Under the Hood: Understanding the Features of Mucin in Pseudomyxoma Peritonei
Abstract
:1. Introduction
- Low-grade mucinous carcinoma peritonei: characterized by low-grade cytology, few mitoses, and scant mucinous tumor epithelium (<20% of tumor volume).
- High-grade mucinous carcinoma peritonei is characterized by the presence of at least one of the following features: high-grade cytology, infiltration of adjacent tissues, invasion of vascular lymphatic vessels or surrounding nerves, cribriform growth, or extensive mucinous tumor epithelium (>20% of tumor volume).
- High-grade mucinous carcinoma peritonei with signet ring cells: characterized by the presence of neoplastic signet ring cells (signet ring cells ≥ 10%).
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buell-Gutbrod, R.; Gwin, K. Pathologic diagnosis, origin, and natural history of pseudomyxoma peritonei. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Morera-Ocon, F.J.; Navarro-Campoy, C. History of pseudomyxoma peritonei from its origin to the first decades of the twenty-first century. World J. Gastrointest. Surg. 2019, 11, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Govaerts, K.; Lurvink, R.J.; De Hingh, I.H.J.T.; Van der Speeten, K.; Villeneuve, L.; Kusamura, S.; Kepenekian, V.; Deraco, M.; Glehen, O.; Moran, B.J.; et al. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur. J. Surg. Oncol. 2021, 47, 11–35. [Google Scholar] [CrossRef]
- Arjona-Sánchez, Á.; Martínez-López, A.; Valenzuela-Molina, F.; Rufián-Andújar, B.; Rufián-Peña, S.; Casado-Adam, Á.; Sánchez-Hidalgo, J.M.; Rodríguez-Ortiz, L.; Medina-Fernández, F.J.; Díaz-López, C.; et al. A Proposal for Modification of the PSOGI Classification according to the Ki-67 Proliferation Index in Pseudomyxoma Peritonei. Ann. Surg. Oncol. 2022, 29, 126–136. [Google Scholar] [CrossRef]
- Floriano, I.; Silvinato, A.; Reis, J.C.; Cafalli, C.; Bernardo, W.M. Efficacy and safety in the use of intraperitoneal hyperthermia chemotherapy and peritoneal cytoreductive surgery for pseudomyxoma peritonei from appendiceal neoplasm: A systematic review. Clinics 2022, 77, 100039. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Li, Y. The Biological Synthesis and the Function of Mucin 2 in Pseudomyxoma Peritonei. Cancer Manag. Res. 2021, 13, 7909–7917. [Google Scholar] [CrossRef]
- Pillai, K.; Akhter, J.; Mekkawy, A.; Chua, T.C.; Morris, D.L. Physical and chemical characteristics of mucin secreted by pseudomyxoma peritonei (PMP). Int. J. Med. Sci. 2017, 14, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.K.; Valle, S.J.; Morris, D.L. Bromelain and acetylcysteine (BromAc®): A novel approach to the treatment of mucinous tumours. Am. J. Cancer Res. 2023, 13, 1522–1532. [Google Scholar]
