adalja, D.; Patel, H.P.; Jauhar, R.; et al. Short term outcomes of rotational atherectomy versus orbital atherectomy in patients undergoing complex percutaneous coronary intervention: A systematic review and meta-analysis. Scand. Cardiovasc. J. 2021, 55, 129–137. [Google Scholar] [CrossRef]
- Bin Song, Y.; Hahn, J.-Y.; Yang, J.H.; Choi, S.-H.; Choi, J.-H.; Lee, S.H.; Jeong, M.-H.; Kim, H.-S.; Lee, J.-H.; Yu, C.W.; et al. Differential Prognostic Impact of Treatment Strategy Among Patients With Left Main Versus Non–Left Main Bifurcation Lesions Undergoing Percutaneous Coronary Intervention: Results from the COBIS (Coronary Bifurca-tion Stenting) Registry II. JACC Cardiovasc. Interv. 2014, 7, 255–263. [Google Scholar] [CrossRef]
- Choi, K.H.; Bin Song, Y.; Lee, J.M.; Park, T.K.; Yang, J.H.; Hahn, J.-Y.; Choi, J.-H.; Choi, S.-H.; Kim, H.-S.; Chun, W.J.; et al. Prognostic Effects of Treatment Strategies for Left Main Versus Non-Left Main Bifurcation Percutaneous Coronary Intervention With Current-Generation Drug-Eluting Stent. Circ. Cardiovasc. Interv. 2020, 13, e008543. [Google Scholar] [CrossRef]
- Dobrzycki, S.; Reczuch, K.; Legutko, J.; Pawłowski, T.; Grygier, M.; Ochała, A.; Wójcik, J.; Buszman, P.; Dudek, D.; Gąsior, M.; et al. Rotational atherectomy in everyday clinical practice. Association of Cardiovascular Interventions of the Polish Society of Cardiology (Asocjacja Interwencji Sercowo-Naczyniowych Polskiego Towarzystwa Kardiologicznego—AISN PTK): Expert opinion. Kardiol. Pol. 2018, 76, 1576–1584. [Google Scholar] [CrossRef]
- Shlofmitz, E.; Jeremias, A.; Shlofmitz, R.A.; Ali, Z.A. Lesion Preparation with Orbital Atherectomy. Interv. Cardiol. 2019, 14, 169–173. [Google Scholar] [CrossRef]
- Garcia-Garcia, H.M.; McFadden, E.P.; Farb, A.; Mehran, R.; Stone, G.W.; Spertus, J.; Onuma, Y.; Morel, M.-A.; van Es, G.-A.; Zuckerman, B.; et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Circulation 2018, 137, 2635–2650. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction. J. Am. Coll Cardiol. 2018, 72, 2231–2264. [Google Scholar]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Sakakura, K.; Taniguchi, Y.; Yamamoto, K.; Tsukui, T.; Jinnouchi, H.; Seguchi, M.; Wada, H.; Fujita, H. Modifiable and unmodifiable factors associated with slow flow following rotational atherectomy. PLoS ONE 2021, 16, e0250757. [Google Scholar] [CrossRef] [PubMed]
- Januszek, R.; Siudak, Z.; Reczuch, K.; Dobrzycki, S.; Lesiak, M.; Legutko, J.; Kleczyński, P.; Rzeszutko, Ł.; Dudek, D.; Bartuś, S. Current trends and procedural outcomes in the era of rotational atherectomy expansion in Poland in the period 2014-2017 (based on the nationwide ORPKI registry). Postep. Kardiol Interwencyjnej 2019, 15, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, K.; Taniguchi, Y.; Yamamoto, K.; Wada, H.; Momomura, S.-I.; Fujita, H. Association of Excessive Speed Reduction with Clinical Factors During Rotational Atherectomy. Cardiovasc. Revascularization Med. 2019, 21, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Januszek, R.; Siudak, Z.; Malinowski, K.P.; Reczuch, K.; Dobrzycki, S.; Lesiak, M.; Hawranek, M.; Gil, R.J.; Witkowski, A.; Wojakowski, W.; et al. Radial versus femoral access in patients treated with percutaneous coronary intervention and rotational atherectomy. Kardiol. Pol. 2020, 78, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Rola, P.; Furtan, Ł.; Włodarczak, S.; Jastrzębski, A.; Barycki, M.; Kędzierska, M.; Szudrowicz, M.; Kulczycki, J.J.; Doroszko, A.; Lesiak, M.; et al. Orbital atherectomy for treatment of calcified coronary artery lesions. First experiences in Poland: Short-term outcomes of the Lower-Silesia Orbital Atherectomy Registry (LOAR). Kardiol. Pol. 2023, 81, 174–176. [Google Scholar] [CrossRef]
- Lee, M.S.; Shlofmitz, E.; Shlofmitz, R.; Sahni, S.; Martinsen, B.; Chambers, J. Outcomes After Orbital Atherectomy of Severely Calcified Left Main Lesions: Analysis of the ORBIT II Study. J. Invasive. Cardiol. 2016, 28, 364–369. [Google Scholar]