- Tong, M.; Jacobs, J.P.; McHardy, I.H.; Braun, J. Sampling of Intestinal Microbiota and Targeted Amplification of Bacterial 16S rRNA Genes for Microbial Ecologic Analysis. Curr. Protoc. Immunol. 2014, 107, 7–41. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, J.T.; Tomlinson, J.S.; Roberts, A.A.; McGonigle, K.F.; Barsky, S.H. Pseudomyxoma Peritonei Is a Disease of MUC2-Expressing Goblet Cells. Am. J. Pathol. 2002, 161, 551–564. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, F.; Gething, S.; Haiba, M.; Brun, E.A.; Sugarbaker, P.H. Clinically aggressive pseudomyxoma peritonei: A variant of a histologically indolent process. J. Surg. Oncol. 2004, 86, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, D.; Kusamura, S.; Baratti, D.; Casali, P.; Younan, R.; Deraco, M. CDX-2 expression in pseudomyxoma peritonei: A clinicopathological study of 42 cases. Histopathology 2006, 49, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Kusamura, S.; Nonaka, D.; Cabras, A.D.; Laterza, B.; Deraco, M. Pseudomyxoma Peritonei. Ann. Surg. 2009, 249, 243–249. [Google Scholar] [CrossRef]
- Mall, A.S.; Chirwa, N.; Govender, D.; Lotz, Z.; Tyler, M.; Rodrigues, J.; Kahn, D.; Goldberg, P. MUC2, MUC5AC and MUC5B in the mucus of a patient with pseudomyxoma peritonei: Biochemical and immunohistochemical study. Pathol. Int. 2007, 57, 537–547. [Google Scholar] [CrossRef]
- Ferreira, C.R.; Carvalho, J.P.; Soares, F.A.; Siqueira, S.A.C.; Carvalho, F.M. Mucinous ovarian tumors associated with pseudomyxoma peritonei of adenomucinosis type: Immunohistochemical evidence that they are secondary tumors. Int. J. Gynecol. Cancer 2008, 18, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.T.; Song, X.; Wei, L.X.; Zhao, P. Histological origin of pseudomyxoma peritonei in Chinese women: Clinicopathology and immunohistochemistry. World J. Gastroenterol. 2011, 17, 3531–3537. [Google Scholar] [CrossRef]
- Chang, M.S.; Byeon, S.J.; Yoon, S.O.; Kim, B.H.; Lee, H.S.; Kang, G.H.; Kim, W.H.; Park, K.J. Leptin, MUC2 and mTOR in Appendiceal Mucinous Neoplasms. Pathobiology 2012, 79, 45–53. [Google Scholar] [CrossRef]
- Breugelmans, T.; Oosterlinck, B.; Arras, W.; Ceuleers, H.; De Man, J.; Hold, G.L.; De Winter, B.Y.; Smet, A. The role of mucins in gastrointestinal barrier function during health and disease. Lancet Gastroenterol. Hepatol. 2022, 7, 455–471. [Google Scholar] [CrossRef]
- Khamzina, Y.; King, M.C.; Nieroda, C.; Merrell, D.S.; Sardi, A.; Gushchin, V. The Role of Microorganisms in Appendiceal Pseudomyxoma Peritonei: A Review. Curr. Oncol. 2022, 29, 3576–3584. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Gilbreath, J.J.; Semino-Mora, C.; Friedline, C.J.; Liu, H.; Bodi, K.L.; McAvoy, T.J.; Francis, J.; Nieroda, C.; Sardi, A.; Dubois, A.; et al. A core microbiome associated with the peritoneal tumors of pseudomyxoma peritonei. Orphanet J. Rare Dis. 2013, 8, 105. [Google Scholar] [CrossRef] [Green Version]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Dohrman, A.; Miyata, S.; Gallup, M.; Li, J.D.; Chapelin, C.; Coste, A.; Escudier, E.; Nadel, J.; Basbaum, C. Mucin gene (MUC 2 and MUC 5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim. Biophys. Acta Mol. Basis Dis. 1998, 1406, 251–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Mohamed, F.; Garcia-Verdugo, I.; Medina, M.; Balloy, V.; Chignard, M.; Ramphal, R.; Touqui, L. A Crucial Role of Flagellin in the Induction of Airway Mucus Production by Pseudomonas aeruginosa. PLoS ONE 2012, 7, e39888. [Google Scholar] [CrossRef]