- Shah, C.A.; Pfau, S.E. Percutaneous Left Main Coronary Intervention: A Review of Plaque Modification in Left Main Percutaneous Coronary Intervention. J. Clin. Med. 2018, 7, 180. [Google Scholar] [CrossRef]
- Goel, S.; Pasam, R.T.; Chava, S.; Gotesman, J.; Sharma, A.; Malik, B.A.; Frankel, R.; Shani, J.; Gidwani, U.; Latib, A. Orbital atherectomy versus rotational atherectomy: A systematic review and meta-analysis. Int. J. Cardiol. 2019, 303, 16–21. [Google Scholar] [CrossRef]
- Angsubhakorn, N.; Kang, N.; Fearon, C.; Techorueangwiwat, C.; Swamy, P.; Brilakis, E.S.; Bharadwaj, A.S. Contemporary Management of Severely Calcified Coronary Lesions. J. Pers. Med. 2022, 12, 1638. [Google Scholar] [CrossRef]
- Galougahi, K.K.; Shlofmitz, E.; Jeremias, A.; Gogia, S.; Kirtane, A.J.; Hill, J.M.; Karmpaliotis, D.; Mintz, G.S.; Maehara, A.; Stone, G.W.; et al. Therapeutic Approach to Calcified Coronary Lesions: Disruptive Technologies. Curr. Cardiol. Rep. 2021, 23, 33. [Google Scholar] [CrossRef]
- Iqbal, M.B.; Arujuna, A.; Ilsley, C.; Archbold, A.; Crake, T.; Firoozi, S.; Kalra, S.; Knight, C.; Lim, P.; Malik, I.S.; et al. Radial versus femoral access is associated with reduced complications and mortality in patients with non-ST-segment-elevation myocardial infarction: An observational cohort study of 10,095 patients. Circ. Cardiovasc. Interv. 2014, 7, 456–464. [Google Scholar] [CrossRef]
- Cantor, W.J.; Mahaffey, K.W.; Huang, Z.; Das, P.; Gulba, D.C.; Glezer, S.; Gallo, R.; Ducas, J.; Cohen, M.; Antman, E.M.; et al. Bleeding complications in patients with acute coronary syndrome undergoing early invasive management can be reduced with radial access, smaller sheath sizes, and timely sheath removal. Catheter. Cardiovasc. Interv. 2006, 69, 73–83. [Google Scholar] [CrossRef]
- Meraj, P.M.; Shlofmitz, E.; Kaplan, B.; Jauhar, R.; Doshi, R. Clinical outcomes of atherectomy prior to percutaneous coronary intervention: A comparison of outcomes following rotational versus orbital atherectomy (COAP-PCI study). J. Interv. Cardiol. 2018, 31, 478–485. [Google Scholar] [CrossRef]
- Włodarczak, S.; Rola, P.; Furtan, Ł.; Barycki, M.; Szudrowicz, M.; Kulczycki, J.J.; Doroszko, A.; Lesiak, M.; Włodarczak, A. Orbital-Tripsy—Orbital atherectomy facilitated by Shockwave Intravascular Lithotripsy: Novel bailout strategy in percutaneous coronary intervention in heavily calcified coronary lesions. Kardiol. Pol. 2023, 81, 296–297. [Google Scholar] [CrossRef]
- Januszek, R.; Siudak, Z.; Malinowski, K.P.; Wańha, W.; Wojakowski, W.; Reczuch, K.; Dobrzycki, S.; Lesiak, M.; Hawranek, M.; Gil, R.J.; et al. Annual operator volume among patients treated using percutaneous coronary interventions with rotational atherectomy and procedural outcomes: Analysis based on a large national registry. Catheter. Cardiovasc. Interv. 2022, 99, 1723–1732. [Google Scholar] [CrossRef]
- Rola, P.; Kulczycki, J.J.; Włodarczak, A.; Barycki, M.; Włodarczak, S.; Szudrowicz, M.; Furtan, Ł.; Jastrzębski, A.; Pęcherzewski, M.; Lesiak, M.; et al. Intravascular Lithotripsy as a Novel Treatment Method for Calcified Unprotected Left Main Diseases—Comparison to Rotational Atherectomy—Short-Term Outcomes. Int. J. Environ. Res. Public Health 2022, 19, 9011. [Google Scholar] [CrossRef]
- Kawamoto, H.; Latib, A.; Ruparelia, N.; Ielasi, A.; D’ascenzo, F.; Pennacchi, M.; Sardella, G.; Garbo, R.; Meliga, E.; Moretti, C.; et al. In-hospital and midterm clinical outcomes of rotational atherectomy followed by stent implantation: The ROTATE multicentre registry. Eurointervention 2016, 12, 1448–1456. [Google Scholar] [CrossRef]
- Bamford, P.; Collins, N.; Boyle, A. A State-of-the-Art Review: The Percutaneous Treatment of Highly Calcified Lesions. Hear. Lung Circ. 2022, 31, 1573–1584. [Google Scholar] [CrossRef]
- Kassimis, G.; Didagelos, M.; De Maria, G.L.; Kontogiannis, N.; Karamasis, G.V.; Katsikis, A.; Sularz, A.; Karvounis, H.; Kanonidis, I.; Krokidis, M.; et al. Shockwave Intravascular Lithotripsy for the Treatment of Severe Vascular Calcification. Angiology 2020, 71, 677–688. [Google Scholar] [CrossRef]