- García-Olmo, D.; Olmedillas-López, S.; Cortés-Guiral, D.; Villarejo, P.; López Rojo, I.; Guadalajara, H.; García Gómez-Heras, S.; García-Arranz, M. The role of mucin cell-free DNA detection as a new marker for the study of acellular pseudomyxoma peritonei of appendicular origin by liquid biopsy. Ther. Adv. Med. Oncol. 2020, 12, 175883592092823. [Google Scholar] [CrossRef] [PubMed]
- Semino-Mora, C.; Testerman, T.L.; Liu, H.; Whitmire, J.M.; Studeman, K.; Jia, Y.; McAvoy, T.J.; Francis, J.; Nieroda, C.; Sardi, A.; et al. Antibiotic Treatment Decreases Microbial Burden Associated with Pseudomyxoma Peritonei and Affects β-Catenin Distribution. Clin. Cancer Res. 2013, 19, 3966–3976. [Google Scholar] [CrossRef] [Green Version]
- Park, S.C.; Shimamura, I.; Fukunaga, M.; Mori, K.I.; Nakai, T. Isolation of Bacteriophages Specific to a Fish Pathogen, Pseudomonas plecoglossicida, as a Candidate for Disease Control. Appl. Environ. Microbiol. 2000, 66, 1416–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimori, E.; Kita-Tsukamoto, K.; Wakabayashi, H. Pseudomonas plecoglossicida sp. nov., the causative agent of bacterial haemorrhagic ascites of ayu, Plecoglossus altivelis. Int. J Syst. Evol. Microbiol. 2000, 50, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.T.; Zhou, S.M.; An, S.W.; Chen, L.; Wang, G.L. Visceral granulomas in farmed large yellow croaker, Larimichthys crocea (Richardson), caused by a bacterial pathogen, Pseudomonas plecoglossicida. J. Fish Dis. 2014, 37, 113–121. [Google Scholar] [CrossRef]
- Huang, L.; Zuo, Y.; Jiang, Q.; Su, Y.; Qin, Y.; Xu, X.; Zhao, L.; Yan, Q. A metabolomic investigation into the temperature-dependent virulence of Pseudomonas plecoglossicida from large yellow croaker (Pseudosciaena crocea). J. Fish Dis. 2019, 42, 431–446. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, Z.; Weng, S.; He, J.; Dong, C. Characterization of a highly lethal barramundi (Lates calcarifer) model of Pseudomonas plecoglossicida infection. Microb. Pathog. 2020, 149, 104516. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Xiong, J.B.; Ding, F.F.; Chen, J. Immune and gut bacterial successions of large yellow croaker (Larimichthys crocea) during Pseudomonas plecoglossicida infection. Fish Shellfish Immunol. 2020, 99, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Porcel, M.; de la Torre, J.; Molina-Henares, M.A.; Daddaoua, A.; Llamas, M.A.; Roca, A.; Carriel, V.; Garzón, I.; Ramos, J.L.; et al. Analysis of the pathogenic potential of nosocomial Pseudomonas putida strains. Front. Microbiol. 2015, 6, 871. [Google Scholar] [CrossRef] [PubMed]



| Age | Sex | Primary Tumor | Perforated | PMP Classification | Preoperative Chemotherapy | Cytoreduction Score | Current Status | Overall Survival (Months) |
|---|---|---|---|---|---|---|---|---|
| 59 | ♂ | LAMN | Yes | Metachronous LMCP (LMCP-1) | No | CC0 | AWR | 84 |
| 44 | ♀ | LAMN | Yes | Synchronous AM (AM-1) | No | CC0 | AWR | 79 |
| 45 | ♂ | LAMN | Yes | Metachronous AM (AM-2) | No | CC0 | AWR | 75 |
| 75 | ♀ | LAMN | Yes | Metachronous AM (AM-3) | No | CC0 | AWR | 72 |
| 73 | ♀ | MCA | No | Metachronous LMCP (LMCP-2) | No | CC0 | AWR | 54 |
| 80 | ♀ | LAMN | No | Synchronous LMCP (LMCP-3) | No | CC1 | DWR | 17 |
| Patients | Samples | Mucin-1 (µg/µL) | Mucin-2 (µg/µL) | Mucin-5AC (µg/µL) |
|---|---|---|---|---|
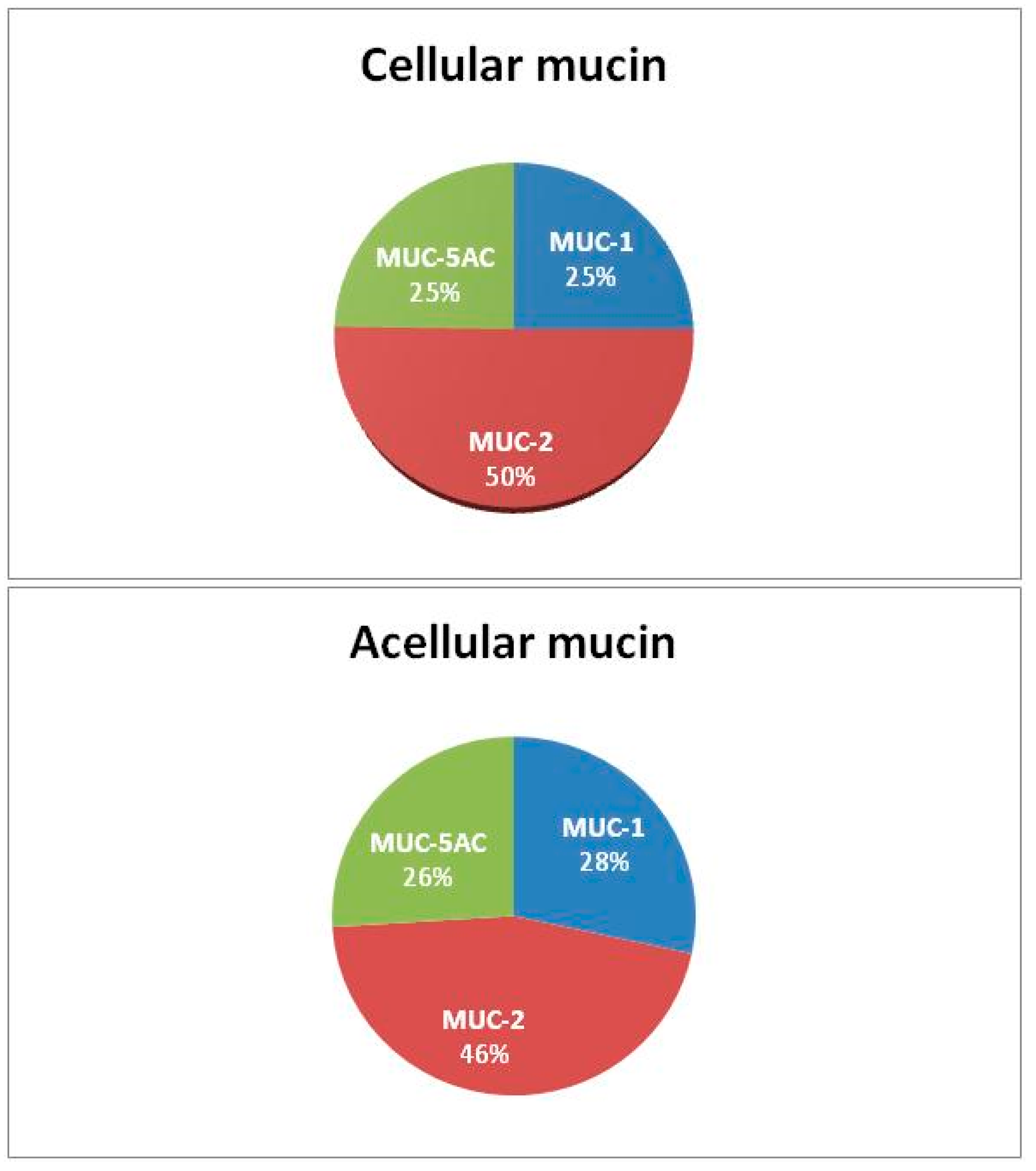
| LMCP-1 | Cellular mucin | 46 | 82 | 40 |
| LMCP-2 | Cellular mucin | 33 | 77 | 31 |
| LMCP-3 | Cellular mucin | 35 | 70 | 42 |
| AM-1 | Acellular mucin | 44 | 68 | 40 |
| AM-2 | Acellular mucin | 44 | 68 | 40 |
| AM-3 | Acellular mucin | 43 | 75 | 40 |
| SAMPLES | MUC-2 | MUC-5AC | MUC-5B | MUC-1 | MUC-6 | MUC-4 | |
|---|---|---|---|---|---|---|---|
| O’Connell (2002) [7] | Appendix, ovarian, and peritoneal tissues (25) | ✓ | ✓ | ||||
| Mohamed (2004) [8] | Peritoneal tissue (11) | ✓ | ✓ | ||||
| Nonaka (2006) [9] | Peritoneal tissue (42) | ✓ | ✓ | ||||
| Mall (2007) [10] | Mucin (cellular) | ✓ | ✓ | ✓ | ✓ | ||
| Ferreira (2008) [11] | Ovarian tissue (28) | ✓ | ✓ | ||||
| Baratti (2009) [12] | Peritoneal tissue (85) | ✓ | ✓ | ||||
| Guo (2011) [13] | Appendix, ovarian, and peritoneal tissues (35) | ✓ | |||||
| Chang (2012) [14] | Appendix tissue (22) | ✓ | ✓ | ||||
| Pillai (2017) [5] | Mucin (16) | ✓ | ✓ | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villarejo-Campos, P.; García-Arranz, M.; Qian, S.; Jiménez de los Galanes, S.; Domínguez-Prieto, V.; Vélez-Pinto, J.F.; Guijo Castellano, I.; Jiménez-Fuertes, M.; Guadalajara, H.; García-Olmo, D. Under the Hood: Understanding the Features of Mucin in Pseudomyxoma Peritonei. J. Clin. Med. 2023, 12, 4007. https://doi.org/10.3390/jcm12124007
Villarejo-Campos P, García-Arranz M, Qian S, Jiménez de los Galanes S, Domínguez-Prieto V, Vélez-Pinto JF, Guijo Castellano I, Jiménez-Fuertes M, Guadalajara H, García-Olmo D. Under the Hood: Understanding the Features of Mucin in Pseudomyxoma Peritonei. Journal of Clinical Medicine. 2023; 12(12):4007. https://doi.org/10.3390/jcm12124007
Chicago/Turabian StyleVillarejo-Campos, Pedro, Mariano García-Arranz, Siyuan Qian, Santos Jiménez de los Galanes, Víctor Domínguez-Prieto, Juan Felipe Vélez-Pinto, Ismael Guijo Castellano, Montiel Jiménez-Fuertes, Héctor Guadalajara, and Damián García-Olmo. 2023. "Under the Hood: Understanding the Features of Mucin in Pseudomyxoma Peritonei" Journal of Clinical Medicine 12, no. 12: 4007. https://doi.org/10.3390/jcm12124007
APA StyleVillarejo-Campos, P., García-Arranz, M., Qian, S., Jiménez de los Galanes, S., Domínguez-Prieto, V., Vélez-Pinto, J. F., Guijo Castellano, I., Jiménez-Fuertes, M., Guadalajara, H., & García-Olmo, D. (2023). Under the Hood: Understanding the Features of Mucin in Pseudomyxoma Peritonei. Journal of Clinical Medicine, 12(12), 4007. https://doi.org/10.3390/jcm12124007