- Rola, P.; Włodarczak, A.; Barycki, M.; Doroszko, A. Use of the Shock Wave Therapy in Basic Research and Clinical Applications—From Bench to Bedsite. Biomedicines 2022, 10, 568. [Google Scholar] [CrossRef]
- Rola, P.; Włodarczak, A.; Barycki, M.; Pęcherzewski, M.; Kulczycki, J.J.; Szudrowicz, M.; Jastrzębski, A.; Furtan, Ł. Shockwave intravascular lithotripsy as a novel strategy for balloon undilatable heavily calcified chronic total occlusion lesions. Cardiol. J. 2021. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, R.S.; Sammour, Y.; Kalra, A.; Reed, G.; Krishnaswamy, A.; Ellis, S.; Nair, R.; Khatri, J.; Kapadia, S.; Puri, R. Excimer Laser Atherectomy in Percutaneous Coronary Intervention: A Contemporary Review. Cardiovasc. Revascularization Med. 2020, 25, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Włodarczak, A.; Rola, P.; Barycki, M.; Kulczycki, J.J.; Szudrowicz, M.; Lesiak, M.; Doroszko, A. Rota-Lithotripsy—A Novel Bail-Out Strategy for Calcified Coronary Lesions in Acute Coronary Syndrome. The First-in-Man Experience. J. Clin. Med. 2021, 10, 1872. [Google Scholar] [CrossRef] [PubMed]
- Rola, P.; Włodarczak, A.; Kulczycki, J.J.; Barycki, M.; Furtan, Ł.; Szudrowicz, M.; Jastrzębski, A.; Pęcherzewski, M.; Doroszko, A.; Lesiak, M. Feasibility of the intravascular lithotripsy in coronary artery disease. Short-term outcomes of the Lower-Silesia Shockwave Registry. Kardiol. Pol. 2021, 79, 1133–1135. [Google Scholar] [CrossRef]
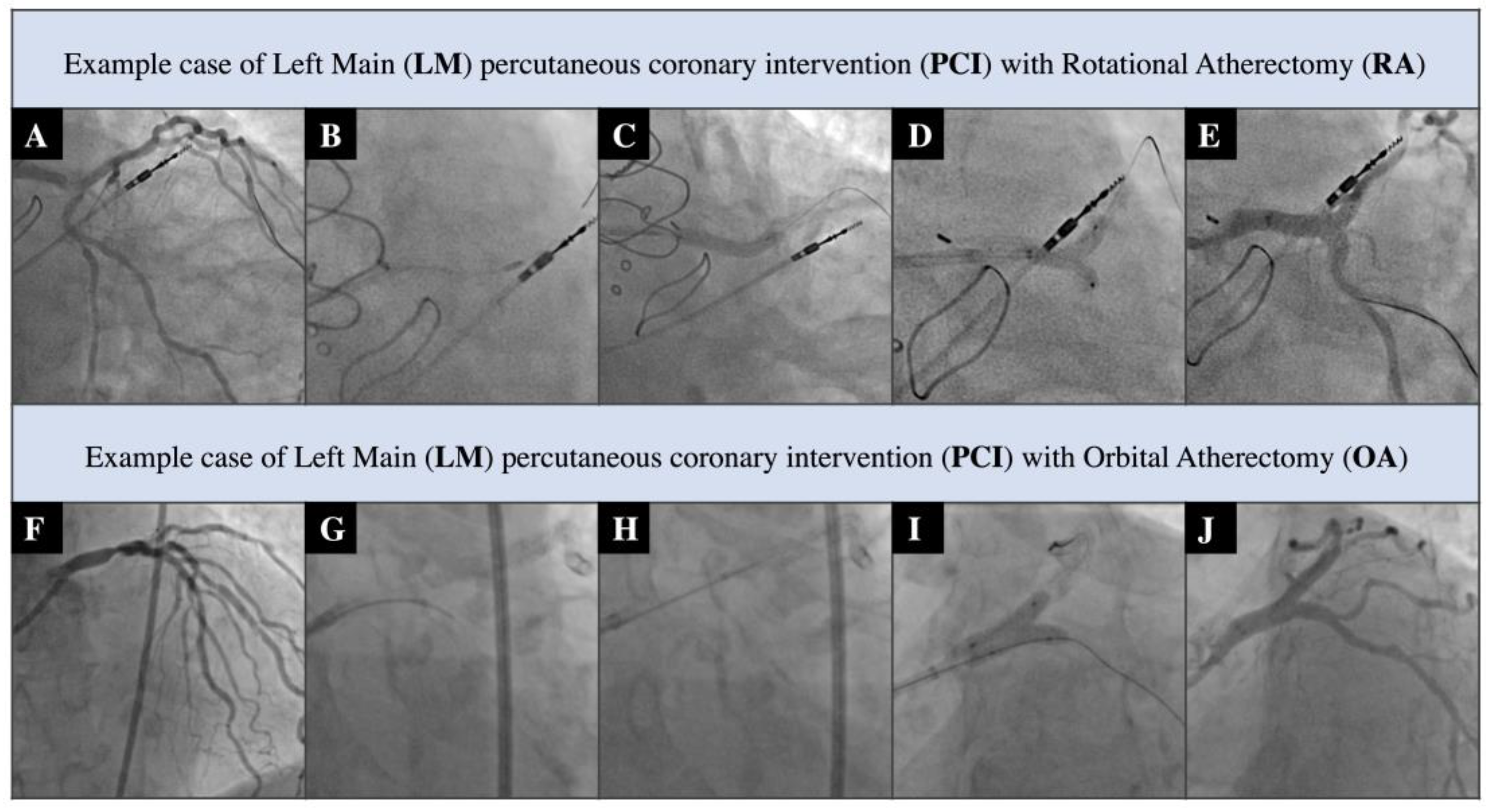
| Rotational Atherectomy (RA) N-30 | Orbital Atherectomy (OA) N-25 | p-Value | |
|---|---|---|---|
| Age | 70.4 ± 8.9 | 68.4 ± 7.9 | 0.398 |
| Gender male (ratio) | 21 (70%) | 17 (68.0%) | 0.584 |
| Stable angina | 13 (43.3%) | 6 (24%) | 0.163 |
| Unstable angina | 7 (23.3%) | 3 (12%) | 0.318 |
| NSTEMI | 9 (30.0%) | 16 (64%) | 0.106 |
| STEMI | 1 (3.4%) | 0 (0%) | 1 |
| Diabetes mellitus | 16 (53.3%) | 11 (44%) | 0.591 |
| Chronic heart failure | 10 (34.5%) | 14 (56%) | 0.170 |
| Hypertension | 26 (86.7%) | 24 (96%) | 0.362 |
| Hyperlipidemia | 21 (70%) | 24 (96%) | 0.015 |
| Atrial Fibrillation | 6 (20%) | 6 (24%) | 1 |
| History of PCI | 16 (53.3%) | 11 (44%) | 0.591 |
| History of MI | 14 (46.7%) | 12 (48%) | 1 |
| History of CABG | 6 (20%) | 2 (8%) | 0.269 |
| COPD | 8 (26.7%) | 10 (40%) | 0.389 |
| CKD | 13 (43.3%) | 6 (24%) | 0. 162 |
| Rotational Atherectomy (RA) N-30 | Orbital Atherectomy (OA) N-25 | p-Value | |
|---|---|---|---|
| SYNTAX I Score | 28 (26–33.1) | 28 (26–36) | 0.874 |
| SYNTAX II—PCI Score | 36.2 ± 8.6 | 45.3 ± 13.3 | 0.005 |
| SYNTAX II PCI four-year mortality | 10.8 (7.7–17.8) | 22.9 (9.3–41.1) | 0.009 |
| Syntax II—CABG Score | 34.2 (28.2–43.6) | 44.3 (37.5–49.3) | 0.010 |
| Syntax II CABG four-year mortality | 10.3 (6.1–20.5) | 21 (12–30) | 0.016 |
| Radial Access | 15 (50%) | 20 (80.0%) | 0.026 |
| 6F Guide Catheter | 3 (10%) | 16 (64%) | 0.015 |
| 7F or larger Guide Catheter | 27 (90%) | 14 (56%) | 0.015 |
| Total ablation time (s) | 180 (140–190) | 225 (200–300) | 0.002 |
| Average number of stents | 2 (2–2.75) | 2 (1–2) | 0.072 |
| Total stent length per procedure (mm) | 75 (50–90.3) | 60 (30–72) | 0.018 |
| Post-dilation—POT | 29 (96.7%) | 25 (100%) | 0.494 |
| Intravascular Guidance | 3 (10%) | 18 (72%) | 0.001 |
| Perforation | 3 (10%) | 1 (4%) | 0.617 |
| Slow/No-flow phenomenon | 2 (6.0%) | 1 (4%) | 1 |
| Administration of catecholamines | 3 (10%) | 3 (12%) | 1 |
| Acetylsalicylic Acid | 30 (100%) | 15 (100%) | 1 |
| Clopidogrel | 19 (63.3%) | 16 (64%) | 1 |
| Ticagrelor | 10 (33.3%) | 7 (28%) | 0.773 |
| Rotational Atherectomy (RA) N-30 | Orbital Atherectomy (OA) N-25 | p-Value | |
|---|---|---|---|
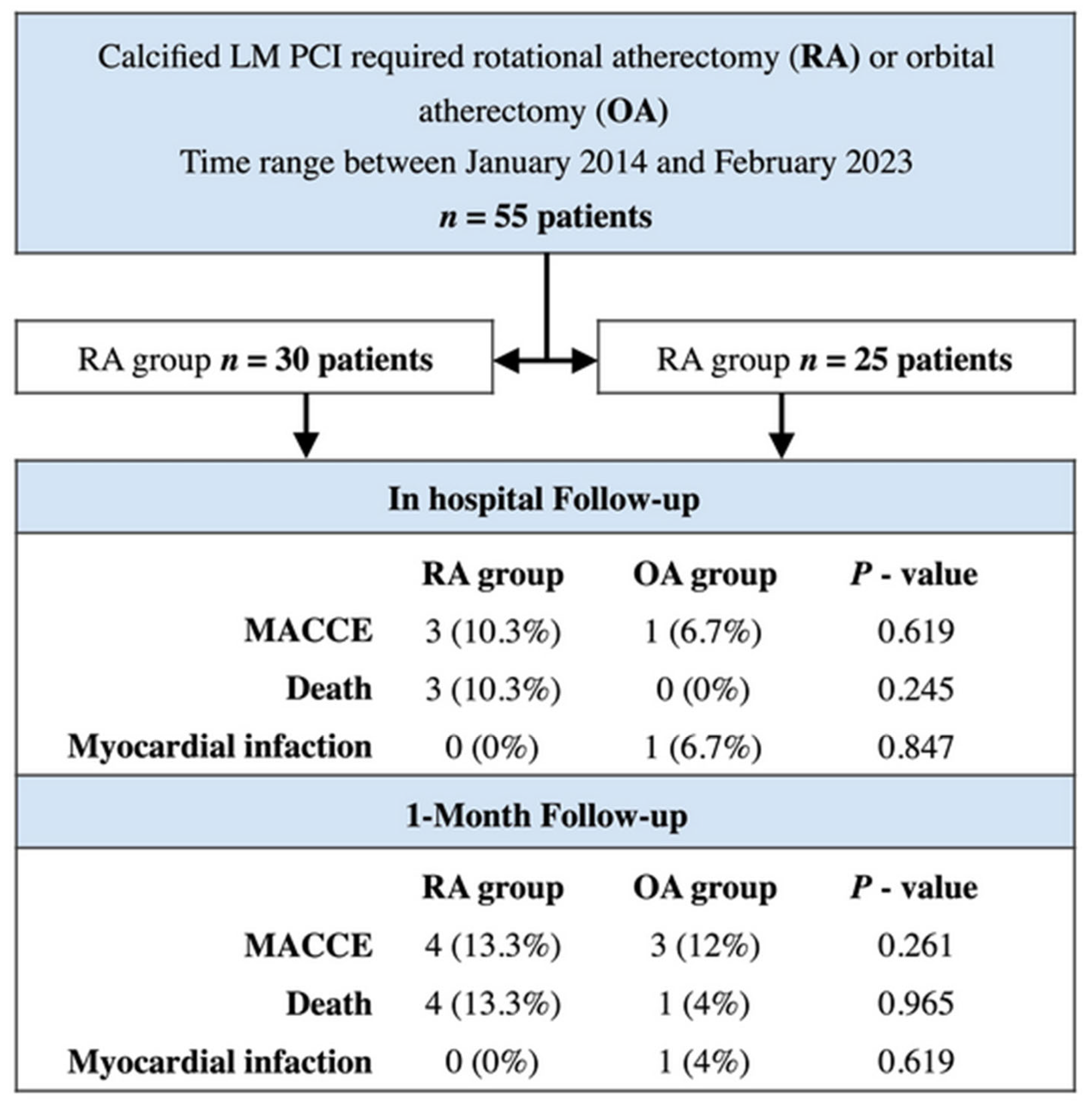
| In-hospital Follow-up | |||
| MACCE | 3 (10.3%) | 1 (6.7%) | 0.619 |
| Death | 3 (10.3%) | 0 (0%) | 0.245 |
| Myocardial infarction | 0 (0%) | 1 (6.7%) | 0.847 |
| Target vessel revascularization | 0 (0%) | 0 (0%) | 0.341 |
| Stent thrombosis | 0 (0%) | 0 (0%) | 0.341 |
| Cerebrovascular episodes | 0 (0%) | 0 (0%) | - |
| Stent restenosis | 0 (0%) | 0 (0%) | - |
| Need for any revascularization | 1 (3.3%) | 2 (8%) | 0.448 |
| 1-Month Follow-up | |||
| MACCE | 4 (13.3%) | 3 (12%) | 0.261 |
| Death | 4 (13.3%) | 1 (4%) | 0.965 |
| Myocardial infarction | 0 (0%) | 1 (4%) | 0.619 |
| Target vessel revascularization | 0 (0%) | 0 (0%) | - |
| Stent thrombosis | 0 (0%) | 0 (0%) | - |
| Cerebrovascular episodes | 0 (0%) | 1 (4%) | 0.619 |
| Stent restenosis | 0 (0%) | 0 (0%) | - |
| Need for any revascularization | 4 (6.9%) | 11 (44%) | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rola, P.; Kulczycki, J.J.; Barycki, M.; Włodarczak, S.; Furtan, Ł.; Kędzierska, M.; Giniewicz, K.; Doroszko, A.; Lesiak, M.; Włodarczak, A. Comparison of Orbital Atherectomy and Rotational Atherectomy in Calcified Left Main Disease: Short-Term Outcomes. J. Clin. Med. 2023, 12, 4025. https://doi.org/10.3390/jcm12124025
Rola P, Kulczycki JJ, Barycki M, Włodarczak S, Furtan Ł, Kędzierska M, Giniewicz K, Doroszko A, Lesiak M, Włodarczak A. Comparison of Orbital Atherectomy and Rotational Atherectomy in Calcified Left Main Disease: Short-Term Outcomes. Journal of Clinical Medicine. 2023; 12(12):4025. https://doi.org/10.3390/jcm12124025
Chicago/Turabian StyleRola, Piotr, Jan Jakub Kulczycki, Mateusz Barycki, Szymon Włodarczak, Łukasz Furtan, Michalina Kędzierska, Katarzyna Giniewicz, Adrian Doroszko, Maciej Lesiak, and Adrian Włodarczak. 2023. "Comparison of Orbital Atherectomy and Rotational Atherectomy in Calcified Left Main Disease: Short-Term Outcomes" Journal of Clinical Medicine 12, no. 12: 4025. https://doi.org/10.3390/jcm12124025
APA StyleRola, P., Kulczycki, J. J., Barycki, M., Włodarczak, S., Furtan, Ł., Kędzierska, M., Giniewicz, K., Doroszko, A., Lesiak, M., & Włodarczak, A. (2023). Comparison of Orbital Atherectomy and Rotational Atherectomy in Calcified Left Main Disease: Short-Term Outcomes. Journal of Clinical Medicine, 12(12), 4025. https://doi.org/10.3390/jcm12124025






